Abstract
Perfusion of 17-alpha-hydroxyprogesterone caproate (17HPC) via the maternal circuit of a dually perfused human placental lobule resulted in the extensive formation of 2 metabolites. On the other hand, human placental microsomes biotransformed 17HPC into 5 monohydroxylated metabolites, which did not correspond to those formed during perfusion. The goal of this investigation was to determine the subcellular localization of the enzymes responsible for the biotransformation of 17HPC during its perfusion in human placenta. Crude subcellular fractions of the human placental tissue were utilized. Six 17HPC metabolites were formed by the placental mitochondrial fraction, of which 4 were identical to those formed by the microsomes; whereas the other 2, namely MM and M19, were formed by the mitochondrial fraction only. The latter metabolites were identical to those formed during 17HPC perfusion, as determined by liquid chromatography–mass spectrometry (LC-MS) analysis. Therefore, these data strongly suggest that the enzymes responsible for the biotransformation of 17HPC during its perfusion are predominantly localized in human placental mitochondria.
Keywords: 17-alpha-hydroxyprogesterone caproate, metabolism, placenta, mitochondria, LC-MS
Introduction
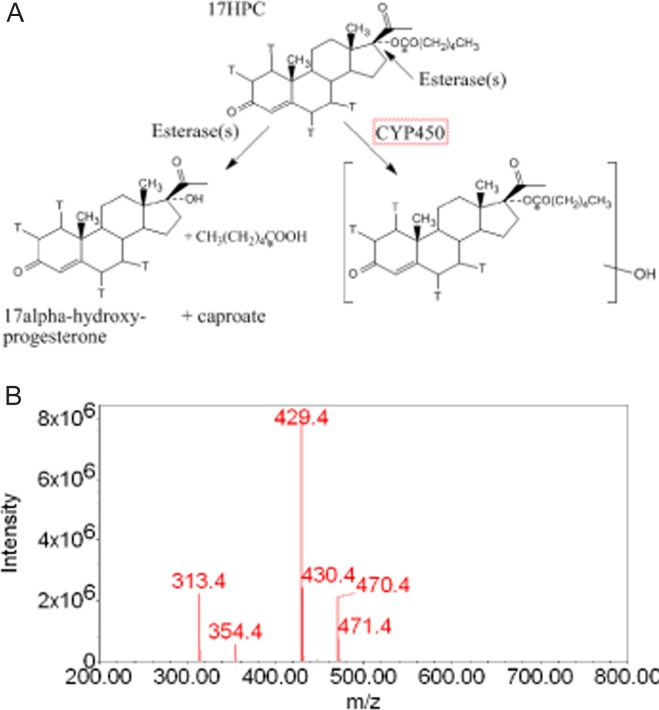
The benefits of 17-alpha-hydroxyprogesterone caproate (17HPC) in reducing the rate of recurrent preterm delivery (<37 weeks of gestation) in high-risk patients has been demonstrated1; however, the mechanism of its action remains unclear. During the last 4 years, the aim of investigations in our laboratory has been to determine the metabolic fate of 17HPC in human placenta, and whether it is a drug or a prodrug with one or more pharmacologically active metabolites. There are at least 3 metabolic pathways for the biotransformation of 17HPC, namely, hydrolysis by plasma and/or tissue esterases to hydroxyprogesterone and caproate, oxidation of 17HPC by cytochrome P450 isozymes, and/or conjugation with other phase 2 metabolic enzymes (Figure 1A).
Figure 1.
A, Structure of 17-alpha-hydroxyprogesterone caproate (17HPC) and 2 of its possible metabolic pathways.3 The position of [3H] and [14C]-radiolabeled atoms in the progesterone and caproate moieties are shown with “T” for tritium and “*” for [14C]. B, Mass spectrum of 17HPC, which revealed its molecular ion at m/z 429 and its progesterone fragment at m/z 313, resulting from a break of the ester bond between the acylcaproate and the hydroxyl-(C-17) of progesterone.4
Recent investigations in our laboratory revealed that 17HPC (dual radioactively labeled with [3H] in progesterone and [14C] in caproate; Figure 1A) was not hydrolyzed in vitro by the following preparations: human plasma esterases, human hepatic enzymes, or term and preterm placental homogenates.2 Moreover, the perfusion of the dually labeled 17-alpha-hydroxy-[3H] progesterone [14C] caproate into human placental lobule (maternal-to-fetal direction) revealed that it was extensively biotransformed into 2 metabolites.3 At the end of the experimental period, 46.3% ± 17.5% of the total radioactivity was retained by the tissue, 21.6% ± 2.3% remained in the maternal circuit, and 17.2% ± 2.2% was transferred into the fetal circuit. In addition, the ratio of the major metabolite formed to the parent compound (17HPC) retained in the tissue was 1:1; while in both the maternal and fetal circuits, the ratio3 of the metabolite to 17HPC was 3:2. Both the metabolites, major and minor, were more polar than the parent compound and retained both [3H] and [14C], indicating that 17HPC was not hydrolyzed to 17-alpha-hydroxyprogesterone and caproate3 during its perfusion. Subsequent studies of 17-alpha-hydroxy-[3H] progesterone [14C] caproate metabolism by human placental microsomes revealed the formation of 5 polar metabolites (namely, M12, M13, M15, M16′, and M17′), which were tentatively identified by liquid chromatography–mass spectrometry (LC-MS) as monohydroxylated derivatives of 17HPC.4 However, the metabolites formed during perfusion were not the same as those formed by placental microsomes as ascertained by their retention times on high-performance liquid chromatography (HPLC).
These data suggested that, in addition to placental microsomal enzymes, the mitochondrial or cytosolic enzymes might also be responsible for the biotransformation of 17HPC and the formation of the metabolites identified in the perfusion medium.
Therefore, the goal of this investigation was to determine the subcellular localization of the enzymes responsible for the biotransformation of 17HPC by placenta during its perfusion.
Materials and Methods
Chemicals and Supplies
All chemicals were purchased from Sigma Chemical Co (St. Louis, Missouri) unless otherwise noted. Acetonitrile (Optima) and methylene chloride were purchased from Fisher Scientific (Fair Lawn, New Jersey). 17-Alpha-hydroxy [1,2,6,7-3H]-progesterone [1-14C] caproate (Figure 1) was custom synthesized by RTI International (Research Triangle Park, North Carolina). The specific activity of 17HPC was 37.3 mCi/mmol for [14C] and 105 mCi/mmol for [3H].
Human Tissues
Human term placentas were obtained from uncomplicated pregnancies immediately after delivery according to a protocol approved by the Institutional Review Board of the University of Texas Medical Branch at Galveston, Texas. Crude subcellular fractions were prepared from placental tissue homogenates as described previously.5 Briefly, dissected villous tissue was rinsed with ice-cold saline and homogenized in 0.1 mol/L potassium phosphate buffer pH 7.4 (Ultra Turrax, Staufen, Germany). Mitochondrial and microsomal fractions were separated by differential centrifugation of the tissue homogenates (10 000g and 104 000g, respectively). The corresponding pellets were resuspended in 0.1 mol/L potassium phosphate buffer (pH 7.4), the cytosol collected, and the protein content of each subcellular fraction was determined (Bio-Rad kit, Hercules, California) using bovine serum albumin as a standard. Each fraction was aliquoted and stored at −80°C until used.
Pools of microsomal and mitochondrial fractions were prepared from 12 human placentas and used in all the experiments reported here. The pool of cytosolic fractions was made from 6 preparations. A pool of 15 donor human liver microsomes was purchased from CellzDirect (Austin, Texas).
Biotransformation of 17HPC by Human Placental Mitochondrial and Cytosolic Fractions
The biotransformation of 17HPC by placental mitochondrial and cytosolic fractions was determined as previously reported.4 Briefly, the reaction solution (total volume of 1 mL in 0.1 mol/L potassium phosphate buffer, pH 7.4) containing 60 µmol/L of 17HPC (4 times its apparent Km value) and 1 mg of mitochondrial or cytosolic protein was preincubated for 5 minutes at 37°C. The reaction was initiated by the addition of Nicotinamide adenine dinucleotide (NADH) [2 mmol/L], or Nicotinamide adenine dinucleotide phosphate-regenerating system (NADPH) [0.4 mmol/L Nicotinamide adenine dinucleotide phosphate (NADP), 4 mmol/L glucose-6-phosphate, 1 U/mL glucose-6-phosphate dehydrogenase, and 2 mmol/L MgCl2]. Incubation was carried out at 37°C for 60 minutes and terminated by adding 100 µL of trichloroacetic acid (10%, weight/volume [w/v]) containing 500 ng/mL testosterone as an internal standard (IS). The reaction solutions at time 0 and 60 minutes of incubation did not include either the NADPH-regenerating system or NADH and served as controls. The biotransformation of 17HPC by human placental and hepatic microsomes was reported previously.4 Since 17HPC metabolites that can be used as standards are not commercially available, human placental and hepatic microsomes were used under the same experimental conditions to prepare “reference samples” for evaluating the biotransformation of 17HPC by the 2 fractions. A range of 17HPC (0-180 µmol/L) concentrations was used to construct saturation curves and to calculate the apparent Km values (the incubation was carried out for 15 minutes). Data reported are a result of at least 3 experiments and each point in duplicate.
Quantitative Determination of 17HPC Metabolites
Both 17HPC (60 µmol/L) and its radioactive isotope ([3H] 1.47 µCi and [14C] 0.52 µCi) were combined and added to the reaction solution containing 1 mg of mitochondrial or cytosolic protein and incubated as described above. The radioactivity in the effluent of the HPLC column was monitored using a β-RAM flow through an in-line detector (model 4, IN/US Systems, Tampa, Florida) connected to the HPLC system and controlled by a ScintFlow SA software program (Tampa, Florida). Aliquots (0.5 mL) of the effluent were collected using a fraction collector (Retriever II, Teledyne Isco, Inc, Lincoln, Nebraska) and analyzed by liquid scintillation spectrometry (1900TR; Packard Instrument, Inc, Shelton, Connecticut). The amounts of metabolites formed were calculated as percentage of the 17HPC added to the reaction solution. The amounts of 17HPC metabolites formed by human placental microsomes were determined under the same experimental condition.
Preparation of Samples
The extraction of 17HPC and its metabolites from the reaction solution was carried out as described previously,4 and the recovery was >85%. Briefly, 17HPC and its metabolites were extracted from the reaction solution twice, each time with 2 volumes of methylene chloride, followed by centrifugation at 3500g for 10 minutes. The organic layers were combined, evaporated to dryness, and the residue was reconstituted in 200 µL of 90% acetonitrile. Aliquots of the samples were injected into the HPLC column for analysis.
Identification of 17HPC Metabolites
Separation of 17HPC and its metabolites was achieved by a reverse phase HPLC column. The structures of the metabolites were tentatively identified using an in-line MS. The metabolites’ retention times and mass spectra were compared with those of the metabolites formed by human hepatic and placental microsomes,4 that is, samples prepared from the reaction solution of human hepatic and placental microsomes served as standards for each analysis. The ratios of the peak areas of the metabolites to that of the IS were used to express the rates of formation of each metabolite by human placental mitochondrial fraction. The amounts of metabolites formed were determined by their content of radioactivity, as described above.
High-Performance Liquid Chromatography Method
The HPLC system consisted of a 1525 Binary HPLC Pump, a 287 dual absorbance detector, and a 717 autosampler (Waters, Milford, Massachusetts). 17-Alpha-hydroxyprogesterone caproate and its metabolites were separated using a reverse phase C18 column (symmetry 5 µm, 4.6 mm, and 150 mm; Waters). Samples were eluted using a mobile phase gradient at a flow rate of 1.2 mL/min, starting with a mobile phase A (30/70 acetonitrile/water containing 0.25% acetic acid) for 3 minutes, then followed by a linear increase up to 80% mobile phase B (10/90 of acetonitrile/water) for a period of 25 minutes, then maintained for another 10 minutes.
Liquid Chromatography–Mass Spectrophotometry Analysis
For LC-MS analysis, HPLC effluent was directed to the MS (Waters EMD 1000 single-quadrupole) interfaced with the HPLC as described previously.4 The total ion current (TIC) was utilized to enable mass spectral characterization of 17HPC and its metabolites. Selected-ion monitoring (SIM) at m/z 429, 445, 461, and 477 was utilized to determine the presence of 17HPC and its mono-, di-, and trihydroxylated metabolites, respectively. For testosterone (IS) SIM was set at m/z 289.
Dual Perfusion of Placental Lobule
Placentas from uncomplicated term pregnancies (n = 5) were obtained immediately after delivery. The experimental conditions for dual perfusion of placental lobule (DPPL) with 17HPC were described previously.3 In the previous report,3 the concentration of 17HPC in the maternal reservoir was 21 ng/mL, which is equal to the mean plasma concentration of the drug in patients with endometrial carcinoma who received a 1000-mg single intramuscular injection.6 In this investigation, the concentration of 17HPC was adjusted to 200 ng/mL to increase the amounts of 17HPC metabolites formed and to achieve a better mass spectra analysis. The perfusion system was used in its closed-closed configuration (recirculation of the media).3 At the end of the perfusion period (4 hours), 4 mL aliquots of maternal and fetal perfusates were collected. To each aliquot, 200 µL of trichloroacetic acid (10%, w/v) containing 500 ng/mL testosterone was added. 17-Alpha-hydroxyprogesterone caproate and its metabolites were extracted from the aliquots of the maternal and fetal perfusates twice with methylene chloride. The organic layers from the 2 extractions were combined and evaporated to dryness. The residue was reconstituted in 200 µL of 90% acetonitrile. Samples were analyzed with LC-MS as described above.
Data Analysis and Statistics
All data are presented as mean ± standard error. The apparent Km values were calculated from the saturation curves using the GraphPad Prism 5 software version 5.01.
Results
Biotransformation of 17HPC by Human Placental Mitochondrial and Cytosolic Fractions
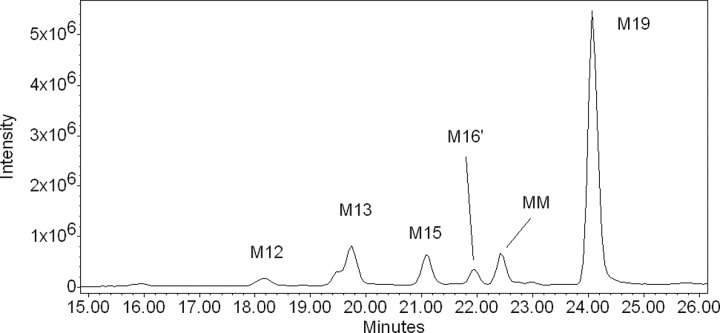
Human placental mitochondrial fractions biotransformed 17HPC into 6 metabolites (Figure 2), namely, M12, M13, M15, M16′, MM, and M19 (M stands for metabolite and the number designates its order of elution on HPLC as previously described for metabolites formed by human hepatic and placental microsomes).4 However, the metabolite MM was only formed by placental mitochondria and, therefore, was not described previously. The formation of the metabolites by placental mitochondrial fractions required the presence of an NADPH-regenerating system. Analysis of the HPLC and MS revealed that the retention times and mass spectra of 4 placental mitochondrial metabolites, namely, M12, M13, M15, and M16′, were identical to those formed by the placental microsomes.4 Accordingly, these mitochondrial metabolites were formed by the introduction of the hydroxyl group into the acyl caproate.
Figure 2.
Extracted ion chromatogram of the metabolites formed (m/z 445) by human placental mitochondria as revealed by LC-MS. Incubation of 17HPC in the presence of human placental mitochondria resulted in the formation of 6 metabolites. Three of them were identical to M12, M13, and M15 formed by human placental microsomes as determined by their retention times and mass spectra.4 One metabolite was identified as M16′ based on the identical retention time and similar mass spectra of M16′ formed by human placental microsomal fractions.4 Metabolite MM was unique to placental mitochondrial fraction. The retention time and mass spectra of M19 were identical to the previously described M19 metabolite formed by human hepatic microsomes.4 LC-MS indicates liquid chromatography–mass spectrometry; 17HPC, 17-alpha-hydroxyprogesterone caproate.
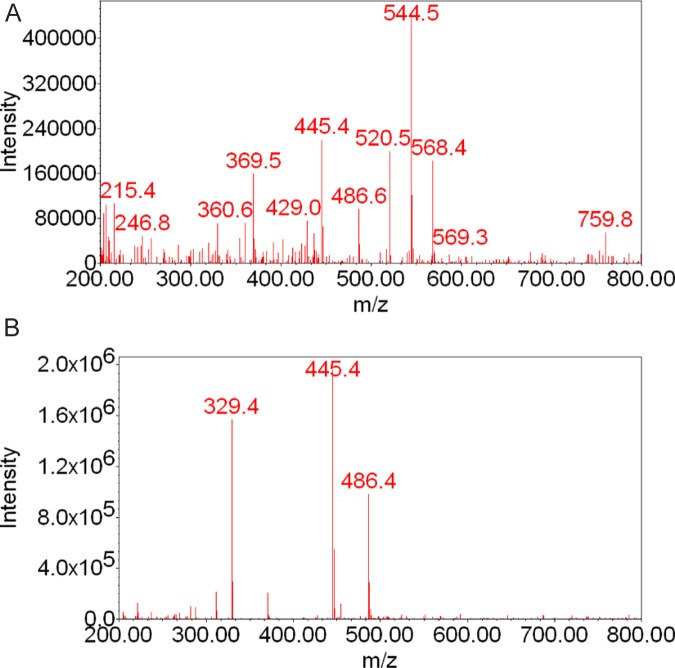
The retention times of metabolites MM and M19 were 22.4 and 24.0 minutes, respectively. These 2 metabolites were not previously identified among those formed by placental microsomes. The fragmentation pattern of MM revealed ions at m/z 360, 369, 445, 520, 544, and 568 with low intensity (Figure 3A). The ions at m/z 445 suggest that MM could be a monohydroxylated 17HPC; however, it cannot be ascertained by the method used. The mass spectrum of M19 revealed the presence of a molecular ion at m/z 445 and a fragment ion at m/z 329, indicating the introduction of the hydroxyl group into the progesterone nucleus of 17HPC (Figures 1B and 3B; the retention time and mass spectrum of this metabolite were identical to those of M19 formed by human hepatic microsomes4).
Figure 3.
Mass spectra of 17HPC metabolites MM (A) and M19 (B) formed by human placental mitochondrial fraction. The fragmentation pattern of MM revealed the presence of the molecular ion at m/z 445, suggesting that MM is a monohydroxylated metabolite of 17HPC. The mass spectrum of M19 revealed the presence of an ion at m/z 329 indicating that the hydroxyl group was introduced into the progesterone moiety of 17HPC. 17HPC indicates 17-alpha-hydroxyprogesterone caproate.
The total amount of 17HPC metabolites formed by placental mitochondrial fraction (2672 ± 111 pmol metabolites/mg protein) was significantly higher than the total amount of metabolites formed by the microsomes (592 ± 27 pmol metabolites/mg protein) under identical experimental conditions (P < .05). The formation of the major mitochondrial metabolite M19 (1538 ± 115 pmol/mg protein) accounted for 57.5% ± 4.5% of the total metabolites formed by placental mitochondrial fraction.
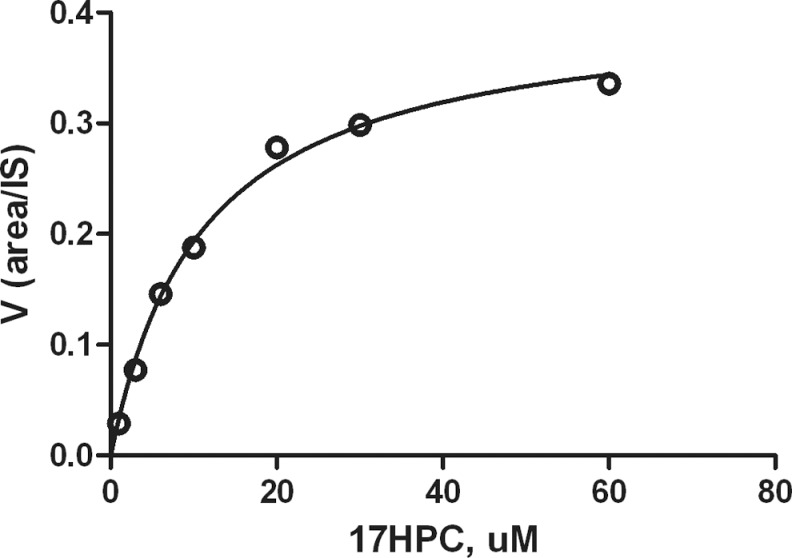
The rate of M19 formation by mitochondrial fraction was dependent on 17HPC concentration and exhibited saturation kinetics (Figure 4). The apparent Km value of 17HPC was 13.2 ± 1.5 µmol/L. The Vmax value of this metabolite was not determined because of the lack of commercially available M19 metabolite. Finally, we were unable to detect the formation of any 17HPC metabolites upon its incubation with the cytosolic fraction under the experimental conditions described.
Figure 4.
M19 formation by placental mitochondrial fractions. The rate of metabolite formation is expressed as a ratio of peak area of metabolite to internal standard (area of metabolite/area of IS). The rate of M19 formation was dependent on 17HPC concentration and exhibited typical saturation kinetics with an approximate apparent Km of 13.2 ± 1.5 µmol/L. 17HPC indicates 17-alpha-hydroxyprogesterone caproate.
Identification of the Metabolites Formed During the Perfusion of 17HPC
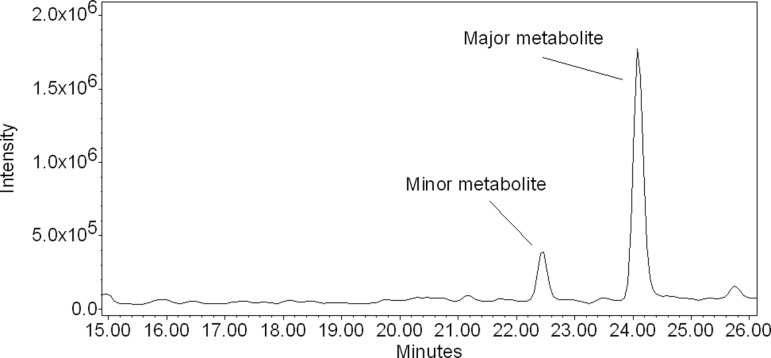
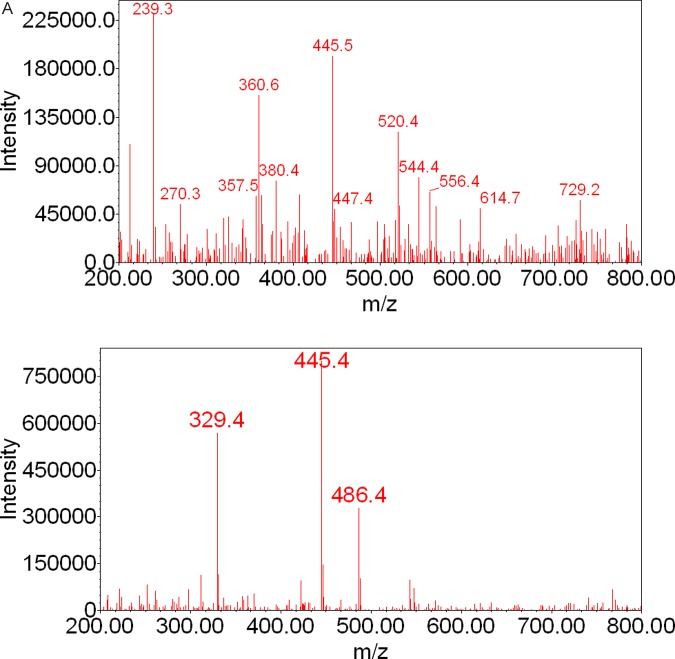
The LC-MS of samples of the perfusion media revealed the presence of 2 peaks with retention times of 22.4 and 24.0 minutes, respectively (Figure 5). The mass spectrum of the minor peak eluted at 22.4 minutes revealed ions at m/z 360, 445, 520, and 544 (Figure 6A). These fragments were also present in the mass spectrum of mitochondrial metabolite MM (Figure 3A). The mass spectrum of the major peak eluted at 24.0 minutes (Figure 6B) revealed ions at m/z 329, 445, and 486 and was identical to the fragmentation pattern of the M19 formed by human placental mitochondrial fraction (Figure 3A).
Figure 5.
Extracted ion chromatogram of the metabolites formed (m/z 445) by the human placental lobule during perfusion of 17HPC. The LC-MS of the perfusion media revealed the presence of 2 peaks with retention times 22.4 and 24.0 minutes, respectively. 17HPC indicates 17-alpha-hydroxyprogesterone caproate; LC-MS, liquid chromatography–mass spectrometry.
Figure 6.
A and B, Mass spectra of 17HPC metabolites formed by the human placental lobule during perfusion. The fragmentation pattern of the minor metabolite revealed ions at m/z 360, 445, 520, and 544. The fragmentation pattern of the major metabolite revealed ions at m/z 329 and 445. The presence of a molecular ion at m/z 445 suggested that these metabolites are monohydroxylated metabolites of 17HPC. The presence of an ion at m/z 329 in the mass spectrum of the major metabolite indicated that hydroxyl group was introduced into the progesterone moiety of 17HPC. 17HPC indicates 17-alpha-hydroxyprogesterone caproate.
Discussion
The perfusion of human placental lobule with 17HPC, radiolabeled with [3H] and [14C], revealed the formation of 2 metabolites3 that were different from the monohydroxylated metabolites of 17HPC formed by placental microsomes.4 These data suggested that the enzymes responsible for the biotransformation of 17HPC during its perfusion are unlikely to be microsomal enzymes. Furthermore, the relatively higher amounts of the metabolites formed during perfusion of 17HPC suggested that the biotransformation of the drug by placental tissue is catalyzed by enzymes of high capacity. Therefore, the aim of this work was to determine the subcellular localization of the enzymes responsible for the biotransformation of 17HPC during its perfusion via the maternal circuit of a dually perfused human placental lobule.
The data obtained in this investigation revealed that 17HPC is biotransformed by placental mitochondrial fractions with the formation of 6 metabolites, namely, M12, M13, M15, M16′, MM, and M19. Retention times and mass spectra of the metabolites M12, M13, M15, and M16′ formed by the mitochondrial fraction were identical to those of the respective metabolites formed by placental microsomes.4 However, the amounts of these placental metabolites formed by the mitochondrial fraction were less than those formed by the microsomes.
Our data on the biotransformation of 17HPC are based on the utilization of crude subcellular factions prepared by differential centrifugation. Hence, the contamination of one fraction by another is possible. Using identical experimental conditions, it was reported that the contamination of mitochondrial fractions by microsomes is approximately 20%, whereas contamination of the microsomal fraction5,7 by mitochondrial fractions is <10%. Therefore, a similar extent of the cross-contamination of the protein used in this investigation cannot be ruled out.
Nevertheless, in view of the following: (1) the metabolites MM and M19 were formed only by the mitochondrial fraction and not by the microsomes and (2) the mitochondrial fraction is more contaminated with microsomes than vice versa; the data strongly suggest that in placental tissue, these 2 metabolites (MM and M19) should have been formed exclusively by mitochondrial enzymes. Moreover, M19 is the major metabolite formed by the mitochondria, and its formation accounted for 57.5% ± 4.5% of the total metabolites formed by the mitochondrial fraction.
The mass spectrum of M19 suggests that it is a monohydroxylated derivative of 17HPC with the hydroxyl group introduced in the progesterone and not in the caproate moiety. The formation of metabolite MM was about 10% ± 2% of the total metabolites formed by the placental mitochondrial fraction, and data of our study are not sufficient to ascertain whether it is a monohydroxylated metabolite. Moreover, based on the similarities revealed between the retention times and mass spectra of the minor and major peaks eluted from the perfusion medium with the metabolites MM and M19, respectively, it can be concluded that the enzymes responsible for the biotransformation of 17HPC by human placenta during its perfusion are most likely localized in the placental mitochondria.
Progesterone and 17HPC reduce the rate of recurrent preterm labor in women with high risk of preterm birth1,8; however, the mechanisms of 17HPC action are still unclear. A recent study demonstrated that the progesterone elicited a rapid in vitro inhibitory response of human uterine tissue contractility.9 In contrast, 17HPC did not bring a direct relaxant effect on human myometrium in vitro,9,10 and in high concentrations stimulated the uterine tissue contractile activity.9 One of the explanations offered by the authors for the nul or stimulating effect of 17HPC was the use of myometrium tissue samples obtained from women undergoing cesarean section late in pregnancy9,10 and from nonpregnant participants,10 rather than preterm myometrium, which could display a different responsiveness to 17HPC. Furthermore, Sexton and coauthors suggested that in vivo administration of 17HPC could result in the formation of a metabolite exerting an uterorelaxant effect.10 Data obtained in our investigation revealed significant activity of placental mitochondrial fractions in the biotransformation of 17HPC into the metabolite M19. Although the question of whether 17HPC is a drug or prodrug still remains to be answered, it could be speculated that changes in the activity of the mitochondrial enzymes in the biotransformation of 17HPC could result in the reported variability in the efficacy of this medication. However, the mitochondrial enzyme responsible for placental metabolism of 17HPC remains to be identified.
Footnotes
This study was presented at the 30th Annual Clinical Meeting of the Society for Maternal–Fetal Medicine, Chicago, IL, February 1-6, 2010.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: supported by NICHP Obstetrics-Fetal Pharmacology Research Units Network grant U10-HD047891.
References
- 1.Meis PJ, Klebanoff M, Thom E, et al. , and National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Prevention of recurrent preterm delivery by 19 alpha-hydroxyprogesterone caproate. N Engl J med. 2003;348(24):2379–2385 [DOI] [PubMed] [Google Scholar]
- 2.Yan R, Fokina V, Hankins GDV, Ahmed MS, Nanovskaya TN. The effect of esterases on 17alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2008;198(2):229.e1–229e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemauer SJ, Yan R, Patrikeeva SL, et al. Transplacental transfer and metabolism of 17-α-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2008;199(2):169.e1–169e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan R, Nanovskaya TN, Zharikova OL, Mattison DR, Hankins GDV, Ahmed MS. Metabolism of 17alpha-hydroxyprogesterone caproate by hepatic and placental microsomes of human and baboon. Biochem pharmacol. 2008;75(9):1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshmukh SV, Nanovskaya TN, Ahmed MS. Aromatase is the major enzyme metabolizing Buprenorphine in human placenta. J Pharmacol Exp Ther. 2003;306(3):1099–1105 [DOI] [PubMed] [Google Scholar]
- 6.Onsrud M, Paus E, Haug E, Kjorstad K. Intramuscular administration of hydroxyprogesterone caproate in patients with endometrial carcinoma: pharmacokinetics and effects on adrenal function. Acta Obstet Gynecol Scand. 1985;64(6):519–523 [DOI] [PubMed] [Google Scholar]
- 7.Clement B, Mau S, Debers S, Havemeyer A. Hepatic, extrahepatic, microsomal, and mitochondrial activation of the N-hydroxylated prodrugs benzamidoxime, guanoxabenz, and RO 48-3656 ([[1-[(2S)-2-[[4-[(hydroxyamino)iminomethyl]benzoyl]amino]-1-oxopropyl]-4-piperidinyl]oxy]-actic acid). Drug Metab Dispos. 2005;33(11):1740–1747 [DOI] [PubMed] [Google Scholar]
- 8.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419–424 [DOI] [PubMed] [Google Scholar]
- 9.Ruddock NK, Shi SQ, Jain S, et al. Progesterone, but not 17-alpha-hydroxyprogesterone caproate, inhibits human myometrial contractions. Am J Obstet Gynecol. 2008;199(4):391.e1–391e7. [DOI] [PubMed] [Google Scholar]
- 10.Sexton DJ, O'Reilly MW, Friel AM, Morrison JJ. Functional effects of 17alpha hydroxyprogesterone caproate (17P) on human myometrial contractility in vitro. Reprod Biol Endocrinol. 2004;2:80 [DOI] [PMC free article] [PubMed] [Google Scholar]