Abstract
Previous studies by our group showed that nicotine delivered via a transdermal nicotine patch significantly enhanced posterior spinal fusion rates in rabbits. Nicotine transdermal patches provide a steady serum level; there may be a dose-dependent effect of nicotine on posterior spinal fusion. In an in vitro cell culture model of rabbit bone marrow–derived osteoblast-like cells, cells were exposed to different concentrations of nicotine (0, 20, 40, 80 ng/mL and 10, 100, 250 μg/mL). Wells were stained with an alkaline phosphatase (ALP) staining kit to determine ALP enzyme activity. Cells were stained with Von Kossa for mineralization. A two-way analysis of variance (ANOVA) using dose and time as variables showed significant differences among groups; post hoc analysis showed that the 100-μg/mL dose of nicotine significantly enhanced ALP activity over controls. A one-way ANOVA using dose as the variable showed that the 100- and 250-μg/mL doses had significantly greater mineralization than controls. Dose-response analysis revealed a statistically significant effect of nicotine dose on ALP activity and Von Kossa activity. The effects of nicotine on spinal fusion may be dose-dependent and due to stimulation of osteoblastic activity. Nicotine may not be responsible for the inhibited bone healing observed in smokers.
Keywords: nicotine, bone healing, spinal fusion, osteoblasts, smoking
Several studies demonstrate that cigarette smoking is detrimental to bone health and impairs bone healing. Gerdhem and Obrant1 showed in elderly women that smoking significantly reduced hip and total body bone mineral density. Andersen et al2 reported that smoking resulted in an odds ratio of nonunion of 2.01 in lumbar fusion patients. Ueng et al3 demonstrated that intermittent exposure to cigarette smoke in rabbits reduced torsional strength of the tibia during tibial lengthening procedures. Laroche et al4 found that smoking significantly reduced serum osteocalcin levels in males, and Nersessian and Arutyunyan5 showed that exposure to cigarette smoke increased osteoclastogenic activity in mice.
Nicotine has been implicated as the agent in cigarette smoke that is responsible for the ill effects of smoking on bone health. Hollinger et al6 reported that nicotine adversely affected bone healing in a rat model, and Wing et al7 showed that chronic exposure to nicotine decreased fusion rates in a rabbit model and that quitting improved fusion rates. Silcox et al8 found that systemic nicotine had a negative effect on bone fusion in a rabbit model of posterior lumbar fusion. Furthermore, Riebel et al9 showed that nicotine increased nonunion in a bone graft model in rabbits and correlated it to reduced vascularization in the bone graft. They did, however, notice a varied response to nicotine among different animals, which they attributed to “predisposition” of the individual animals.
The mechanism by which nicotine affects bone health and healing is not clearly known. Theiss et al10 showed that gene expression of type I and II collagen, bone morphogenic protein (BMP)-2, BMP-4, BMP-6, basic fibroblast growth factor, and vascular endothelial growth factor were reduced by systemic administration of nicotine. Nicotine reduced vascularization to autologous cancellous bone grafts in the anterior chamber of the eye in a rabbit model.11 Feitelson et al12 reported that nicotine increased the effect of norepinephrine in constricting bone vasculature. To the contrary, however, Heeschen et al13 found nicotine to be a potent stimulator of angiogenesis in three different animal models not involving bone, and Clouse et al14 found that results of coronary artery bypass grafts in a canine model were unaffected by nicotine.
In a previous animal study designed to evaluate the effects of direct current electrical stimulation on spinal fusion in rabbits exposed to nicotine, we found that both the nicotine control group of animals (fusion + nicotine administration) and the direct current stimulation group (fusion + nicotine + direct current stimulator) had increased fusion rates compared with the negative control group (fusion alone).15 In light of the above information, we found this intriguing, as this was contrary to what other groups had found.6 7 8 9 To resolve this issue, the current study was undertaken to see if the effects of nicotine on bone could be dose-dependent and if this could be demonstrated in an in vitro evaluation of osteoblast activity.
Materials and Methods
Establishing a Bone Marrow–Derived Osteoblast-Like Cell Culture System
Bone marrow was isolated from adult male New Zealand white rabbit femurs via aspiration with an 18-gauge needle. The cells in the bone marrow were expanded in monolayer culture and split and subcultured according to standard protocols developed for rat models.16 17 Approximately 5 × 104 cells were placed in each treatment well of a 24-well plate and exposed to one of three treatments: (1) minimum essential medium (MEM); (2) 40 ng/mL dexamethasone and MEM; or (3) 50 ng/mL BMP-2, 40 ng/mL dexamethasone, and MEM. The noncontrol treatments were given additional mineralizing agents of 2.8 × 10−4 mol/L concentration of L-ascorbic acid and 10 mmol/L concentration of β-glycerophosphate starting on day 7 and every media change thereafter.18 19 The media was changed every 3 days. Wells were stained with an alkaline phosphatase staining kit (Sigma, St. Louis, MO) to determine the activity of the alkaline phosphatase enzyme, an early indicator of osteogenesis,20 and Von Kossa,21 which indicates mineralization, a late stage of osteogenesis. The amount of staining was quantified as follows: Digital images of the stained wells were obtained at low power such that the entire well could be accommodated in one picture frame (as can be seen in Figs. 1 2 3 4). The images were imported into image analysis software (Image J: http://rsbweb.nih.gov/ij/). Using a custom written macro, it was possible to measure the area of the image that was stained. The measured area depended on the threshold value used for the image (portions of the image with staining intensity below the threshold would be ignored; thus the higher the threshold value, the lower the stained area). The appropriate threshold value was determined from the control samples; in the control samples, the threshold value was adjusted until the stained area became 0.
Figure 1.
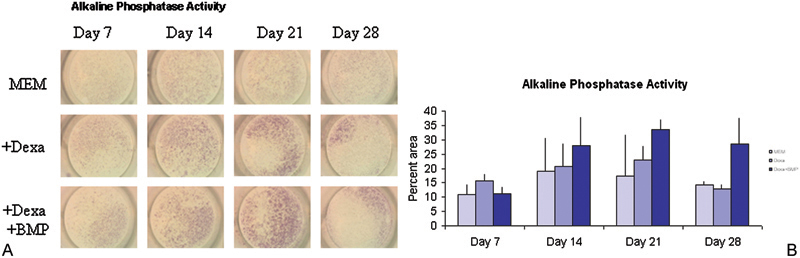
(A) Rabbit bone marrow–derived cells were cultured in monolayer under different conditions and tested for alkaline phosphatase activity (proportional to red color) at different times. (B) Alkaline phosphatase enzyme activity increased through day 21 and declined by day 28. Analysis of variance showed that some groups were significantly different from others (f = 0.0021 at p = 0.05 level) in terms of alkaline phosphatase activity. MEM, minimum essential medium; Dexa, dexamethasone; BMP, bone morphogenic protein.
Figure 2.
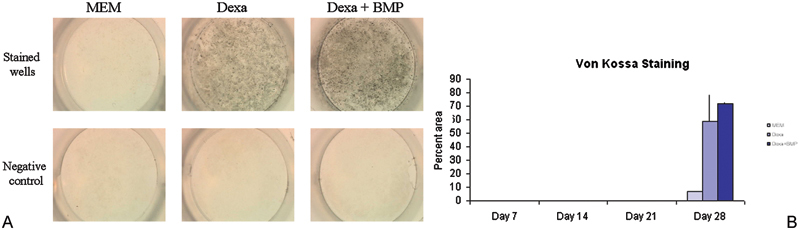
(A) Rabbit bone marrow–derived cells were cultured in monolayer and tested for calcium deposition by Von Kossa staining (black) at different time points. In the negative controls, a chelator of calcium was added before Von Kossa staining. The clear wells of the negative control indicated that the stain seen was due to deposited calcium and was not an artifact. (B) Von Kossa staining was not observed through day 21 but appeared at day 28. Treatment groups showed significantly higher mineralization than controls on day 28 (p = 0.001). MEM, minimum essential medium; Dexa, dexamethasone; BMP, bone morphogenic protein.
Figure 3.
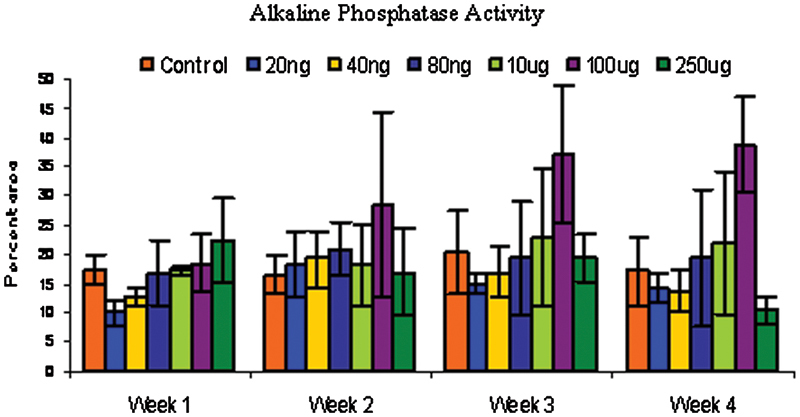
Rabbit bone marrow–derived cells were cultured in monolayer under different conditions and tested for alkaline phosphatase activity at different time points. Alkaline phosphatase enzyme activity was significantly enhanced by nicotine at 100-μg/mL dose over the control at p < 0.05.
Figure 4.
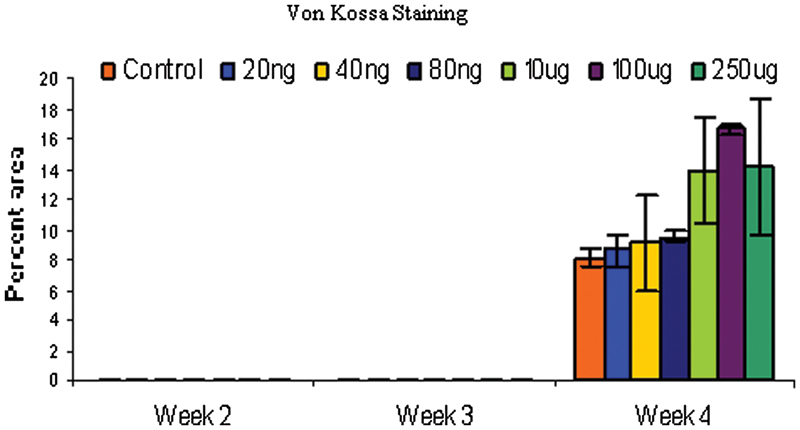
Rabbit bone marrow–derived cells were cultured in monolayer and tested for calcium deposition by Von Kossa staining at different time points. Von Kossa staining was not observed through day 21 but appeared at day 28. Both 100 and 250 μg/mL treatments showed significantly higher mineralization than control on day 28 (p < 0.05).
Effects of Nicotine on Bone Marrow–Derived Osteoblast-Like Cells
The cell culture conditions were optimized based on previous experience with these cells. Approximately 3 × 103 cells were placed in each treatment well of a 24-well plate and culture medium containing MEM and 40 ng/mL dexamethasone was added. All treatments were given 2.8 × 10−4 mol/L concentration of L-ascorbic acid and 10 mmol/L concentration of β-glycerophosphate after day 7 and with every media change after that. Media were changed every 3 days. This regimen was shown to induce osteogenesis in the cells. Cells were exposed to one of six concentrations of nicotine: 20, 40, 80 ng/mL and 10, 100, 250 μg/mL. Control cells were not exposed to nicotine. Wells were stained with an alkaline phosphatase staining kit (Sigma) to determine the activity of the alkaline phosphatase enzyme. Cells were also stained with Von Kossa to determine mineralization.
Results
Establishing a Bone Marrow–Derived Osteoblast-Like Cell Culture System
The trend in the data showed that alkaline phosphatase activity increased over a 3-week period with a decline in activity seen at week 4 (Figs. 1A, 1B). Von Kossa staining was not observed at sampling times of weeks 1 through 3. Week 4 showed a positive stain for Von Kossa (Figs. 2A, 2B) with the MEM + BMP-2 + dexamethasone treatment having the largest percent of area coverage (p = 0.001). This mimicked the behavior of other preosteoblastic cells that have been induced to undergo osteogenesis.17 22
Effects of Nicotine on Bone Marrow–Derived Osteoblast-Like Cells
Control cells behaved as expected, with increasing alkaline phosphatase activity up to 3 weeks and dropping off slightly at 4 weeks. As expected, there was no appreciable Von Kossa staining up to 3 weeks but after 4 weeks, the control cells stained positively for Von Kossa. A two-way analysis of variance (ANOVA) using dose and time as variables showed significant differences among groups (f = 0.0021). A post hoc analysis (least squares means differences Student t test) among groups showed that the 100-μg/mL dose of nicotine significantly enhanced alkaline phosphatase activity over controls (Fig. 3). Von Kossa staining was not observed at sampling times of weeks 1 through 3. Week 4 showed a positive stain for Von Kossa (Fig. 4). A one-way ANOVA using dose as the variable was performed, and the 100- and 250-μg/mL dose had significantly greater mineralization than controls.
The dose-response analysis revealed a statistically significant (p = 0.001) effect of nicotine dose on alkaline phosphatase activity and Von Kossa activity at 4 weeks (Figs. 5A, 5B). The alkaline phosphatase activity at 1, 2, and 3 weeks showed the same trends but the results were not statistically significant.
Figure 5.
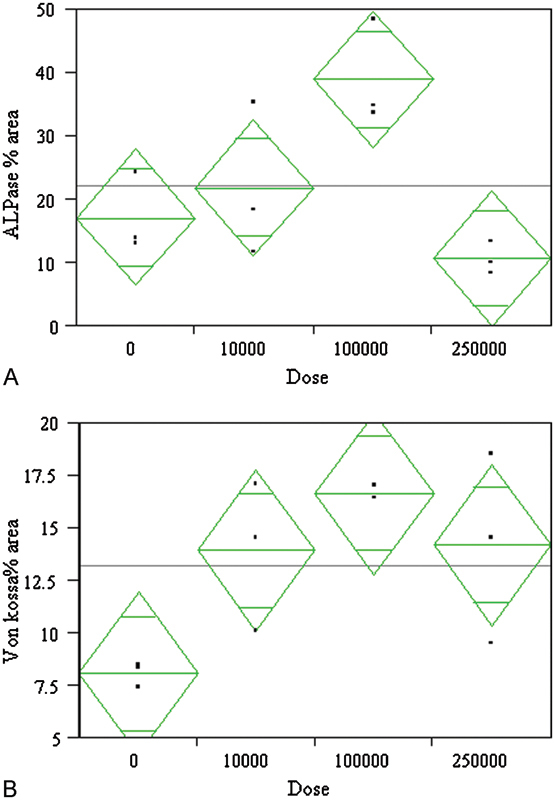
A dose-response analysis showed a statistically significant effect of dose on osteoblastic activity markers (A) alkaline phosphatase (ALPase) activity (p = 0.0139) and (B) Von Kossa (p = 0.0352) staining after 4 weeks of culture. The doses are shown in ng/mL concentrations. The peak effect was seen for the 100-μg/mL dose and dropped off for higher doses. The bars in the diamond represent mean and 95% confidence intervals.
Discussion
Our study demonstrated a statistically significant effect of nicotine dose on osteoblast activity in vitro. Bone marrow–derived osteoblastic cells increase alkaline phosphatase activity in the presence of nicotine, although statistically significant effects were only seen for high doses in the μg/mL range. The maximum effect was observed at the 100-μg/mL dosage. Mineralization was enhanced when nicotine was administered with higher dosages, resulting in more mineralization (100- and 250-μg/mL dosages). In a similar study, nicotine has been shown to have a significant dose-dependent effect on the activity of osteoblastic cell lines with increased alkaline phosphatase activity and deposition of calcium.23 Fang et al24 showed that nicotine suppressed proliferation and increased alkaline phosphatase activity in UMR 601 osteoblast-like cells. Walker et al.25 showed that nicotine had a direct effect on osteoblasts that includes increased expression of osteopontin. In that study, cells expressed the nicotine receptor α 4 unit and the effects of nicotine were blocked by receptor antagonists. Rothem et al reported a dose-dependent effect of nicotine on bone metabolism in osteoblastic cells, although low-dose nicotine (equivalent to a light smoker) up-regulated expression of osteocalcin, type I collagen, and alkaline phosphatase, and a high concentration inhibited expression of these genes.26 Kim et al27 showed a bimodal effect of nicotine on the proliferation and osteoblastic differentiation of mesenchymal stem cells derived from alveolar bone marrow. These studies support the fact that nicotine can have a positive role in mesenchymal stem cells and osteoblast function and may act in a dose-dependent manner. Thus, the increased fusion rates we previously observed in rabbits exposed to nicotine in vivo might be explained partially by enhanced osteoblastic activity due to nicotine.
The dose of nicotine exposure in the current study, however, is substantially higher than that administered during our prior in vivo study.15 This disparity in doses that causes responses in the in vivo and in vitro systems continues to be a concern and demands further investigation. One potential explanation could be that the bone marrow aspirate we used contains many cell types, only a small number of which are osteoblast progenitors, and thus a higher dose is required to elicit a statistically significant difference. The sensitivity of the assay could also be a factor. However, the trend is consistent with dosage, although very high doses were required for statistical significance. The fact that our in vivo and in vitro data trend in the same direction despite dose differences is encouraging.
Gullihorn et al,36 working with an osteoblastic cell line, showed statistically significant responses of nicotine for as low as 12.5 ng/mL, which is well within the circulating levels of nicotine in smokers. Kim et al27 showed bimodal responses that changed as the concentration of nicotine shifted between the 1 to 2 mmol/L range. Thus the specific cell-culture model seems to heavily influence the dosages at which the effects of nicotine are seen.
Interestingly, in many models of posterior spinal fusion, in vivo administration of nicotine has been shown to decrease fusion rates. Decreased angiogenesis measured by decreased vascular ingrowth into autogenous cancellous bone grafts9 and inhibition of the expression of cytokines associated with neovascularization10 have been observed in these models. However, in other settings, such as in tumor or ischemia models, nicotine is a potent enhancer of angiogenesis.13 The overall relationship between bony fusion rates and nicotine may depend on many factors including both angiogenesis and osteoblastic activity. Nicotine seems to positively affect osteoblasts and negatively affect vascularization in bone and increase angiogenesis in other models.
The effects of nicotine alone on bone health are not conclusive. High doses of nicotine administered via mini–osmotic pumps (1.5 to 4 times as much as heavy smokers) administered for 12 weeks caused no difference in bone mass and strength in female rats,28 although bone mineral density was lowered in male rats given nicotine via drinking water in a different study.29 In growing female rats, nicotine had no effect on bone mineral content, bone strength, or markers of bone metabolism such as vitamin D, calcitonin, and parathyroid hormone.30 In adult female rats, a limited effect was noticed for high concentrations of nicotine.31 Gotfredsen et al reported that long-term (6 months) exposure to nicotine did not have a detrimental effect on osseointegration of titanium implants in a rabbit osteotomy model.32
Numerous clinical studies have documented deleterious effects of smoking on bone healing and spinal fusion.2 33 34 35 One must be careful, however, to differentiate smoking exposure from nicotine exposure. Gullihorn et al reported that although exposure of osteoblast-like cells to nicotine elicited a dose-dependent increase in metabolic activity, preparations composed of cigarette smoke condensate with equivalent levels of nicotine showed reduced alkaline phosphatase activity and decreased total protein and collagen synthesis.36 Skott et al reported the results of exposure to nicotine, tobacco extract, and both in a rat femur fracture model.37 When mechanically testing the fracture healing, they found that ultimate torque and torque at yield point in rats receiving tobacco extract alone were decreased 21% and 23%, respectively, compared with the control (saline infusion) group and 20% and 26%, respectively, compared with the nicotine-only group. The combined group (tobacco extract plus nicotine) demonstrated an 18% torque reduction compared with the nicotine only group, and no difference was found between the tobacco-only group and the combined group.37 This suggests that smoking, rather than just nicotine, may be the prime culprit. Some authors have even hypothesized that low-dose nicotine exposure may be utilized to reduce the incidence of osteoporosis.38
Conclusions
In conclusion, the results of this study suggest that the effects of nicotine on osteoblast activity are complex and dose-dependent and may not always be detrimental. More research needs to be performed before the effects of nicotine on healing bone can be determined. Nicotine may not be responsible for the inhibited bone healing observed in smokers.
Footnotes
Disclosures John C. France, None Scott D. Daffner, None Stacey Waugh, None Timothy L. Norman, None Nilay Mukherjee, None
References
- 1.Gerdhem P, Obrant K J. Effects of cigarette-smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int. 2002;13:932–936. doi: 10.1007/s001980200130. [DOI] [PubMed] [Google Scholar]
- 2.Andersen T, Christensen F B, Laursen M, Høy K, Hansen E S, Bünger C. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001;26:2623–2628. doi: 10.1097/00007632-200112010-00018. [DOI] [PubMed] [Google Scholar]
- 3.Ueng S W, Lee M Y, Li A F, Lin S S, Tai C L, Shih C H. Effect of intermittent cigarette smoke inhalation on tibial lengthening: experimental study on rabbits. J Trauma. 1997;42:231–238. doi: 10.1097/00005373-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Laroche M, Lasne Y, Felez A. et al. [Osteocalcin and smoking] Rev Rhum Ed Fr. 1994;61:433–436. [PubMed] [Google Scholar]
- 5.Nersessian A K, Arutyunyan R M. The comparative clastogenic activity of mainstream tobacco smoke from cigarettes widely consumed in Armenia. Mutat Res. 1994;321:89–92. doi: 10.1016/0165-1218(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 6.Hollinger J O, Schmitt J M, Hwang K, Soleymani P, Buck D. Impact of nicotine on bone healing. J Biomed Mater Res. 1999;45:294–301. doi: 10.1002/(sici)1097-4636(19990615)45:4<294::aid-jbm3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Wing K J, Fisher C G, O'Connell J X, Wing P C. Stopping nicotine exposure before surgery. The effect on spinal fusion in a rabbit model. Spine. 2000;25:30–34. doi: 10.1097/00007632-200001010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Silcox D H III, Daftari T, Boden S D, Schimandle J H, Hutton W C, Whitesides T E Jr. The effect of nicotine on spinal fusion. Spine. 1995;20:1549–1553. doi: 10.1097/00007632-199507150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Riebel G D, Boden S D, Whitesides T E, Hutton W C. The effect of nicotine on incorporation of cancellous bone graft in an animal model. Spine. 1995;20:2198–2202. doi: 10.1097/00007632-199510001-00004. [DOI] [PubMed] [Google Scholar]
- 10.Theiss S M, Boden S D, Hair G, Titus L, Morone M A, Ugbo J. The effect of nicotine on gene expression during spine fusion. Spine. 2000;25:2588–2594. doi: 10.1097/00007632-200010150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Daftari T K, Whitesides T E Jr, Heller J G, Goodrich A C, McCarey B E, Hutton W C. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine. 1994;19:904–911. doi: 10.1097/00007632-199404150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Feitelson J B, Rowell P P, Roberts C S, Fleming J T. Two week nicotine treatment selectively increases bone vascular constriction in response to norepinephrine. J Orthop Res. 2003;21:497–502. doi: 10.1016/S0736-0266(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Jang J J, Weis M. et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 14.Clouse W D, Yamaguchi H, Phillips M R. et al. Effects of transdermal nicotine treatment on structure and function of coronary artery bypass grafts. J Appl Physiol. 2000;89:1213–1223. doi: 10.1152/jappl.2000.89.3.1213. [DOI] [PubMed] [Google Scholar]
- 15.France J C, Norman T L, Buchanan M M. et al. Direct current stimulation for spine fusion in a nicotine exposure model. Spine J. 2006;6:7–13. doi: 10.1016/j.spinee.2005.05.380. [DOI] [PubMed] [Google Scholar]
- 16.Goseki M, Omi N, Oida S, Ezawa I, Sasaki S. Voluntary exercise increases osteogenetic activity in rat bones. Bull Tokyo Med Dent Univ. 1995;42:1–8. [PubMed] [Google Scholar]
- 17.Rickard D J, Kazhdan I, Leboy P S. Importance of 1,25-dihydroxyvitamin D3 and the nonadherent cells of marrow for osteoblast differentiation from rat marrow stromal cells. Bone. 1995;16:671–678. doi: 10.1016/8756-3282(95)00099-y. [DOI] [PubMed] [Google Scholar]
- 18.Malaval L, Modrowski D, Gupta A K, Aubin J E. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- 19.Satomura K, Krebsbach P, Bianco P, Gehron Robey P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J Cell Biochem. 2000;78:391–403. [PubMed] [Google Scholar]
- 20.Sheehan D, Hrapchak B. St. Louis, MO: The CV Mosby Company; 1980. Theory and Practice of Histotechnology. [Google Scholar]
- 21.Yamanouchi K, Satomura K, Gotoh Y. et al. Bone formation by transplanted human osteoblasts cultured within collagen sponge with dexamethasone in vitro. J Bone Miner Res. 2001;16:857–867. doi: 10.1359/jbmr.2001.16.5.857. [DOI] [PubMed] [Google Scholar]
- 22.Gori F, Thomas T, Hicok K C, Spelsberg T C, Riggs B L. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522–1535. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 23.Yuhara S, Kasagi S, Inoue A, Otsuka E, Hirose S, Hagiwara H. Effects of nicotine on cultured cells suggest that it can influence the formation and resorption of bone. Eur J Pharmacol. 1999;383:387–393. doi: 10.1016/s0014-2999(99)00551-8. [DOI] [PubMed] [Google Scholar]
- 24.Fang M A, Frost P J, Iida-Klein A, Hahn T J. Effects of nicotine on cellular function in UMR 106-01 osteoblast-like cells. Bone. 1991;12:283–286. doi: 10.1016/8756-3282(91)90077-v. [DOI] [PubMed] [Google Scholar]
- 25.Walker L M, Preston M R, Magnay J L, Thomas P B, El Haj A J. Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone. 2001;28:603–608. doi: 10.1016/s8756-3282(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 26.Rothem D E, Rothem L, Soudry M, Dahan A, Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab. 2009;27:555–561. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- 27.Kim B S, Kim S J, Kim H J. et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012;90:109–115. doi: 10.1016/j.lfs.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Akhter M P, Iwaniec U T, Haynatzki G R, Fung Y K, Cullen D M, Recker R R. Effects of nicotine on bone mass and strength in aged female rats. J Orthop Res. 2003;21:14–19. doi: 10.1016/S0736-0266(02)00107-9. [DOI] [PubMed] [Google Scholar]
- 29.Broulik P D, Jaráb J. The effect of chronic nicotine administration on bone mineral content in mice. Horm Metab Res. 1993;25:219–221. doi: 10.1055/s-2007-1002080. [DOI] [PubMed] [Google Scholar]
- 30.Iwaniec U T, Fung Y K, Cullen D M, Akhter M P, Haven M C, Schmid M. Effects of nicotine on bone and calciotropic hormones in growing female rats. Calcif Tissue Int. 2000;67:68–74. doi: 10.1007/s00223001099. [DOI] [PubMed] [Google Scholar]
- 31.Iwaniec U T, Fung Y K, Akhter M P. et al. Effects of nicotine on bone mass, turnover, and strength in adult female rats. Calcif Tissue Int. 2001;68:358–364. doi: 10.1007/s00223-001-0011-8. [DOI] [PubMed] [Google Scholar]
- 32.Gotfredsen K, Lindh C H, Berglundh T. Does longstanding nicotine exposure impair bone healing and osseointegration? An experimental study in rabbits. J Biomed Mater Res B Appl Biomater. 2009;91:918–923. doi: 10.1002/jbm.b.31475. [DOI] [PubMed] [Google Scholar]
- 33.Castillo R C Bosse M J MacKenzie E J Patterson B M; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures J Orthop Trauma 200519151–157. [DOI] [PubMed] [Google Scholar]
- 34.Lee T C, Ueng S W, Chen H H. et al. The effect of acute smoking on spinal fusion: an experimental study among rabbits. J Trauma. 2005;59:402–408. doi: 10.1097/01.ta.0000174918.38764.00. [DOI] [PubMed] [Google Scholar]
- 35.Krannitz K W, Fallat L M, Schwartz S M. Radiographic healing of conservative versus operative management of supination-external rotation II fractures in a smoking and premature weight-bearing population. J Foot Ankle Surg. 2007;46:218–222. doi: 10.1053/j.jfas.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Gullihorn L, Karpman R, Lippiello L. Differential effects of nicotine and smoke condensate on bone cell metabolic activity. J Orthop Trauma. 2005;19:17–22. doi: 10.1097/00005131-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Skott M, Andreassen T T, Ulrich-Vinther M. et al. Tobacco extract but not nicotine impairs the mechanical strength of fracture healing in rats. J Orthop Res. 2006;24:1472–1479. doi: 10.1002/jor.20187. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Chen F, Yun F, Chen J. Low level nicotine: a novel approach to reduce osteoporosis incidence. Med Hypotheses. 2010;74:1067–1068. doi: 10.1016/j.mehy.2009.12.024. [DOI] [PubMed] [Google Scholar]


