Abstract
Introduction: Bisphosphonates are well known above all for their use in the treatment of osteoporosis. They also play an important role as accompanying therapy for advanced tumour diseases with extensive spread into the skeletal system. Their adjuvant use in the treatment of breast cancer without bony metastases is currently a subject of controversial discussion. The objective of the present evaluation is to describe the use of bisphosphonates in the therapy for breast cancer. We will show how frequently bisphosphonates are used, which bisphosphonates are preferred and what specific features patients under bisphosphonate therapy exhibit. Methods and Materials: The pseudonymous data set from the biobank of the German PATH foundation was used for the evaluation. From the total collective, 2492 patients were selected after consideration of the inclusion and exclusion criteria. The selected patient collective was divided into two groups (with and without bisphosphonate therapy) and the two groups compared with one another with the help of descriptive statistics. Results: 17.5 % of the 2492 patients had prescriptions for a bisphosphonate as part of their therapy. The most frequently administered bisphosphonate was zoledronate. Pathological (induced by tumour therapy) osteoporosis was the most frequently stated indication among the bisphosphonate patients, followed by consumption starting prior to the breast cancer therapy and treatment of bony metastases. Patients under bisphosphonate and antihormonal therapy frequently received an aromatase inhibitor as the active principle in the antihormonal therapy whereas patients under antihormonal therapy but without bisphosphonates more frequently received tamoxifen as active principle. Ten of the 2492 patients reported receiving bisphosphonate therapy as prophylaxis for bony metastases without a documented and approved indication. Use of bisphosphonates in the course of the GAIN, ICE, SUCCESS or, respectively, NATAN trials was reported by 29 of the 2492 patients. Conclusions: In the PATH collective, bisphosphonates were employed above all for the treatment of (tumour therapy-induced) osteoporosis and bony metastases. Off-label use and participation in clinical trials played only a minor role in this patient collective. Against the background of the uncertain data status for the adjuvant use of bisphosphonates, the development (and use) of standardised, validated questionnaires to record the indications for and frequency of use of bisphosphonate therapy is recommended.
Key words: bisphosphonates, breast cancer, therapy, biobank
Abstract
Zusammenfassung
Einleitung: Die Bisphosphonate sind vor allem für ihren Einsatz bei der Behandlung einer Osteoporose bekannt. In der Therapie des Mammakarzinoms spielen sie als begleitende Therapie bei fortgeschrittenen, auf das Skelett ausgedehnten Tumorerkrankungen eine wichtige Rolle. Kontrovers diskutiert wird zurzeit der adjuvante Einsatz bei primären Brustkrebserkrankungen ohne ossäre Metastasen. Das Ziel dieser Auswertung ist es, den Einsatz der Bisphosphonate in der Therapie des Mammakarzinoms zu beschreiben. Es soll gezeigt werden, wie oft die Bisphosphonate eingesetzt werden, welche Bisphosphonate bevorzugt eingesetzt werden und welche besonderen Merkmale Patientinnen mit einer Bisphosphonattherapie aufweisen. Methoden und Materialien: Für die Auswertung wurde der pseudonymisierte Datensatz aus der Biobank der deutschen Stiftung PATH verwendet. Aus dem Gesamtkollektiv wurden unter Berücksichtigung der Ein- und Ausschlusskriterien 2492 Patientinnen ausgewählt. Das ausgewählte Patientenkollektiv wurde in 2 Gruppen (mit und ohne Bisphosphonattherapie) aufgeteilt und mithilfe der deskriptiven Statistik miteinander verglichen. Ergebnisse: 17,5 % der 2492 Patientinnen wurde im Rahmen der Therapie ein Bisphosphonat verordnet. Das am häufigsten eingesetzte Bisphosphonat war Zoledronat. Die pathologische (tumortherapieinduzierte) Osteoporose war die am häufigsten genannte Indikation unter den Bisphosphonat-Patientinnen, gefolgt von der Einnahme bereits vor der Brustkrebstherapie und der Behandlung von Knochenmetastasen. Patientinnen mit Bisphosphonat- und Antihormontherapie erhielten häufiger einen Aromatasehemmer als Wirkstoff der Antihormontherapie, während Patientinnen mit einer Antihormontherapie, aber ohne Bisphosphonattherapie häufiger Tamoxifen als Wirkstoff erhielten. Eine Bisphosphonattherapie zur Vorbeugung von Knochenmetastasen ohne dokumentierte, zugelassene Indikation berichteten 10 von 2492 Patientinnen. Der Bisphosphonateinsatz im Rahmen der GAIN-, ICE-, SUCCESS- bzw. NATAN-Studie wurde von 29 der 2492 Patientinnen berichtet. Schlussfolgerungen: Im PATH-Kollektiv werden die Bisphosphonate vor allem für die Behandlung einer (tumortherapieinduzierten) Osteoporose und die Behandlung von Knochenmetastasen eingesetzt. Der Einsatz im Off-Label-Use und die Studienteilnahme spielt in diesem Patientenkollektiv eine untergeordnete Rolle. Vor dem Hintergrund der unsicheren Datenlage zum adjuvanten Einsatz der Bisphosphonate ist die Entwicklung (und Anwendung) standardisierter, validierter Fragebögen zur Erhebung der Frequenz und Indikation einer Bisphosphonattherapie zu empfehlen.
Schlüsselwörter: Bisphosphonate, Brustkrebs, Therapie, Biobank
Introduction
Among women, breast cancer with a proportion of 32.1 % of all new cancer diseases is the most frequent tumour disease in Germany. Every year 72 000 women are afflicted with and 17 200 women die of breast cancer 1.
The standard therapeutic modalities include surgical treatment, radiotherapy, chemotherapy, antihormonal therapy and/or molecular biological therapy. Surgical treatment is often followed by the adjuvant use of one of the above-mentioned systemic therapies 2. In cases of advanced breast cancer, e.g., in the treatment of metastatic breast cancer, an additional therapy to the standard treatment strategy may be indicated, e.g., with drugs containing a bisphosphonate as active principle such as zoledronate, ibandronate, pamidronate and clodronate, which are approved as therapy for advanced tumour diseases spreading to the skeleton and for tumour-induced hypercalcaemia 3, 4, 5, 6. They are administered for osteolytic metastases, bone pain due to metastases, and manifest osteoporosis induced by tumour therapy 2.
Bisphosphonates can be differentiated between nitrogen-containing (e.g., zoledronate) and not nitrogen-containing (e.g., clodronate) substances. The two groups differ not only in their chemical structures but also in their modes of action. The not nitrogen-containing bisphosphonates are metabolised by the cells into non-hydrolysable, cytotoxic ATP analogues, which thus inhibit ATP-dependent enzymes. In this way bone resorption is reduced and apoptosis of osteoclasts occurs. The more potent nitrogen-containing bisphosphonates, on the other hand, inhibit an enzyme in the mevalonate cycle and thus prevent the further activation and binding of important signalling substances of the osteoclasts 7. The lack of messenger substances has effects on cell morphology, the cytoskeleton and other important cell features and can lead to cell death 8.
Bisphosphonates have a high affinity for bone tissue and accumulate to differing extents in the bone 8. For a long time it was assumed that bisphosphonates act exclusively on osteoclasts and inhibit bone resorption there. However, products of the mevalonate cycle are also highly important for other cells, including tumour cells 7. In two large European trials (ABCSG-12, ZO-FAST) a positive effect on the time period up to first appearance of a recurrence (ABCSG: ipsi-, contralateral; ZO-FAST: only ipsilateral) or to death upon concomitant administration of zoledronate as add-on to a standard therapy was demonstrated for non-metastatic breast cancer 9, 10. The AZURE trial, on the other hand, did not show any exclusive advantage with regard to the frequency of recurrences and deaths due to any cause 11. However, bisphosphonates have not yet been approved for adjuvant use in patients without any skeletal complications. Although a direct anti-tumour activity has not yet been unequivocally demonstrated and further results from on-going trials have to be awaited, the adjuvant use of bisphosphonates has already been recommended by the Committee for Gynaecological Oncology [Arbeitsgemeinschaft Gynäkologische Onkologie e. V. (AGO e. V.)]. In the guidelines published in March 2011 the use of zoledronate was exclusively positively assessed for postmenopausal patients with primary breast cancer and premenopausal patients under solely anti-endocrine therapy 12.
The bisphosphonates are already in use for cases of advanced breast cancer with skeletal complications, their use for locally limited breast cancer is still under discussion. The aim of the present article is to analyse the applications and frequency of use of bisphosphonates in the therapy for breast cancer on the basis of the data collected in the tumour bank of the PATH foundation. The differences between patients receiving and not receiving bisphosphonate therapy have been analysed.
Material and Methods
PATH Biobank
PATH, the Patientsʼ Tumor Bank of Hope, is a German charitable foundation that was founded by breast cancer patients in 2002. An objective of the foundation is the collection of breast cancer tissue not only for patients but also for research. Besides the preservation of tumour tissue, normal tissue and serum in a decentrally organised tumour bank, PATH also collects clinical data on tumours (reported by the respective breast cancer centres), sociodemographic data and information on disease course and applied therapy (from the patients in the framework of follow-up interviews). The information is collected in a central databank, which is annotated in the biobank (Fig. 1). At present PATH cooperates with seven certified breast centres in Germany (cf. Acknowledgements; see also http://www.stiftungpath.org/kooperationspartner/kooperationskliniken) in which the patients are recruited.
Fig. 1.
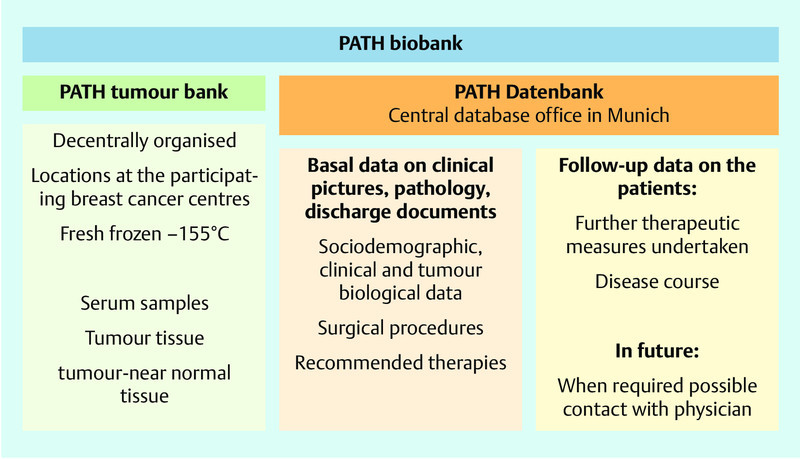
Structure of the PATH biobank, biomaterial stocks, and database.
The patients each gave their written agreement to the storage, later contacts for the purpose of follow-up and data storage as well as further use of samples and data for research purposes in pseudonymous form. The described process was presented to an ethics committee and the Bavarian Data Protection Commissioner. It was approved by the ethics committee of the University of Bonn.
PATH follow-up
Since 2009 the women are contacted by telephone (exception: patients of 2004–2007 were contacted in writing 3–5 years after diagnosis) about 2 years after the diagnosis, if not successful then in writing. The telephone interview is carried out following a standardised guideline and in future should be repeated at 1–2 year intervals. At the time point of the investigation described here all women participation in the years 2004 to 2009 were contacted in the follow-up.
During the telephone interview the patients were asked, among others, about a possible bisphosphonate therapy, if appropriate the reasons for taking it, a possible therapy pause as well as the name of the bisphosphonate and these data were recorded.
Prior to starting data collection at follow-up, the PATH study centre decided that in those cases where patients reported taking bisphosphonates before the onset of tumour disease this should be documented as being independent of the indication (e.g., also for “prophylaxis against bone pain”), as being “in use before the onset of breast cancer disease”. For the purposes of the present evaluation, a patient was assigned to the subgroup “study patients” when she reported consumption of bisphosphonates in the course of a clinical trial on the adjuvant use of bisphosphonates. If the use of the bisphosphonate was reported to be for prophylactic purposes against bone metastases the patient was assigned to the subgroup “off-label use” for the present evaluation.
Selection of the patient collective
The data set made available by the PATH foundation in April 2012 contained information about 5625 patients. This data set contained the details of 22 men and 1436 women whose diagnoses were not made in the period 2004–2009. Both groups were excluded from the evaluation. 4167 women who received the diagnosis breast cancer in the period 2004 to 2009 (potentially successful follow-up, see above) were defined as the basal study population. From this group 1565 had to be excluded because of the lack of follow-ups together with further 101 women due to a lack of or invalid replies to the question on the consumption of bisphosphonate.
Thus there remained for the analysis 436 patients who were taking bisphosphonates (BP[+]) and 2056 patients who were not taking bisphosphonates (BP[−]).
Furthermore, two subgroup analyses were carried out. (1) Patients who had received an antihormonal therapy were divided into patients with aromatase inhibitor therapy (AI and AI plus GnRH) and patients with tamoxifen therapy (TAM und TAM plus GnRH). The two groups were compared with regard to the frequency of bisphosphonate use and the indication for the prescribed bisphosphonate. Excluded from these subgroups were patients with a switch in therapy (TAM two years, then AI three years) and a usually named extended adjuvant therapy (TAM five years, then AI) (n = 298). (2) A second subgroup analysis was performed for the subgroups with differing indications for the bisphosphonate therapy. Here distinctions were made between the trial subgroup (ICE, GAIN, NATAN, and SUCCESS), the off-label use group and the group with “regular indications for the use of bisphosphonates”. Because of the widely differing case numbers of the individual groups this evaluation was purely descriptive.
Statistical analyses
Qualitative data are described with absolute and relative frequencies and quantitative data with mean values and standard deviations. The data were evaluated using SPSS Version 20. The valid data of the two main groups, BP(+) und BP(−), were tested for statistical significance with the help of explorative, univariate analyses (χ2 test, Fisherʼs test). The significance level was set at p = 0.05.
Results
After application of the inclusion and exclusion criteria 2492 patients remained from the total collective (n = 5625) of the PATH database. Those patients excluded from the evaluation due to a lack of follow-up data are representative with regard to age of the examined group but, in comparison, exhibited a somewhat more advanced disease stage (data not shown).
Use of bisphosphonates
In the patient collective 436 (17.5 %) patients received a bisphosphonate. The women reported zoledronate, followed by alendronate and ibandronate as the most frequently prescribed bisphosphonates (Table 1).
Table 1 Use of bisphosphonates on the basis of patient reports in follow-up (absolute and relative frequencies).
| BP(+) (n = 436) | |
|---|---|
| Formulation | |
| Zoledronate | 132 (37.0) |
| Ibandronate | 70 (19.6) |
| Pamidronate | 6 (1.7) |
| Alendronate | 94 (26.3) |
| Risedronate | 43 (12.0) |
| Combination of 2 BPs | 12 (3.4) |
| Unknown | 79 |
| Point in time of BP use | |
| Already prior to cancer disease | 63 (21.5) |
| Adjuvant (postoperative) | 201 (68.6) |
| Trial participation (ICE, GAIN, NATAN, SUCCESS) | 22 (7.5) |
| Already prior to disease + trial participation | 7 (2.4) |
| Unknown | 143 |
| Indication | |
| Treatment of bone metastases | 42 (11.4) |
| Treatment of bone pain | 12 (3.3) |
| Preventative (prior to disease/operation) | 61 (16.5) |
| Prophylaxis against bone pain | 36 (9.8) |
| Treatment of pathological osteoporosis | 179 (48.5) |
| Trial participation (ICE, GAIN, NATAN, SUCCESS) | 29 (7.9) |
| Off-label use/prophylaxis against bone metastases | 10 (2.7) |
| Unknown | 69 |
Of the 436 women under bisphosphonate therapy, 293 (57.1 %) provided information on the point in time of use. 63 women reported use already before the onset of breast cancer, 22 women reported use within the framework of a clinical trial. In addition, seven women reported use prior to disease as well as participation in a clinical trial after the diagnosis had been made. Further 201 women reported starting BP use after surgical treatment (Table 1).
The most frequent indication for bisphosphonate therapy reported by the women during the interviews is for treatment of a pathological (induced by tumour therapy) osteoporosis (48.5 %), followed by use prior to start of breast cancer therapy (16.5 %). This was followed by treatment of bone metastases (11.4 %). 9.8 % of the patients who received a bisphosphonate therapy, reported its use for prophylactic purposes against bone pain. The use of bisphosphonates within the framework of clinical trials was reported by 7.9 % of the women. The SUCCESS trial (14/29), followed by GAIN (6/29) and NATAN (7/29) trials were mentioned most often. Merely two women took part in the ICE trial. Furthermore, 2.7 % of the women reported off-label use as prophylaxis against bone metastases (Table 1).
For the two indications, treatment of bone metastases and prophylaxis against bone pain or, respectively, use prior to the start of breast cancer therapy, zoledronate was mentioned most frequently by the patients. For use in cases of tumour therapy-induced osteoporosis with onset after the occurrence of breast cancer and for use in the treatment of bone pain alendronate was mentioned most frequently (Fig. 2).
Fig. 2.
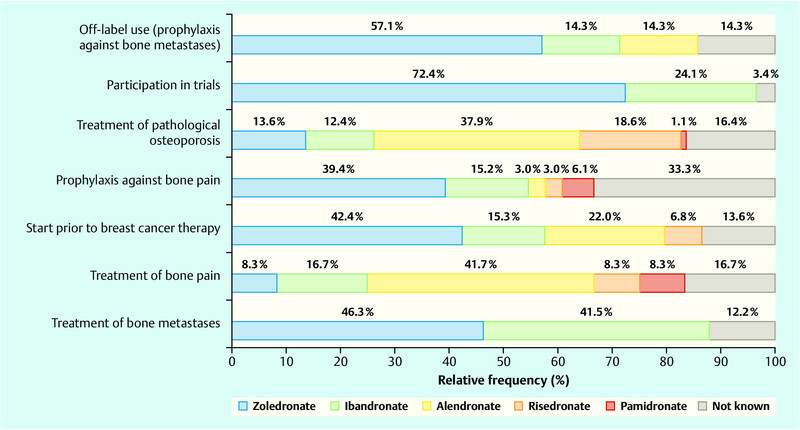
Use of the different bisphosphonates according to indication.
Oral administration of the bisphosphonates was reported by 42 women (eight of whom started use already prior to the cancer disease, 31 women under treatment for pathological osteoporosis and one woman who reported taking ibandronate as treatment for bone metastases).
Of the 12 women who reported taking two formulations, seven patients mentioned a change of the bisphosphonate. In two cases the reasons for taking two different bisphosphonates were not known. In one case the patient reported taking zoledronate and pamidronate alternately since 2009 as prophylaxis against bone metastases.
Subgroup analyses—bisphosphonate therapy
There are in part marked differences between the women with and without bisphosphonate therapy (Table 2). The TNM stage reported by the centres (tumour size, local lymph node involvement, presence of metastases at first diagnosis) is more advanced for those patients taking bisphosphonates. Breast-sparing operations are performed less frequently on women taking bisphosphonates. In addition, women under bisphosphonate therapy more frequently suffer from distant metastases, which most often attack the bone tissue of patients taking bisphosphonates (66.7 %) (Table 3). It should be noted that the higher use of bisphosphonates in cases of metastatic breast cancer is just due to the presence of distant metastases (confounding by indication).
Table 2 Patient characteristics recorded by the breast centre at the time of recruitment/diagnosis (absolute and relative frequencies; mean values and SD).
| total (n = 2 492) | BP(−) (n = 2 056) | BP(+) (n = 436) | p value | Approved indication (n = 397) | Trial participation (n = 29) | Off-label use (n = 10) | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis | 59.6 ± 11.2 | 59.6 ± 11.2 | 59.5 ± 11.5 | 0.932 | 60.4 ± 11.3 | 51.1 ± 9.0 | 50.4 ± 12.1 |
| Menopause status | |||||||
| PremenopausalPerimenopausalPostmenopausalNo data | 370 (17.1)49 (2.3)1 743 (80.6)330 | 306 (17.1)39 (2.2)1 446 (80.7)265 | 64 (17.3)10 (2.7)297 (80.1)65 | 0.824 | 55 (15.9)8 (2.3)283 (81.8)51 | 6 (35,3)1 (5,9)10 (58,8)12 | 3 (37,5)1 (12,5)4 (50,0)2 |
| ER status | |||||||
| NegativePositiveNo data | 407 (16.7)2 037 (83.3)48 | 326 (16.2)1 691 (83.9)39 | 81 (19.0)346 (81.0)9 | 0.174 | 64 (16.4)326 (83.6)7 | 9 (32.1)19 (67.9)1 | 8 (88.9)1 (11.1)1 |
| PR status | |||||||
| NegativePositiveNo data | 651 (26.6)1 796 (73.4)45 | 521 (25.8)1 497 (74.2)38 | 130 (30.3)299 (69.7)7 | 0.062 | 110 (28.1)281 (71.9)6 | 12 (42.9)16 (57.1)1 | 8 (80.0)2 (20.0)0 |
| Her2-neu | |||||||
| NegativePositiveNo data | 2 156 (88.4)282 (11.6)54 | 1 782 (88.6)229 (11.4)45 | 374 (87.6)53 (12.4)9 | 0.560 | 342 (87.9)47 (12.1)8 | 23 (82.1)5 (17.9)1 | 9 (90.0)1 (10.0)0 |
| First diagnosis | |||||||
| NoYesNo data | 171 (6.9)2 292 (93.1)29 | 142 (7.0)1 892 (93.0)22 | 29 (6.8)400 (93.2)7 | 0.917 | 27 (6.9)364 (93.1)6 | 2 (7.1)26 (92.9)1 | 0 (0.0)10 (100.0)0 |
| Tumour size | |||||||
| pTispT0pT1pT2pT3pT4Tx | 14 (0.6)39 (1.6)1 598 (65.2)709 (28.9)67 (2.7)25 (1.0)40 | 14 (0.7)29 (1.4)1 341 (66.2)577 (28.5)47 (2.3)17 (0.8)31 | 0 (0.0)10 (2.3)257 (60.1)132 (30.9)20 (4.7)8 (1.9)9 | 0.003 | 0 (0.0)10 (2.6)237 (61.1)118 (30.4)15 (3.9)8 (2.1)9 | 0 (0.0)0 (0.0)13 (44.8)11 (37.9)5 (17.2)0 (0.0)0 | 0 (0.0)0 (0.0)7 (70.0)3 (33.0)0 (0.0)0 (0.0)0 |
| Lymph node involvement | |||||||
| pN0pN1pN2pN3Nx | 1 622 (67.3)569 (23.6)147 (6.1)71 (2.9)83 | 1 378 (69.4)461 (23.2)98 (4.9)49 (2.5)70 | 244 (57.7)108 (25.5)49 (11.6)22 (5.2)13 | < 0.001 | 231 (60.0)98 (25.5)37 (9.6)19 (4.9)12 | 9 (32.1)6 (21.4)12 (42.9)1 (3.6)1 | 4 (40.0)4 (40.0)0 (0.0)2 (20.0)0 |
| Metastases at first diagnosis | |||||||
| pM0pM1Mx | 2 135 (98.2)40 (1.8)119 | 1 779 (99.2)14 (0.8)100 | 356 (93.2)26 (6.8)19 | < 0.001 | 319 (92.5)26 (7.5)19 | 26 (100.0)0 (0.0)0 | 10 (100.0)0 (0.0)0 |
| Grading | |||||||
| G1G2G3no data | 323 (14.2)1 467 (64.4)487 (21.4)214 | 285 (15.1)1 206 (63.9)395 (20.9)170 | 38 (9.7)261 (66.8)92 (23.5)44 | 0.017 | 37 (10.2)248 (68.3)78 (21.5)34 | 1 (4.3)12 (52.2)10 (43.5)6 | 0 (0.0)2 (33.3)4 (66.7)4 |
| Resection margins | |||||||
| R0R1R2no data | 2 411 (98.8)29 (1.2)1 (< 0.1)51 | 1 994 (99.0)20 (1.0)1 (< 0.1)41 | 417 (97.9)9 (2.1)0 (0.0)10 | 0.138 | 379 (97.9)8 (2.1)0 (0.0)10 | 29 (100.0)0 (0.0)0 (0.0)0 | 9 (90.0)1 (10.0)0 (0.0)0 |
| Lymph vessel involvement | |||||||
| L0L1No date | 1 854 (77.9)526 (22.1)112 | 1 552 (79.1)411 (20.9)93 | 302 (72.4)115 (27.6)19 | 0.003 | 275 (72.6)104 (27.4)18 | 24 (85.7)4 (14.3)1 | 3 (30.0)7 (70.0)0 |
| Breast-sparing therapy | |||||||
| NoyesNo data | 513 (20.8)1 950 (79.2)29 | 385 (18.9)1 650 (81.1)21 | 128 (29.9)300 (70.1)8 | < 0.001 | 112 (28.8)277 (71.2)8 | 11 (37.9)18 (62.1)0 | 5 (50.0)5 (50.0)0 |
Table 3 Therapies received and disease course on the basis of patient reports at follow-up (absolute and relative frequencies).
| Total (n = 2 492) | BP(−) (n = 2 056) | BP(+) (n = 436) | p value | Approved indication (n = 397) | Trial participation (n = 29) | Off-label use (n = 10) | |
|---|---|---|---|---|---|---|---|
| Chemotherapy | |||||||
| NoYesNo data | 1 100 (48.3)1 177 (51.7)215 | 941 (49.9)943 (50.1)172 | 159 (40.5)234 (59.5)43 | 0.001 | 158 (44.5)197 (55.5)42 | 0 (0.0)29 (100.0)0 | 1 (11.1)8 (88.9)1 |
| Radiation | |||||||
| NoYesNo data | 276 (11.1)2 215 (88.9)1 | 221 (10.8)1 834 (89.2)1 | 55 (12.6)381 (87.4)0 | 0.275 | 50 (12,6)347 (87,4)0 | 4 (13.8)25 (86.2)0 | 1 (10.0)9 (90.0)0 |
| Antihormonal therapy (AHT) | |||||||
| NoYesNo data | 392 (16.0)2 051 (84.0)49 | 319 (15.8)1 699 (84.2)38 | 73 (17.2)352 (82.8)11 | 0.513 | 58 (15,0)328 (85,0)11 | 9 (31.0)20 (69.0)0 | 6 (60.0)4 (40.0)0 |
| Drugs of AHT | |||||||
| AIAI plus GnRH analoguesTAM 5 years, then AITAM 2 years, then AI 3 yearsTAMTAM plus GnRH analoguesNo data | 1 026 (53.5)1 (< 0.1)4 (0.2)294 (15.3)409 (21.3)182 (9.5)135 | 813 (51.1)0 (0.0)4 (0.3)268 (16.9)357 (22.5)148 (9.3)109 | 213 (65.3)1 (0.3)0 (0.0)26 (8.0)52 (16.0)34 (10.4)26 | < 0.001 | 204 (67.5)0 (0.0)0 (0.0)23 (7.6)44 (14.6)31 (10.3)26 | 7 (35.0)1 (5.0)0 (0.0)3 (15.0)7 (35.0)2 (10.0)9 | 2 (50.0)0 (0.0)0 (0.0)0 (0.0)1 (25.0)1 (25.0)6 |
| Herceptin | |||||||
| NoYesNo data | 2 202 (89.4)262 (10.6)28 | 1 825 (89.6)212 (10.4)19 | 377 (88.3)50 (11.7)9 | 0.437 | 344 (88.7)44 (11.3)9 | 24 (82.8)5 (17.2)0 | 9 (90.0)1 (10.0)0 |
| Later recurrence | |||||||
| NoYesNo data | 2 403 (98.3)41 (1.7)48 | 1 986 (98.5)30 (1.5)40 | 417 (97.4)11 (2.6)8 | 0.143 | 383 (98.2)7 (1.8)7 | 27 (96.4)1 (3.6)1 | 7 (70.0)3 (30.0)0 |
| Later metastases | |||||||
| NoYesNo data | 2 357 (96.5)85 (3.5)49 | 1 983 (98.3)34 (1.7)39 | 374 (87.8)51 (12.2)10 | < 0.001 | 339 (87.4)49 (12.6)9 | 26 (92.9)2 (7.1)1 | 9 (90.0)1 (10.0)0 |
| Localisation of metastases | |||||||
| BoneLiverLungsBrainPleuraBone marrowOther organsNo data | 38 (47.0)10 (12.0)17 (20.5)9 (10.8)1 (1.2)1 (1.2)6 (7.2)3 | 5 (15.6)5 (15.6)13 (40.6)6 (18.8)0 (0.0)0 (0.0)3 (9.4)1 | 34 (66.7)5 (9.8)4 (7.8)3 (5.9)1 (2.0)1 (2.0)3 (5.9)0 | < 0.001 | 33 (68.8)5 (10.4)4 (8.3)3 (6.2)0 (0.0)1 (2.1)2 (4.2)1 | 1 (50.0)0 (0.0)0 (0.0)0 (0.0)1 (50.0)0 (0.0)0 (0.0)0 | 0 (0.0)0 (0.0)0 (0.0)0 (0.0)0 (0.0)0 (0.0)1 (100.0)0 |
Those women who reported in the telephone interview that they were prescribed bisphosphonates in off-label use, i.e., for prophylactic use against bone metastases (BP[+]-Off-Label Use; n = 10), are on average 10 years younger than the women without bisphosphonate therapy or, respectively, those with bisphosphonate therapy for other indications (Table 2). In addition they are markedly more frequently ER/PR negative and have a more favourable tumour stage distribution. Even so only 50 % undergo breast-sparing operations. Furthermore, three of these women reported a recurrence and one distant metastases at follow-up (Table 3).
Those women who participated in the ICE, GAIN, NATAN or SUCCESS trials were on average eight years younger than the comparison groups BP−/BP+ (total) and BP with approved indication. 32.1 and 42.9 % were ER negative or, respectively, PR negative. Although the tumour stage distribution was more favourable than in the comparison groups, these women more frequently exhibited lymph node involvement, thus merely 32.1 % were free from lesions in their lymph nodes. The proportion of women undergoing breast-sparing surgery amounted to 62.1 %. Later recurrences and metastases during the follow-up were reported by 1 and 2 women, respectively. Of the 29 trial participants, 72.4 % received zoledronate. All trial patients reported undergoing chemotherapy.
Subgroup analyses—antihormonal therapy
On assessment of the group of women under antihormonal therapy it is seen in comparison that more patients with concomitant bisphosphonate therapy reported in the follow-up interview the consumption of exclusively one aromatase inhibitor as breast cancer therapeutic agent (Table 3). A comparison of the patients with bisphosphonate and aromatase inhibitor therapy (mean age: 63.3 ± 9.12 years) with those under bisphosphonate and tamoxifen therapy (mean age: 52.5 ± 13.2 years) revealed differences with regard to their further therapy and the indications for bisphosphonate therapy. Patients under bisphosphonate and tamoxifen therapy more frequently reported that they had received bisphosphonates within the framework of a clinical trial or, respectively, had started bisphosphonate therapy prior to the onset of cancer disease. On the other hand, they mentioned treatment of a pathological (tumour therapy-induced) osteoporosis as indication for the bisphosphonate therapy less frequently than did patients with bisphosphonate and aromatase inhibitor therapy (Fig. 3). Chemotherapy was also reported more frequently by women under bisphosphonate and aromatase inhibitor therapy.
Fig. 3.
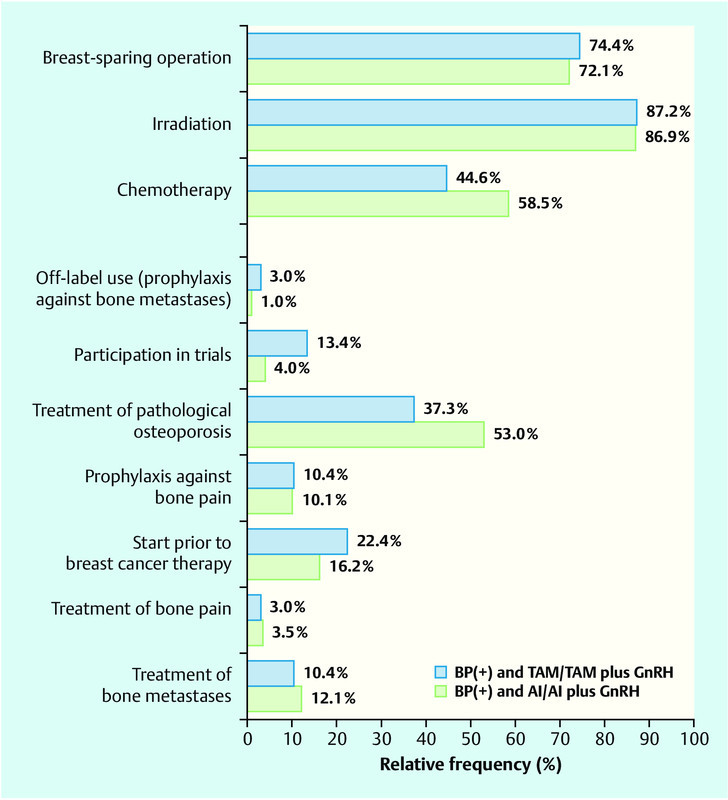
Comparison of women under bisphosphonate therapy (BP[+]) with tamoxifen or, respectively, with aromatase inhibitor.
Discussion
On the basis of the collective from the PATH biobank, the use of bisphosphonates in patients with breast cancer has been investigated.
Less than 20 % of the interviewed patients reported a bisphosphonate therapy. Zoledronate with 37.0 % was the most frequently mentioned drug. With the exception of the indications “treatment of pathological osteoporosis” and “treatment of bone metastases”, it was the most frequently named bisphosphonate for all other indications. Alendronate is one of the drugs for treatment of tumour therapy-induced osteoporosis for which a fracture protective action has been best demonstrated 13. Also the PATH women mentioned alendronate most frequently for the treatment of osteoporosis that developed during the tumour disease (tumour therapy-induced osteoporosis). In contrast, the bisphosphonate zoledronate – which is approved for this indication – was the most frequently mentioned drug for the treatment of bone metastases. Coldronate, which is only available for oral administration, was not mentioned by the PATH women although it is indicated for the treatment of metastatic breast cancer 4. It was shown that of the 42 women who received an orally available bisphosphonate only one reported as indication the treatment of bone metastases (ibandronate). The oral administration of bisphosphonates is associated with side effects and characterised by a poor bioavailability of the respective bisphosphonate. A possible reason for the low usage of orally available bisphosphonates in the treatment of bone metastases or bone pain is that the more laborious i. v. administration in breast cancer patients can be combined with chemotherapy.
The differences between the subgroups with or, respectively, without bisphosphonate therapy in regard to the characteristics reported by the breast cancer centres can be explained by the indications for bisphosphonates. In the BP(+) group the TNM stage is more advanced. In comparison to the BP(−) group lymph node involvement and distant metastases were more frequently found at first diagnosis in the BP(+) group. Also in the follow-up distant metastases were reported more frequently in the group taking bisphosphonates, and in more than 50 % bone tissue had been attacked. These results confirm that in cases with pre-existing bone metastases, there is a preference to employ bisphosphonates in the treatment. However, the evaluation also shows that bisphosphonates are principally used in breast cancer patients to treat tumour therapy-induced osteoporosis (Table 1). The use of bisphosphonates in the treatment of osteoporosis became established in the 1990s and has since progressed to be the first choice therapy. The efficacy of bisphosphonates has been confirmed in several clinical trials 14. Standard therapies such as chemotherapy and antihormonal therapy often lead to an early onset of menopause, a loss of bone mineral density and high bone remodelling, similar to the clinical picture of manifest osteoporosis 15, 16.
As expected, the results show that among the patients with reported antihormonal therapy and a concomitantly reported bisphosphonate consumption exclusively one aromatase inhibitor is employed as compared to patients who report a sole antihormonal therapy. The aromatase inhibitors belong to a new group of active principles for breast cancer therapy and, above all, are prescribed for postmenopausal hormone receptor-positive patients 2. A more detailed consideration of patients under bisphosphonate and aromatase inhibitor therapy reveals that the women with aromatase inhibitor therapy receive a chemotherapy more often than women under bisphosphonate and tamoxifen therapy. The patients with bisphosphonate and aromatase inhibitor therapies are on average almost 10 years older, which can be explained by the preferred use of aromatase inhibitors in postmenopausal patients 2. Patients under aromatase inhibitor therapy are at a higher risk for skeletal complications than patients under tamoxifen therapy 17. This clearly demonstrates that for these patients the risk of a pathological (tumour therapy induced) osteoporosis is greater than for patients with alternative antihormonal therapy. The E-ZO-FAST study and other trials have shown that the increased usage of bisphosphonates in patients under aromatase inhibitor therapy leads to an increase in bone density so that the occurrence of skeletal complications is reduced 16, 18. However, it must be considered that PATH women with aromatase inhibitor therapy and bisphosphonate therapy are on average 60 years old and mostly postmenopausal. Thus, two of the tumour therapy-independent risk factors for osteoporosis are already fulfilled. Furthermore, as a rule bone density measurements are made when there is an indication for on-going aromatase inhibitor therapy in order to determine the risk for osteoporosis and to initiate possible preventative measures 2. This can result in osteoporosis being diagnosed earlier and more frequently than in patients under tamoxifen therapy.
Just recently there has been much discussion about the adjuvant use of bisphosphonates for primary breast cancer without documented indications and beyond the current approved clinical situations. None of the bisphosphonates currently on the market have been approved for these new therapeutic options. In December 2010 Novartis® withdrew an application to extend the approval. The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) could not recommend an extension of approval at that time since an advantage could not be sufficiently demonstrated 19. The work to record the follow-up data of the PATH foundation began in 2008 and only in 2009 did data collection for the follow-up start. At that time the usage for primary breast cancer was not under such intensive discussion as today. Many larger trials were not yet completed and only few results were available. Thus, the question of the adjuvant use of bisphosphonates was not then included in the data collection of the PATH foundation. For the evaluation in the present contribution the information on the use in cases of primary breast cancer with unapproved indication was extracted from the free texts of the follow-up documentation. Thus it must be taken into account that some patients may not have been identified due to the lack of details in the free texts. Furthermore, it should be noted that, in our evaluation due to the above-mentioned lack of data in the tumour data base, prophylaxis against bone metastases is used as a definition for off-label use and details on the participation in the GAIN, ICE, NATAN or SUCCESS trials on the adjuvant usage of bisphosphonates are used as a definition for participation in trials. Even so a comparison was made between patients under bisphosphonate therapy with off-label use/prophylaxis against bone metastases, trial participation and the patients under bisphosphonate therapy for approved indications. The evaluation revealed that the women with off-label use/prophylaxis against bone metastases were on average 10 years younger. The application for extension of approval by Novartis was made for the treatment of hormone receptor-positive tumours 19. However, the majority of PATH women in the off-label group are hormone receptor-negative. The reasons why only 50 % of the women reporting off-label use were given breast-sparing therapy in spite of the slightly more favourable TNM stage unfortunately cannot be deduced from the available data. Women in the group of trial participants are also on average eight years younger. It is possible that younger women are more open to new therapy options and accept recommendations from their physicians (for trial participation or for prophylactic use of bisphosphonates). Furthermore, it is feasible that younger women with advanced lymph node involvement are offered this therapy more frequently. An exact statement about the frequency and patient characteristics for the off-label or, respectively, trial participation usage cannot be made due to the above-mentioned facts. For off-label applications the patients themselves must pay for the expensive drugs due to the lack of approval since off-label use is only reimbursed by the health insurances under well justified exceptions. The ambiguous trial situation and financial aspects are facts that probably speak against a prophylactic therapy with bisphosphonates.
One of the strengths of our investigational health-care study is the large size of the collective which with a total of 2492 patients is able to provide meaningful results. A further strength of the study is that it was carried out in cooperation with the German PATH foundation, the recruiting centres as well as an external evaluation agency. Among the weaknesses of the study is that the trial participants of the PATH foundation possibly differ slightly from a normal collective (younger age at disease onset, different tumour stage distributions compared to all other breast cancer patients in Germany 1). Furthermore, the data are based in part on self-reported patient details so that the question investigated here (use of bisphosphonates) cannot be answered on the basis of data confirmed by clinics or physicians. However, other health-care studies have shown that patients, and especially breast cancer patients, can provide valid 20 and reliable 21, 22, 23 information about their therapy. A further problem concerns the information on tumour therapy-induced osteoporosis. From the available data it cannot be determined whether osteoporosis was detected to a larger extent due to the more frequently performed bone density measurements in patients under aromatase inhibitor therapy, whether it existed already prior to tumour therapy or whether it really did occur as a consequence of the tumour therapy. Also, as already mentioned above, the registration of off-label use/prophylaxis against bone metastases and the details of the group of trial participants were not acquired by direct data collection by means of standardised and validated questionnaires but were rather extracted from the free text responses; this weakens the value of the results about usage in the absence of an approve indications.
Conclusion
On the basis of data from the PATH collective it may be assumed that bisphosphonates are used in the therapy for breast cancer above all for the treatment of skeletal complications. Also among the PATH breast cancer patients tumour therapy-induced osteoporosis is one of the main reasons for their use. Applications in off-label use and among trial participants play a minor role in this patient collective. Against the background of the uncertain data status on adjuvant use the development (and usage) of standardised and validated questionnaires to register the frequency of and indications for bisphosphonate therapy is highly recommended.
Acknowledgements
We heartily thank all patients who gave their consent to store samples in PATH and who shared their disease histories with us. Without their support this research project would not have been possible. In addition we are grateful to all participating gynaecologists and pathologists in the cooperating centre in Bonn (PATH contact partners: Prof. Dr. Uwe-Jochen Göhring, Prof. Dr. Walter Kuhn), Dortmund (PD Dr. Georg Kunz), Bochum/Herne (Prof. Dr. Clemens Tempfer, Dr. H. Y. Ergönenc), Kassel (Prof. Dr. Thomas Dimpfl), Marburg (Prof. Dr. Ute-Susann Albert), Offenbach (Prof. Dr. Christian Jackisch) and Regensburg (Prof. Dr. Olaf Ortmann) (see also http://www.stiftung-path.org/kooperationspartner/kooperationskliniken) for their engagement. Finally we thank Dr. Elke Faust for critically reviewing this manuscript.
Footnotes
Conflict of Interest The PATH foundation is supported in the financing of its creation and running by donations and sponsorships from private citizens and companies, this applies also in part to the tumour and data banks. We are grateful to all of them. Among the donors and sponsors are: Amgen, Munich; AstraZeneca, Wedel; Hans Anzeneder, Burghausen; Bristol-Myers Squibb, Munich; Prof. Reinhard Büttner, University of Bonn; Förderverein Robert Janker Krebsstiftung e. V., Bonn; GlaxoSmithKline, Munich; Henkel Foundation, Düsseldorf; MammaMia, das Brustkrebsmagazin; Dr. Patrizia Mikulcik, Bad Homburg; Notaries Zimmermann and Hauschild, Düsseldorf; Novartis Pharma, Nürnberg; Pfizer Oncology, Berlin; Pierre Fabre, Freiburg; Revierinitiative Bochum Herne; Roche Pharma, Grenzach; Unterweger Healthcare Communication, Hamburg (see also http://www.stiftungpath.org/kooperationspartner). There are no conflicts of interest in this work because the evaluation was performed by an external and independent agency (Institute for Social Medicine and Epidemiology; Institute for Cancer Epidemiology). Private citizens and companies that support PATH did not provide any finances or financial support with regard to this evaluation. The external evaluation agency (E. Fick, A. Waldmann, A. Katalinic) also had no contact with any supporting companies or private citizens.
Supporting Information
German supporting informations for this article
References
- 1.Kaatsch P, Spix C, Katalinic A, Robert Koch-Institut und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V.; 2012. Krebs in Deutschland 2007/2008. 8th edn; p. 134. [Google Scholar]
- 2.Kreienberg R, Albert U, Follmann M, Deutschen Krebsgesellschaft e.V. und Deutschen Krebshilfe e.V.; 2012. Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. [Google Scholar]
- 3.Rote Liste® Service GmbH Fachinfo Zometa 4 mg/5 ml Rote Liste Service GmbH; 2011http://www.fachinfo.de/data/fi/jsearch?praepStand: 03.11.2011 [Google Scholar]
- 4.Rote Liste® Service GmbH Fachinfo Ostac 520 mg Rote Liste Service GmbH; 2011http://www.fachinfo.de/data/fi/jsearch?praepStand: 03.11.2011 [Google Scholar]
- 5.Rote Liste® Service GmbH Fachinfo Bondronat Rote Liste Service GmbH; 2011http://www.fachinfo.de/data/fi/jsearch?praepStand: 03.11.2011 [Google Scholar]
- 6.Rote Liste® Service GmbH Fachinfo Aredia Rote Liste Service GmbH; 2011http://www.fachinfo.de/data/fi/jsearch?praepStand: 03.11.2011 [Google Scholar]
- 7.Holen I, Coleman R E. Bisphosphonates as treatment of bone metastases. Current Pharmaceutical Design. 2010;16:1262–1271. doi: 10.2174/138161210791034003. [DOI] [PubMed] [Google Scholar]
- 8.Russell R G. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119 02:S150–S162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 9.Gnant M, Mlineritsch B, Stoeger H. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABSCG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 10.Eidtmann H, Boer de R, Bundred N. et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 11.Coleman R E, Marshall H, Cameron D. et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 12.Nitz U, Maass N. Arbeitsgemeinschaft für Gynäkologische Onkologie e.V. AGO; 2011. Leitlinien-Diagnostik und Therapie primärer und metastasierter Mammakarzinome – Bisphosphonate und der RANKL-Antikörper Denosumab. [Google Scholar]
- 13.Dachverband der deutschen Osteologie Prophylaxe, Diagnostik und Therapie der Osteoporose bei Erwachsenen Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften – AWMF; 2010http://www.awmf.org/uploads/tx_szleitlinien/034-003_S3_Prophylaxe__Diagnostik_und_Therapie_der_Osteoporose_bei_Erwachsenen_lang_10-2009_12-2012.pdfStand: 26.02.2013 [Google Scholar]
- 14.Russell R G. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Coleman R E, McCloskey E V. Bisphosphonates in oncology. Bone. 2011;49:71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Hadji P, Aapro M S, Body J J. et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22:2546–2555. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 17.Regan M M, Price K N, Giobbie-Hurder A. et al. Interpreting Breast International Group (BIG) 1-98: a randomized, double-blind, phase III trial comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive, early breast cancer. Breast Cancer Res. 2011;13:209. doi: 10.1186/bcr2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llombart A, Frassoldati A, Paija O. et al. Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer. 2012;12:40–48. doi: 10.1016/j.clbc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.European Medicine Agency Withdrawal of Zometa-Letter European Medicines Agency; 2010http://www.ema.europa.eu/docs/en_GB/document_library/Other/2011/01/WC500101331.pdfStand: 26.02.2013 [Google Scholar]
- 20.Ritterhoff N L. Lübeck: Medizinische Fakultät, Institut für Krebsepidemiologie e.V., Universität zu Lübeck; 2010. Wie gut kennen Patienten ihre Krankheit und Behandlung? Ein Vergleich von Patientenangaben, Arztangaben und Registerdaten in der onkologischen Versorgung. [Google Scholar]
- 21.Waldmann A, Dreckschmidt J, Pritzkuleit R. et al. Test-Retest Reliabilität des OVIS-Fragebogens – Ein Instrument zur Evaluation der onkologischen Versorgung aus Patientensicht. Gesundheitswesen. 2010;72:707–713. doi: 10.1055/s-0029-1242787. [DOI] [PubMed] [Google Scholar]
- 22.Slanger T, Mutschelknauss E, Kropp S. et al. Test-retest reliability of self-reported reproductive and lifestyle data in the context of a German case-control study on breast cancer and postmenopausal hormone therapy. Ann Epidemiol. 2007;17:993–998. doi: 10.1016/j.annepidem.2007.07.094. [DOI] [PubMed] [Google Scholar]
- 23.Adelstein B A, Irwig L, Macaskill P. et al. A self administered reliable questionnaire to assess lower bowel symptoms. BMC Gastroenterol. 2008;8:8. doi: 10.1186/1471-230X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
German supporting informations for this article


