Abstract
The purpose of radiation therapy (RT) for patients with spinal metastases is pain relief and control of paralysis. The aim of the present study was to assess pain relief using RT and to evaluate prognostic factors for pain control. We evaluated 97 consecutive patients, of mean age 62.7 years (range 28 to 86), with spinal metastases that had been treated by RT. We evaluated the effects of RT using pain level assessed using a drug grading scale based on the World Health Organization standards. The following potential prognostic factors for pain control of RT were evaluated using multivariate logistic regression analysis: age, gender, tumor type, performance status (PS), number of spinal metastases, and a history of chemotherapy. Among the 97 patients who underwent RT for pain relief, 68 patients (70.1%) presented with pain reduction. PS (odds ratio: 1.931; 95% confidence interval: 1.244 to 2.980) was revealed by multivariate logistic regression analysis to be the most important prognostic factor for pain control using RT. In conclusion, we found that RT was more effective for patients with spinal metastases while they maintained their PS.
Keywords: radiation therapy, spinal metastases, multivariate logistic regression analysis, pain relief
Spinal metastases are the most common type of bone metastases, and the occurrence rate has been estimated at 40% among the patients with bone metastasis.1 Paralysis caused by spinal cord compression and back pain due to the destruction of the spinal column decrease patients' abilities to perform activities of daily living (ADL). Treatment modalities for spinal metastases are limited, because cancer is systemic disease. Therefore, allopathy and palliative therapy form the basis of treatment for spinal metastases. Chemotherapy is a systemic therapy for spinal metastases, and radiation therapy (RT) and surgery are local therapies. The indication of surgery for spinal metastases is controversial, because there are some factors that influence treatment outcome, such as the type of original lesion, staging, and prognosis. Tokuhashi et al established a scoring system for the preoperative evaluation of the prognosis of metastatic spinal tumors.2 3 4 Tomita et al also proposed a surgical strategy for patients with spinal metastases.5 However, patients with spinal metastases are individuals, with unique prognoses and general conditions, hopes of residual life, beliefs, and families' hopes. As a consequence, surgeons have difficulty in deciding the optimal treatment for patients with spinal metastases. Successful treatment of spinal metastases, which can be defined as maintaining or restoring ambulation and controlling severe pain, may prolong survival and improve abilities to perform ADL.6
The purpose of RT for patients with spinal metastases is pain relief, control of paralysis, and preservation of the symptom associated with spinal metastases. Pain control is the most important aspect of treatment for patients with spinal metastases. Some authors have reported that 60 to 90% of patients with spinal metastases experienced pain relief after RT.7 8 9 10 The indication and timing of RT for the treatment of spinal metastases are thought not to be dependent on certain factors, such as the original lesion, tumor type, staging, tumor size, and multiple metastases.11 12 However, little attention has been paid to the prognostic factors that influence the effectiveness of RT for spinal metastases. Identification of these factors is considered very important for optimizing the therapeutic effectiveness and reducing the side effects of RT. The aim of the present study was to assess the clinical outcome of RT for spinal metastases in a retrospective case control study and to identify prognostic factors for pain control in patients with spinal metastases treated with RT.
Materials and Methods
From January 2000 through October 2005, we retrospectively evaluated 97 consecutive patients with spinal metastases treated by RT at our hospital. The demographic data were obtained from medical charts. Spinal metastases were detected using magnetic resonance imaging, computed tomography, or bone scintigraphy. The RT dosage was a total 30 Gy (radiation schedule; 10 × 3 Gy given in 2 weeks). Patients with a previous history of RT were excluded from our study. If severe complications associated with RT occurred during the 2-week treatment period, RT was discontinued. We decided the indication of RT for pain control by considering the following factors: (1) back pain that was not controlled with any drugs, (2) patients' hope, and (3) prognosis.
The pain grades were assessed using a drug grading level based on World Health Organization (WHO) standards. Drug grading using the WHO scale was as follows: 0, no drug; 1, nonsteroidal anti-inflammatory drugs; 2, weak opioid; 3, strong opioid. The drug grading is shown in Table 1. The criteria for pain reduction were defined as: (1) reduction of drug grading level; (2) reduction of drug dose; and (3) no change of drug but improvement in performance status (PS).13 Pain levels were assessed prior to RT for spinal metastases and at the completion of RT. Patients were followed until death, and the survival rate after RT was evaluated using the Kaplan-Meier life table estimation method. We evaluated the prognostic score in accordance with the method proposed by Tokuhashi (Table 2).3
Table 1. Drug Grading using WHO scale.
| WHO Scale | Drug Type | Example |
|---|---|---|
| 0 | None | — |
| 1 | Nonopioid | NSAIDS |
| 2 | Weak opioid | Codeine, buprenorphine, pentazocine |
| 3 | Strong opioid | Morphine, fentanyl |
NSAIDS, nonsteroidal anti-inflammatory drugs; WHO, World Health Organization.
Table 2. Tokuhashi's Evaluation System for the Prognosis of Metastatic Spine Tumor.
| Variable | Score |
|---|---|
| General condition (performance status) | |
| Poor (PS 3, 4) | 0 |
| Moderate (PS 2) | 1 |
| Good (PS 0, 1) | 2 |
| No. of extraspinal bone metastases foci | |
| ≥ 3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| No. of metastases in the vertebral body | |
| ≥ 3 | 0 |
| 2 | 1 |
| 1 | 2 |
| Metastases to the major internal organs | |
| Unremovable | 0 |
| Removable | 1 |
| No metastases | 2 |
| Primary site of the cancer | |
| Lung, stomach, bladder, esophagus, pancreas | 0 |
| Liver, gallbladder, unidentified | 1 |
| Others | 2 |
| Kidney, uterus | 3 |
| Rectum | 4 |
| Thyroid, breast, prostate, carcinoid tumor | 5 |
| Palsy | |
| Complete (Frankel A, B) | 0 |
| Incomplete (Frankel C, D) | 1 |
| None (Frankel E) | 2 |
Criteria of predicted prognosis: total score 0–8 ≤ 6 mo; total score 9–11 ≥ 6 mo; total score 12–15 ≤ 1 y. PS, performance status.
Analysis of Prognostic Factors for Pain Control
The following potential prognostic factors were evaluated using multivariate logistic regression analysis: age, gender, cancer type classified using Tokuhashi's evaluation system,3 PS13 (Table 3), number of spinal metastases, and history of chemotherapy for systemic cancer control.
Table 3. Performance Status.
| Score | Description |
|---|---|
| 0 | Fully active, able to carry on all predisease activities without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light housework, office work |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care, confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled; cannot carry on any self-care; totally confined to bed or chair |
Statistical Analysis
Clinical outcomes were evaluated using the Mann-Whitney U test. Differences were considered statistically significant when the p value was <0.05. To identify influence factors for pain control using RT, the relationships between the categorical variables were analyzed using the χ2 test. The odds ratios (ORs) for significant variables and 95% confidence intervals (CIs) were calculated by univariate and multivariate logistic regression analysis. SAS software (version 9; SAS Institute Inc., Cary, NC) was used for all statistical analyses. This study was approved by the institutional review boards of the first author's institution.
Results
Patient Demographic Data
The patient group consisted of 51 men and 46 women, with a mean age of 62.7 years (range 28 to 86). Primary lesions included lung cancer in 30 patients, breast cancer in 20, hepatocellular carcinoma in 10, prostate cancer in 7, renal cell carcinoma in 6, and other cancers in 24 (Table 4). Classification of the cancer type using Tokuhashi's evaluation system3 was as follows: score 0 (n = 36 patients, 37.1%); score 1 (n = 15 patients, 15.5%); score 2 (n = 7 patients, 7.2%); score 3 (n = 8 patients, 8.3%); score 4 (n = 4 patients, 4.1%); and score 5 (n = 27 patients, 27.8%; Table 4). Ten patients had spinal metastases treated by RT in the cervical region, 8 in the cervicothoracic region, 40 in the thoracic region, 13 in the thoracolumbar region, 22 in the lumbar region, and 4 in the sacral region. The number of spinal metastases in each patient was as follows: solitary spinal metastases in 28 patients (28.8%); two metastases in 21 patients (21.7%); and more than three metastases in 28 patients (49.5%). Thirty-two patients among the 97 patients (33.0%) had a history of chemotherapy, the purpose of which was to control the cancer's advancement.
Table 4. Original Lesion and Classification of Cancer Type by Tokuhashi's Evaluation System.
| Number of Patients | |
|---|---|
| Original lesion | |
| Lung | 30 |
| Breast | 20 |
| Liver | 10 |
| Prostate | 7 |
| Kidney | 6 |
| Rectum | 4 |
| Stomach | 4 |
| Gall bladder | 3 |
| Uterus | 2 |
| Esophagus | 1 |
| Bladder | 1 |
| Unidentified | 2 |
| Others | 7 |
| Total | 97 |
| Tokuhashi's score | |
| 0 | 36 (37.1%) |
| 1 | 15 (15.5%) |
| 2 | 7 (7.2%) |
| 3 | 8 (8.3%) |
| 4 | 4 (4.1%) |
| 5 | 27 (27.8%) |
| 97 (100%) | |
PS, performance status; RT, radiation therapy.
PS before RT was score 0 in 12 patients (12.4%), score 1 in 23 patients (23.7%), score 2 in 29 patients (29.9%), score 3 in 18 patients (18.6%), and score 4 in 15 patients (15.4%; Table 5). PS in 19 among the 97 patients (19.6%) improved after RT. However, PS deteriorated in 8 patients (8.2%).
Table 5. PS before and after RT.
| PS | Before RT, n (%) | After RT, n (%) |
|---|---|---|
| 0 | 12 (12.4%) | 18 (18.6%) |
| 1 | 23 (23.7%) | 21 (21.6%) |
| 2 | 29 (29.9%) | 25 (25.8%) |
| 3 | 18 (18.6%) | 18 (18.5%) |
| 4 | 15 (15.4%) | 15 (15.5%) |
| Total | 97 (100%) | 97 (100%) |
Tokuhashi's Scoring System for Preoperative Evaluation of Spinal Metastases and Survival Rate
Eighteen patients with scores of more than 9 points (one patient with over 12 points and 17 patients with 9 to 11 points) had a survival prognosis of over 6 months. Seventy-nine among 97 patients (81.5%) had scores of less than 8 points and were expected to have a poor prognosis (Fig. 1). The Kaplan-Meier curve indicated that the median survival time in the entire group was 13 weeks (Fig. 2).
Figure 1.
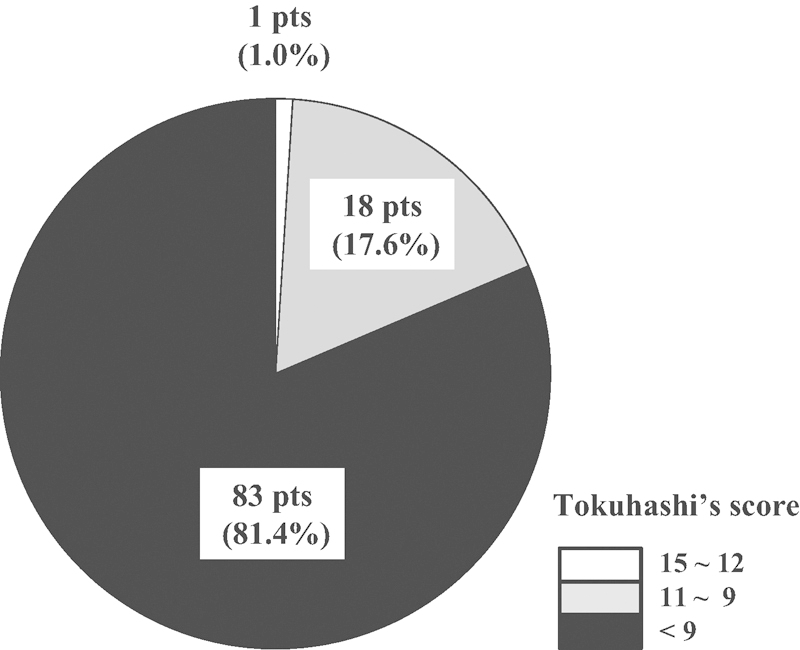
Predicted prognosis before radiation therapy assessed using Tokuhashi's scoring system: score of 12–15 predicted prognosis ≥ 1 year; score of 9–11, predicted prognosis of 6 months to ≤1 year; score of 8 predicted prognosis ≤ 6 months.
Figure 2.
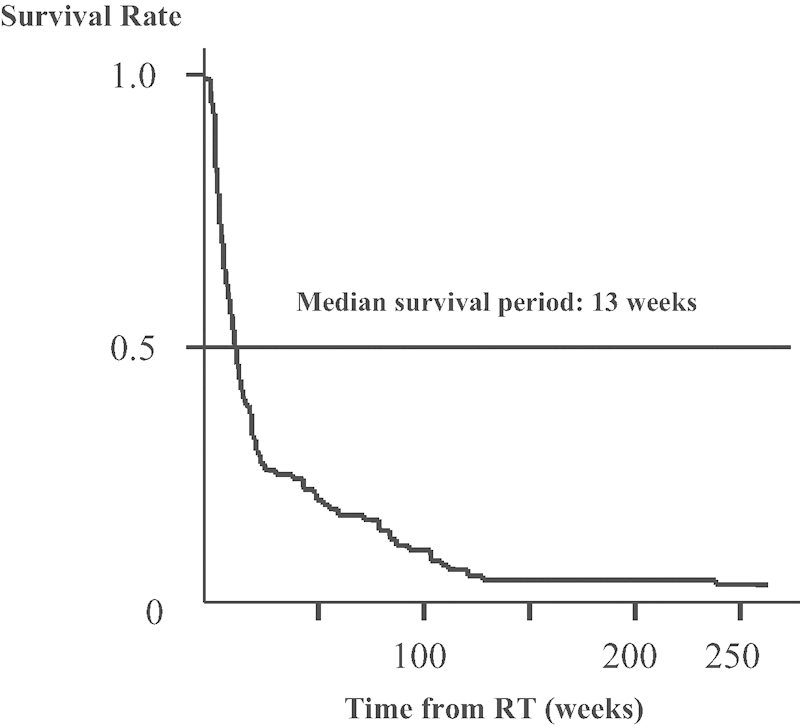
Patient survival curve after radiation therapy (RT) for spinal metastases.
Outcome of RT
Among the 97 patients who underwent RT for pain relief, 68 patients (70.1%) presented with pain reduction. Fifty-eight (59.8%) of these 97 patients presented with a reduction in drug grading level or a reduction of drug dose, and 19 patients (19.6%) exhibited an improvement in PS. Complications associated with RT occurred in 13 patients (13.4%). Nausea and vomiting were evident in 12 of these 13 patients, and liver dysfunction occurred in one patient.
Multivariate Analysis of Prognostic Factors for Pain Control Using RT
We conducted an analysis of the following potential prognostic factors: age, gender, cancer type, PS, number of spinal metastases, and a history of chemotherapy for systemic cancer control. The PS (OR: 1.931, 95% CI: 1.244 to 2.98) was revealed by multivariate logistic regression analysis to be the most important prognostic factor. The univariate and multivariate data are summarized in Table 6.
Table 6. Univariate and Multivariate Analysis Prognostic Factors for Pain Control Using RT.
| Factors | Odds Ratio | p Value | 95% CI |
|---|---|---|---|
| Univariate analysis | |||
| PS | 2.014 | 0.0009 | 1.33–3.05 |
| Chemotherapy | 0.574 | 0.250 | 0.22–1.48 |
| No. of spinal metastases | 0.753 | 0.520 | 0.32–1.79 |
| Original lesion | 0.529 | 0.173 | 0.21–1.32 |
| Age | 1.004 | 0.838 | 0.46–1.23 |
| Sex | 0.957 | 0.921 | 0.40–2.27 |
| Multivariate analysis | |||
| PS | 1.931 | 0.003 | 1.24–3.00 |
| Chemotherapy | 0.637 | 0.461 | 0.19–2.11 |
| No. of spinal metastases | 0.861 | 0.780 | 0.30–2.45 |
| Original lesion | 0.765 | 0.083 | 0.57–1.04 |
| Age | 0.993 | 0.779 | 0.94–1.05 |
| Sex | 1.212 | 0.719 | 0.43–3.44 |
p < 0.05: significant difference. CI, confidence interval; PS, performance status; RT, radiation therapy.
Discussion
There is no doubt that RT plays an important role in the treatment for bone metastases. The mechanism of pain relief by RT is still unclear, although it is thought to be related to tumor shrinkage or to depressed cytokine production by inflammatory cells surrounding the tumor cell.14 15 16 Tong et al noted that a total RT dose of 30 Gy given in 10 fractions provided pain relief for 77 to 84% of patients with multiple bone metastases during the 12 to 20 months after treatment.7 Maranzano and Latini reported the clinical outcome of a total RT dose of 30 Gy administered in 10 fractions to 255 patients with spinal metastases.10 They found that pain relief occurred in 82% of all patients treated. The Radiation Therapy Oncology Group reported that several RT dose regimens, such as 8 Gy in one fraction, 20 Gy in five fractions, and 30 Gy in 10 fractions, resulted in complete pain relief in 57% of all patients for 6 months after treatment.9 Zaidat and Ruff prospectively examined the effect of RT in combination with glucocorticoid therapy on 139 patients with spinal metastases.8 They reported that the level of pain was significantly reduced after treatment. In this study, 80 patients (82.5%) had a Tokuhashi score of less than 8 points, which indicated a prognostic survival period of less than 6 months. Actually, the median survival period for this group was 13 weeks. The patients in this study had a poorer prognosis in comparison with these previous reports.7 8 10 Despite this poor prognosis, among the 97 patients who underwent RT for pain relief, 68 patients (70.1%) presented with pain reduction. Our data concerning pain control is in agreement with previous studies. Additionally, mild complications (nausea) occurred in only 12 patients, with the exception of radiation hepatitis in one patient. Therefore, RT is a less invasive and effective treatment for pain relief in patients with spinal metastases even if they have poor prognosis. One potential limitation of our study is that we did not evaluated pain relief with pain grading scale such as the visual analogue scale, but used drug grading, dose level, and PS. However, in clinical circles, the methods of pain evaluation used in our study are believed to accurately reflect the patients' condition.
Some important factors in relation to the surgical prognosis for spinal metastases have been reported.17 18 Ogihara et al examined prognostic factors for patients with spinal metastases from lung cancer.17 These authors pointed out that the PS for non–small cell lung cancer and a history of chemotherapy in small cell lung cancer are useful factors to indicate whether surgical intervention is required. Hirabayashi et al reported that ambulation is the most important factor for postoperative prognosis in patients who had received palliative surgery for spinal metastases.18 Tokuhashi et al used PS, number of bone metastases, number of spinal metastases, metastases of major internal organs, type of original lesion, and palsy as factors in their evaluation systems for the prognosis of metastatic spinal tumors.2 3 These authors proved that their system was effective in their prospective study.4 Therefore, PS is thought to be important factor in the prognosis of surgical outcome for spinal metastases. On the other hand, Maranzano et al reported that early diagnosis was important for the prognosis of patients who underwent RT for spinal metastases.19 Zaidat and Ruff found that ambulatory ability before RT and steroid therapy were the most important factors for pain relief and prognosis.8 In our study, we evaluated the following potentially prognostic factors using multivariate logistic regression analysis: age, gender, cancer type, PS, number of spinal metastases, and a history of chemotherapy. PS (OR: 1.931, 95% CI: 1.244 to 2.98) was found to be the most important prognostic factor for pain control using RT. Our analysis does not conflict with previous studies. It seems reasonable to suppose that patients with spinal metastases would be able to achieve important ADL if they underwent early diagnosis and early treatment prior to loss of PS.
The goal of treatment for spinal metastases is to obtain a better ADL during the survival period. RT was revealed to be a less invasive and effective treatment for the pain control associated with spinal metastases. However, multivariate logistic regression analysis indicated that PS was the most important prognostic factor for pain control using RT. In conclusion, we confirmed that RT is more effective for patients with spinal metastases while they maintain their PS.
Footnotes
Disclosures Akira Matsumura, None Manabu Hoshi, None Masatsugu Takami, None Takahiko Tashiro, None Hiroaki Nakamura, None
References
- 1.Klimo P Jr, Schmidt M H. Surgical management of spinal metastases. Oncologist. 2004;9:188–196. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 2.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 4.Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine. 2009;34:69–73. doi: 10.1097/BRS.0b013e3181913f19. [DOI] [PubMed] [Google Scholar]
- 5.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Ruff R L, Lanska D J. Epidural metastases in prospectively evaluated veterans with cancer and back pain. Cancer. 1989;63:2234–2241. doi: 10.1002/1097-0142(19890601)63:11<2234::aid-cncr2820631130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Tong D, Gillick L, Hendrickson F R. The palliation of symptomatic osseous metastases: final results of the Study by the Radiation Therapy Oncology Group. Cancer. 1982;50:893–899. doi: 10.1002/1097-0142(19820901)50:5<893::aid-cncr2820500515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Zaidat O O, Ruff R L. Treatment of spinal epidural metastasis improves patient survival and functional state. Neurology. 2002;58:1360–1366. doi: 10.1212/wnl.58.9.1360. [DOI] [PubMed] [Google Scholar]
- 9.Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up Radiother Oncol 199952111–121. [PubMed] [Google Scholar]
- 10.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 11.Ferris F D, Bezjak A, Rosenthal S G. The palliative uses of radiation therapy in surgical oncology patients. Surg Oncol Clin N Am. 2001;10:185–201. [PubMed] [Google Scholar]
- 12.Loblaw D A, Laperriere N J. Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998;16:1613–1624. doi: 10.1200/JCO.1998.16.4.1613. [DOI] [PubMed] [Google Scholar]
- 13.Oken M M, Creech R H, Tormey D C. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 14.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 15.Coussens L M, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choong P FM. The molecular basis of skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S19–S31. doi: 10.1097/01.blo.0000093839.72468.da. [DOI] [PubMed] [Google Scholar]
- 17.Ogihara S, Seichi A, Hozumi T. et al. Prognostic factors for patients with spinal metastases from lung cancer. Spine. 2006;31:1585–1590. doi: 10.1097/01.brs.0000222146.91398.c9. [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi H, Ebara S, Kinoshita T. et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 19.Maranzano E, Latini P, Checcaglini F. et al. Radiation therapy in metastatic spinal cord compression. A prospective analysis of 105 consecutive patients. Cancer. 1991;67:1311–1317. doi: 10.1002/1097-0142(19910301)67:5<1311::aid-cncr2820670507>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]


