Abstract
Study Design This case report describes an acute Schmorl's node (SN) in an elite monofin athlete during exercise. The patient presented with severe back pain and leg numbness and was managed successfully with conservative treatment.
Objective The aim of our communication was to describe a rare presentation of a common pathological condition during an intense sport.
Background Swimming is not generally considered to be a sport activity that leads to spinal injuries. SNs are usually asymptomatic lesions, incidentally found on imaging studies. There is no correlation between swimming and symptomatic SN formation.
Case Report A 16-year-old monofin elite athlete suffered from an acute nonradiating back pain during extreme exercise. His back pain was associated with a fracture of the superior L5 end plate and an acute SN at the L5 vertebral body with perilesional bone marrow edema. The pain resolved with nonsteroidal anti-inflammatory drugs and bed rest. The athlete had an excellent outcome and returned to his training activities 6 months after his incident.
Conclusion SN should be considered in the differential diagnosis of severe back pain, especially in sport-related injuries. SNs present with characteristic imaging findings. Due to the benign nature of these lesions, surveillance-only management may be the best course of action.
Keywords: acute Schmorl's node/giant, monofin swimming, MR imaging/diagnosis, conservative treatment
Herniation of disk material through the end plates and into the vertebral body is a relatively common pathological condition known as a “Schmorl's node” (SN). These nodes are usually found incidentally during imaging studies for degenerative disorders or traumatic conditions of the lumbar spine.1 However, in a minority of cases, patient symptoms have been attributed to acute SN formations; these nodes are usually traumatic in nature.2 Minor trauma may be the cause of SN in osteoporotic patients as the result of the weakened end plate surface microfractures.1 2
Trauma-related sport injuries of the spine have been extensively described in the literature. Swimming is among the sports that may cause spinal injuries. The most common mechanism of injury involves axial loading of the cervical spine, which then causes injury to the bones and spinal cord.3 However, despite the relative high frequency of traumatic SN formation, their formation has not been correlated with swimming.4
We report on a very rare case of an adolescent with acute onset of back pain during strenuous monofin swimming. There are no reports in the literature regarding the occurrence, imaging follow-up, and outcome of symptomatic acute SN associated with monofin swimming.
Case Report
The patient was initially referred from another hospital, where the initial diagnosis based also on the magnetic resonance (MR) imaging examination was vertebral hemangioma. Vertebroplasty was suggested, and this was the reason for further evaluation to a tertiary medical center.
A 16-year-old male athlete presented at the outpatient clinic of our department, 1 week after the onset of a sudden, acute, lower back pain accompanied by transient bilateral leg numbness during monofin training. The pain was severe and sharp in nature, and it forced the young athlete to suspend exercise. The patient remained bedridden after this incident, and his pain did not improve over time. The pain was worse at night, and it prevented the patient from sleeping. There were no bladder or bowel disturbances noted by the patient or his family. His past medical history was unremarkable. The interview did not reveal any yellow psychological flags or any hidden benefits for the patient and his family.
During the clinical examination, the patient was in apparent agony. There was an obvious spasm at the paravertebral muscles, which were tender during palpation. Percussion over the spinous process of the L5 vertebrae reproduced his complaints. His neurological examination during the initial presentation did not correlate with his degree of distress. The muscle power in all key muscle groups was normal and equal in both sides. There were no sensory disturbances in pain sensation, light touches, or deep proprioceptive sensations. His reflexes were recorded as normal. There were no findings related to other organ systems, and laboratory examination did not yield any significant findings.
The plain radiographs of the patient showed a lucent lesion extending craniocaudally through the L5 vertebral body. A focal depression was evident in the L5 upper epiphyseal plate. The lysis was surrounded by a thin sclerotic margin, which suggested the presence of a nonaggressive lesion (Fig. 1A). The MR imaging examination was also reevaluated and the diagnosis of a giant SN was suggested, based on the low-signal-intensity signal on T1-weighted and high on T2-weighted images. On T2-weighted images, there was increased signal surrounding the lesion anteriorly and to the right, in keeping with reactive bone marrow edema. This area, as well as the internal wall of the lesion, enhanced following administration of contrast medium. The lesion was associated with a dehydrated-degenerative intervertebral disk L4–L5. Thus, a final diagnosis of an acute giant SN following a fatigue fracture of the L5 superior end plate was established (Fig. 1B–G).
Figure 1.
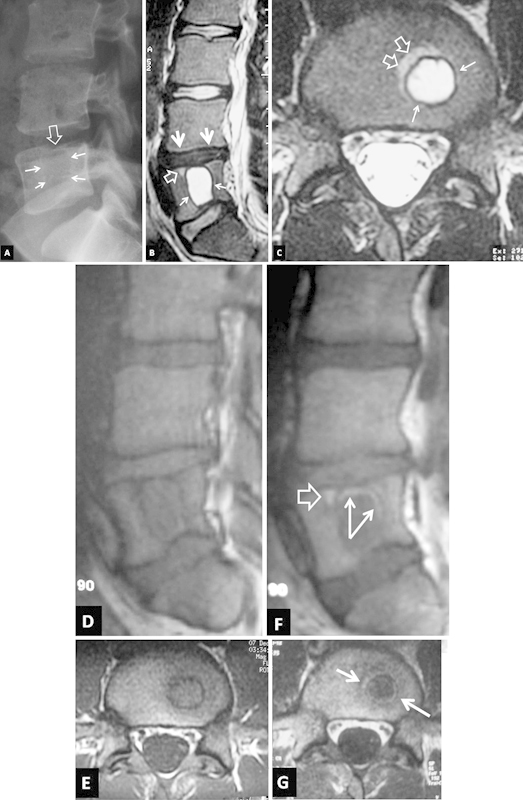
Imaging 1 week after injury. (A) The lateral plain film shows a lytic lesion with a thin sclerotic border (arrows). A focal depression is also evident in the L5 upper epiphyseal plate (open arrow). The sagittal (B) and axial (C) T2-weighted magnetic resonance (MR) images show a large cystic area with a low signal intensity border corresponding to a giant Schmorl's node (arrows). Reactive bone marrow edema is seen anterior to the lesion (open arrows). The L4–L5 intervertebral disc is degenerated (thick arrowheads). The sagittal (D) and axial (E) T1-weighted MR images and the corresponding images following intravenous contrast medium administration (F, G) show a “ringlike” enhancement within the sclerotic border (arrows) and anterior to the lesion (open arrow).
The patient was treated conservatively with bed rest and nonsteroidal anti-inflammatory medication. During this treatment, he was examined at the outpatient department on a weekly basis. The pain subsided gradually 3 weeks after its onset. By week 6, the young athlete was fully mobilized and was able to return to his daily activities. He was allowed to return to his sport activities after 6 months. MR imaging was repeated 10 months after the incident (Fig. 2). The parasagittal short T1 inversion recovery (STIR) and axial T2-weighted MR images showed a reduction in lesion size regarding the craniocaudal diameter, resolution of the surrounding bone marrow edema, and increased focal high signal intensity at the nucleus pulposus in the L4–L5 disc. This was consistent with partial regression of the herniated disc material. The contrast-enhanced transverse T1-weighted MR image showed persistent enhancement of the outer ring of the lesion.
Figure 2.
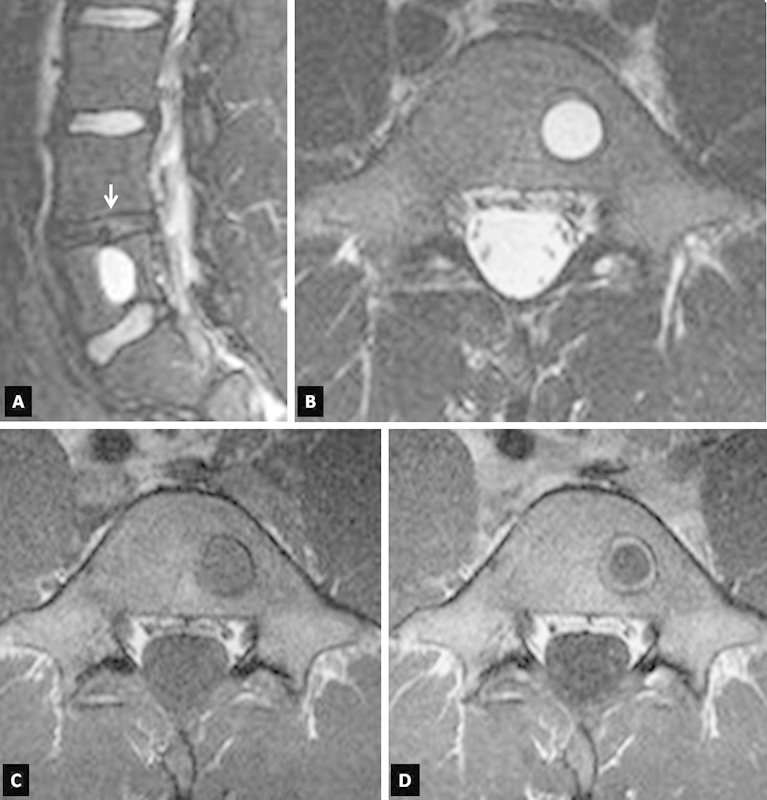
The sagittal short T1 inversion recovery (STIR) (A) and axial T2-weighted (B) magnetic resonance (MR) images 10 months after the onset of symptoms show a minor reduction of the size of the lesion and a minor increase in the signal within the nucleus pulposus of the L4–L5 disc (arrow). However, there is no surrounding edema in the bone marrow. The axial T1-weighted MR images, before (C) and after (D) contrast medium administration, show the persistent “ringlike” enhancement within the sclerotic border of the lesion.
Discussion
SNs are intravertebral herniations of disk material through areas of weakness into the vertebral endplate. These benign pathological entities are also known as “intraosseous disc herniations” or “Geipel hernias.” They were initially described and extensively studied by Christian George Schmorl in 1927, as a potential cause of Scheuermann's kyphosis.5 Since then, their exact role in spine pathology and Scheuermann's disease has remained vague.
The incidence of SN has been reported to be as high as 76% in postmortem studies.6 However, population-based studies seem to present more realistic estimates of ~10 to 16% incidence in the general population.1 They are predominantly found among males (54.3%) and European-Americans.2 There are significant variations in the size of SNs, but their average size has been reported to be ~7 × 9 mm.7 The most common anatomic site of SN occurrence is the thoracolumbar spine, especially in the central third of the superior end plate.2 7 Various pathogenic mechanisms have been proposed, including developmental and degenerative processes, infection, malignancy, and trauma.8 The association of SN and Scheuermann's kyphoscoliosis has been repeatedly reported.5 9 Nevertheless, the exact etiology and pathogenesis of the SN remains largely unknown. Most chronic SNs demonstrate quiescent clinical symptomatology. However, the acute nodes are usually painful.10 11 In the clinical-radiological differential diagnosis list for SN, several benign and malignant conditions are included, including vertebral hemangiomas, multiple myeloma, and bone metastases from lungs, breast, and prostate. A proper diagnostic workup should include both detailed clinical examination and high-quality MR imaging.12 SNs usually appear as irregularities of the vertebral contour or as small radiolucent lesions of the vertebral bodies that are limited by reactive sclerosis and connected to the intervertebral disk. However, in rare occasions, a node may appear as a diagnostically challenging large cystic lesion, which may be confused with other cystlike lesions of the vertebral body. Giant SNs pose further diagnostic difficulties.13 The indolent natural course of SN does not justify aggressive intervention in most cases. However, there have been reports of cases that required ramus communicans nerve blocking, navigation-assisted fluoroscopic lumbar vertebroplasty,11 14 or anterior interbody fusion.12
For our literature review, we performed a PubMed search using the keywords “acute” or “painful” AND “Schmorl's node,” “intraosseous disc herniation,” “Geipel herniation,” and “intravertebral disc herniation.” The search was limited to articles written in English, but there were no temporal limitations. The gathered articles were reviewed for relevance to the keywords, and their references were further scrutinized for relevant articles. Altogether, there were 22 articles reporting on 76 patients suffering from painful SN (Table 1). The latter can be either acute or chronic in nature. It is worth noting that Lipson et al published the first report on a painful SN in 1985, more than half a century after Schmorl's initial description.5 15
Table 1. A Summary Table of the Published Studies Related to Acute Schmorl's Nodes (SN).
| Author (year) | No. | Age (years) | Gender | Pathology | Back Pain History | Mechanism | Presentation | Diagnosis | Laboratory Exams | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipson et al15 (1985) | 1 | 49 | M | Painful C5 SN | − | − | Progressive right neck pain | Tomography: lucency surrounded by sclerosis Discometrics: reproduced the pain |
Normal | ACDF | Gradual reduction of pain in three months |
| McCall et al27 (1985) | 8 | 16.4 (12–22) | 6M, 2F | L1-L2: 4 T12-L1: 2 L2-L3: 1 L4-L5: 1 |
Paralyric scoliosis (1), Scheurmann's kyphosis (3), spondylo-lysis (2) | Acute vertical compression (blast explosion, motor vehicle accident, rugby injuries, falls) | Acute progressive back-pain, limited radiation to the thigh | Radiographs: end-plate fracture Discography: reproduced pain |
− | Conservative | − |
| Kornberg20 (1988) | 1 | 27 | M | L4 | − | Forced lumbar flexion | Acute lumbar pain | MRI: intraosseousdisc heriation | Normal | − | − |
| Walters et al17 (1991) | 1 | 14 | F | L4 | − | Competitive exercise | Acute low back-pain | MRI: inflammation and edema around the SN | − | Conservative | Improvement over four months, return to sports |
| Takahashi and Takata33 (1994) | 5 | 14–81 | 2M, 3F | Lumbar | − | − | Low back pain | MRI: T1 low intensity, T2 high intensity | − | 3 conservative, 2 ALIF | Improvement over three to four months |
| Tosi et al19 (1996) | 1 | 16 | F | T12 and L1 SN leading to fibrocartilage-nous embolism | Good health | Hand-standing | Acute back-pain, progressive paraplegia, complete sensory loss under T12 | MRI: intraosseous disc herniation | Normal | Conservative | Complete paraplegia |
| Leibner and Floman26 (1998) | 1 | 19 | M | L4 SN | Fall from height before 6 years of age | Motor vehicle accident | Acute low back pain | CT: osteolytic lesion with sclerotic margins and tunneling of the L4 vertebral body | − | − | − |
| Seymour et al18 (1998) | 8 | 49 (13-63) | 3M, 5F | SN at: T6 (1) T9 (1) T10 (3) T11 (3) L1 (1) L2 (3) L3 (1) |
− | Injury related in 2/8 | Acute or exacerbation of chronic back pain | MRI: intraosseous disc herniation with perinodal edema | No infection or malignancy | Conservative | Significant reduction of edema in 7–10 months, complete edema resolution and fatty degeneration in 18 months |
| Grive et al21 (1999) | 2 | 68 and 38 | 1M, 1F | T11, L3 | L5-S1 spondylolisthesis, plasmacy-toma | Dorsolubar flexion injury, physical exercise | Acute back pain without sciatica | CT: radiolucent rim around a sclerotic area MRI: low signal in T1 and high signal in T2 surrounded by edema and inflamation |
Normal | Conservative | Gradual pain reduction by the second month |
| Khashaba31 (2000) | 1 | 14 | F | L4 | − | Motor vehicle accident | Acute abdominal pain and lower spinal pain, painful flexion | CT: irregular lucent are in the VB MRI: end-plate defect and intraosseous disc herniation |
− | − | − |
| Leone et al29 (2000) | 1 | 76 | F | T12-L3 | Pre-B acute lympobla-stic leukemia | − | Chronic back pain | MRI | Lymphobla-stic leukemia | Conservative | Death due to primary disorder |
| Wagner et al32 (2000) | 14 | 30 (19-45) | 12M, 2F | Thoraco-lumbar region | − | 9 motor vehicle accidents, 5 ski jumping | Acute low back pain without radiculopathy | MRI: end plate defects and marrow edema | − | Conservative | Improvement |
| Hasegawa et al10 (2004) | 1 | 55 | F | L3 | Recurrent low back pain | No obvious injury | Acute exacerbation low back pain | Discography demonstrated the leakage into the VB, MRI | − | L2-L3 interbody fusion | Improvement |
| Masala et al14 (2006) | 23 | 72.5 (61–84) | 7M, 16F | Chronic painful spinal SN | − | − | Back pain for 6 months | CT, MRI | − | Percutaneous vertebroplasty | 18 improved, 3 did not worsen |
| Crawford and Van der Wall28 (2007) | 1 | 57 | F | SN at L4 | Scheurmann's disease | Gardening plus minor trauma | Acute back pain | Bone scintigraphy: increased uptake MRI: bone marrow edema |
Normal | Conservative | Substantial edema resolution after 18 months |
| Park et al24 (2007) | 1 | 52 | F | SN at T8 | Mid thoracic back pain radiating to right scapula | Fall | Acute exacerbation of the back pain | MRI: Contrast enhancing lesion marrow edema | − | Conservative | Reduction of the edema but persistence of the enhancement at 5 and 9 months; no enhancement by the 26th month; clinical improvement |
| Fucuta et al12 (2009) | 1 | 19 | M | SN at T11, T12 | Back pain for over two years | Soccer player, no obvious injury | Chronic progressive pain | MRI, discography reproduced the pain | − | Anterior interbody fusion | Return to play |
| Pilet et al22 (2009) | 1 | 36 | M | SN at L3 and L4 | Long history of back pain, mini-discectomy at L5-S1 before 17 years of age | Discography-induced SN | Acute back pain, different from the pre-existing one | MRI: subchondral bone marrow edema, no enhancement | Normal CRP, high ESR | Conservative | Schmorl node by the 7th week; persistence of findings by the 5th month |
| Wegner and Markwalder11 (2009) | 1 | 31 | M | L4 SN | Healthy | No obvious injury | Progressive lumbalgia | MRI: marked rim of bone edema at the VB | − | Orthosis followed by fluoronavigation-assisted vertebroplasty | Improvement |
| Jang et al23 (2010) | 1 | 82 | M | L4 SN | − | No obvious injury | Acute back pain | MRI: SN with adjacent marrow edema | Normal | Rami communicantes nerve block | Improvement |
| Mittal et al25 2010) | 1 | 10 | M | Acute painful SN at T12 and L1 | Acute calcific discitis | Yoga session | − | MRI: SN with adjacent marrow edema and calcification | − | Conservative | Improvement |
| Paterakis et al (2011) | 1 | 16 | M | L5 | No past medical problems | Monofin swimming | Acute back pain | MRI: SN with adjacent marrow edema | Normal | Conservative | Improvement |
M, male; F, female; CT, computerized tomography; MRI, magnetic resonance imaging; VB, vertebral body.
Our PubMed search yielded 44 patients suffering from acute SN. Acute SN can affect all age groups except for infants and toddlers. They are more common in males than females (1.8:1), and they usually occur in the thoracolumbar region, with the upper and lower end plates equally affected.1 2 16 Patients suffering from acute SN usually present with acute back pain. They can also present as an acute exacerbation of a chronic back pain or nonhabitual pain that is different in nature from the previously existing pain. The pain can radiate to the abdomen and thighs and is usually worsened by trunk movements. The absence of radiculopathy or other neurological findings is remarkable.17 18 19 20 21 22 23 24 25 The medical history of the patients is often omitted, but it may include trauma (fall from a height),26 degenerative disorders (spondylolisthesis, degenerative disc disease, scoliosis),21 22 27 congenital disorders (spondylolysis and Scheuermann's kyphosis),21 27 28 or neoplasia (plasmacytoma).29 30
With a few exceptions, a traumatic mechanism occurs before the formation of an acute SN. Of the 44 published cases of an acute SN, 41 patients (93%) reported some kind of injury prior to the onset of symptoms. The mechanical stress caused by the injury leads to failure of the end plates and herniation of disc material within the vertebral body. Falls, motor-vehicle accidents, and sport activities are among the traumatic mechanisms described in the literature.17 18 19 20 21 24 25 26 27 31 32 Additionally, Pilet et al described an acute SN after lumbar discography.22 Similarly, Tosi et al reported the case of a 16-year-old girl who developed complete paraplegia a few hours after performing handstands because of a fibrocartilaginous embolism from a newly formed SN to the spinal cord.19
Many diagnostic imaging modalities are included in the armamentarium of a clinician. Plain radiographs depict end plate fractures and can be used to highlight vertebral body osteolytic lesions.27 Both findings can be examined in more detail with the use of spinal computed tomography, which also show the sclerotic margins of lesions.21 26 MR imaging is the examination method of choice because it is able to clearly show an end plate defect. Moreover, the typical finding of an SN is an intravertebral lesion of low intensity in TI-weighted images and high signal intensity in T2-weighted images. These images are representative of intradiscal herniation and a surrounding zone of edema and inflammation. Contrast enhancement is not a constant finding on MR imaging of SN.17 18 19 20 21 22 23 24 25 Discography is not among the standard examination procedures because it has been surpassed by less invasive examinations. When performed, it reproduces the pain of the patient and shows the leakage into the vertebral body.10 27 Finally, there are reports describing increased radioisotope uptake in bone scintigraphy, but this examination is not routinely performed.28 The interesting imaging findings presented herein include the surrounding bone marrow edema and its enhancement, resolution of the edema in keeping with clinical improvement, and persistence of the ring enhancement in the periphery of the lesion at the 10-month follow-up MR imaging.
The differential diagnosis list includes neoplastic and inflammatory disorders. A battery of laboratory examinations, including tumor markers and nonspecific inflammatory markers such as C-reactive protein and white blood cell count, have been measured within normal ranges in patients with SN, with the exception of the erythrocyte sedimentation rate, which has been found to be elevated in some patients, including the patient in this study.18 22 25
The most common treatment protocol is bed rest and analgesics. Symptoms usually resolve after a variable period of time, ranging from 2 to 6 months. Clinical improvement occurs with a gradual decrease of the vertebral body edema and inflammatory reactions. However, in some cases, the SNs do not become chronic by radiological examination until 9 months after the initial formation of the node. The last stage of an SN is fatty degeneration, which takes place 18 months after the formation of the node. At this point, the intraosseous herniation has become chronic, and the patients are allowed to fully resume their daily activities, including return to competitive sports.18 19 21 22 24 25 27 28 29 32 33 However, there are a few reports on the surgical treatment of acute SN. Hasegawa et al described a retroperitoneal excision of an L3 SN followed by an L3–L4 Z-fusion in a 55-year-old woman. According to the authors, the patient improved dramatically immediately after surgery, was able to remove the brace after 1 month, and could fully cope with daily activities by the second postoperative follow-up.10 Similarly, Fukuta et al used anterior interbody fusion to manage thoracic SN in an athlete to allow for an early return to play.12 Wenger and Markwalder chose to perform navigation-assisted fluoroscopic lumbar vertebroplasty to treat a 31-year-old patient with progressive lumbalgia with good results.11 Jang et al successfully treated an 82-year-old man suffering from an L4 acute SN with the use of ramus communicans nerve blocking using mepivacaine and triamcinolone.23
Conclusion
SN should be considered in the differential diagnosis of elite athletes with severe back pain. However, the diagnosis is set by the characteristic radiological findings. It is worth attempting a conservative treatment with anti-inflammatory medications and bed rest until the SN turns into a chronic node before attempting more aggressive treatment modes, such as vertebroplasty or anterior lumbar interbody fusion.
Footnotes
Disclosures Konstantinos N. Paterakis, None Alexandros G. Brotis, None Efthimios Dardiotis, None Georgios M. Hadjigeorgiou, None Theofilos Karachalios, None Kostas N. Fountas, None Apostolos Karantanas, None
References
- 1.Mok FP, Samartzis D, Karppinen J, Luk KD, Fong DY, Cheung KM. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine. 2010;35:1944–1952. doi: 10.1097/BRS.0b013e3181d534f3. [DOI] [PubMed] [Google Scholar]
- 2.Dar G, Peleg S, Masharawi Y, Steinberg N, May H, Hershkovitz I. Demographical aspects of Schmorl nodes: a skeletal study. Spine. 2009;34:E312–E315. doi: 10.1097/BRS.0b013e3181995fc5. [DOI] [PubMed] [Google Scholar]
- 3.Boden BP Jarvis CG Spinal injuries in sports Neurol Clin 20082663–78., viii [DOI] [PubMed] [Google Scholar]
- 4.Karantanas A. Berlin, Heidelberg: Springer-Verlag; 2011. Common injuries in water sports; pp. 289–316. [Google Scholar]
- 5.Schmorl G. Die pathologische Anatomie der Wirbelsaule. Verh Dtsch Orthop Ges. 1927;21:3–41. [Google Scholar]
- 6.Hilton RC, Ball J, Benn RT. Vertebral end-plate lesions (Schmorl's nodes) in the dorsolumbar spine. Ann Rheum Dis. 1976;35:127–132. doi: 10.1136/ard.35.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu HT, Morrison WB, Schweitzer ME. Edematous Schmorl's nodes on thoracolumbar MR imaging: characteristic patterns and changes over time. Skeletal Radiol. 2006;35:212–219. doi: 10.1007/s00256-005-0068-y. [DOI] [PubMed] [Google Scholar]
- 8.Peng B, Wu W, Hou S, Shang W, Wang X, Yang Y. The pathogenesis of Schmorl's nodes. J Bone Joint Surg Br. 2003;85:879–882. [PubMed] [Google Scholar]
- 9.Paajanen H, Alanen A, Erkintalo M, Salminen JJ, Katevuo K. Disc degeneration in Scheuermann disease. Skeletal Radiol. 1989;18:523–526. doi: 10.1007/BF00351753. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa K, Ogose A, Morita T, Hirata Y. Painful Schmorl's node treated by lumbar interbody fusion. Spinal Cord. 2004;42:124–128. doi: 10.1038/sj.sc.3101506. [DOI] [PubMed] [Google Scholar]
- 11.Wenger M, Markwalder TM. Fluoronavigation-assisted, lumbar vertebroplasty for a painful Schmorl node. J Clin Neurosci. 2009;16:1250–1251. doi: 10.1016/j.jocn.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Fukuta S, Miyamoto K, Iwata A. et al. Unusual back pain caused by intervertebral disc degeneration associated with Schmorl node at Th11/12 in a young athlete, successfully treated by anterior interbody fusion: a case report. Spine. 2009;34:E195–E198. doi: 10.1097/BRS.0b013e318198c60cXXX. [DOI] [PubMed] [Google Scholar]
- 13.Coulier B. Giant fatty Schmorl's nodes: CT findings in four patients. Skeletal Radiol. 2005;34:29–34. doi: 10.1007/s00256-004-0858-7. [DOI] [PubMed] [Google Scholar]
- 14.Masala S, Pipitone V, Tomassini M, Massari F, Romagnoli A, Simonetti G. Percutaneous vertebroplasty in painful schmorl nodes. Cardiovasc Intervent Radiol. 2006;29:97–101. doi: 10.1007/s00270-005-0153-6. [DOI] [PubMed] [Google Scholar]
- 15.Lipson SJ, Fox DA, Sosman JL. Symptomatic intravertebral disc herniation (Schmorl's node) in the cervical spine. Ann Rheum Dis. 1985;44:857–859. doi: 10.1136/ard.44.12.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfirrmann CWA, Resnick D. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology. 2001;219:368–374. doi: 10.1148/radiology.219.2.r01ma21368. [DOI] [PubMed] [Google Scholar]
- 17.Walters G, Coumas JM, Akins CM, Ragland RL. Magnetic resonance imaging of acute symptomatic Schmorl's node formation. Pediatr Emerg Care. 1991;7:294–296. doi: 10.1097/00006565-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Seymour R, Williams LA, Rees JI, Lyons K, Lloyd DC. Magnetic resonance imaging of acute intraosseous disc herniation. Clin Radiol. 1998;53:363–368. doi: 10.1016/s0009-9260(98)80010-x. [DOI] [PubMed] [Google Scholar]
- 19.Tosi L Rigoli G Beltramello A Fibrocartilaginous embolism of the spinal cord: a clinical and pathogenetic reconsideration J Neurol Neurosurg Psychiatry 19966055–60. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornberg M. MRI diagnosis of traumatic Schmorl's node. A case report. Spine. 1988;13:934–935. doi: 10.1097/00007632-198808000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Grivé E, Rovira A, Capellades J, Rivas A, Pedraza S. Radiologic findings in two cases of acute Schmörl's nodes. AJNR Am J Neuroradiol. 1999;20:1717–1721. [PMC free article] [PubMed] [Google Scholar]
- 22.Pilet B, Salgado R, Van Havenbergh T, Parizel PM. Development of acute schmorl nodes after discography. J Comput Assist Tomogr. 2009;33:597–600. doi: 10.1097/RCT.0b013e318188598b. [DOI] [PubMed] [Google Scholar]
- 23.Jang JS, Kwon HK, Lee JJ, Hwang SM, Lim SY. Rami communicans nerve block for the treatment of symptomatic Schmorl's nodes: a case report. Korean J Pain. 2010;23:262–265. doi: 10.3344/kjp.2010.23.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park P, Tran NK, Gala VC, Hoff JT, Quint DJ. The radiographic evolution of a Schmorl's node. Br J Neurosurg. 2007;21:224–227. doi: 10.1080/02688690701317169. [DOI] [PubMed] [Google Scholar]
- 25.Mittal P, Saggar K, Sandhu P, Gupta K. Case report: acute calcific discitis with intravertebral disc herniation in the dorsolumbar spine. Indian J Radiol Imaging. 2010;20:205–207. doi: 10.4103/0971-3026.69360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibner ED, Floman Y. Tunneling Schmorl's nodes. Skeletal Radiol. 1998;27:225–227. doi: 10.1007/s002560050371. [DOI] [PubMed] [Google Scholar]
- 27.McCall IW, Park WM, O'Brien JP, Seal V. Acute traumatic intraosseous disc herniation. Spine. 1985;10:134–137. doi: 10.1097/00007632-198503000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Crawford BA, van der Wall H. Bone scintigraphy in acute intraosseous disc herniation. Clin Nucl Med. 2007;32:790–792. doi: 10.1097/RLU.0b013e318149ee54. [DOI] [PubMed] [Google Scholar]
- 29.Leone A, Cerase A, Equitani F, Pagano L. Tunneling Schmorl's nodes in an elderly woman treated for acute lymphoblastic leukemia. Skeletal Radiol. 2000;29:660–663. doi: 10.1007/s002560000272. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Suzuki S, Ishiiwa H, Yamato M, Ueda Y. Schmorl's node developing in the lumbar vertebra affected with metastatic carcinoma: correlation magnetic resonance imaging with histological findings. Spine. 2003;28:E503–E505. doi: 10.1097/01.BRS.0000099388.63504.4D. [DOI] [PubMed] [Google Scholar]
- 31.Khashaba A. Acute intraosseous disc herniation. Injury. 2000;31:271–272. doi: 10.1016/s0020-1383(99)00297-1. [DOI] [PubMed] [Google Scholar]
- 32.Wagner AL, Murtagh FR, Arrington JA, Stallworth D. Relationship of Schmorl's nodes to vertebral body endplate fractures and acute endplate disk extrusions. AJNR Am J Neuroradiol. 2000;21:276–281. [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi K, Takata K. A large painful Schmorl's node: a case report. J Spinal Disord. 1994;7:77–81. doi: 10.1097/00002517-199407010-00011. [DOI] [PubMed] [Google Scholar]


