Abstract
The objective of this study was to evaluate the efficacy of a microendoscopic spinal decompression surgical technique using a novel approach for the treatment of lumbar spinal canal stenosis (LSCS). The following modifications were made to the conventional microendoscopic bilateral decompression via the unilateral approach: the base of the spinous process was first resected partially to secure a working space, so as not to separate the spinous process from the lamina. The tip of the tubular retractor was placed at the midline of the lamina, where laminectomy was performed microendoscopically. A total of 126 stenotic levels were decompressed in 70 patients. The mean operating time per level was 77.0 minutes, and the mean intraoperative blood loss per level was 15.0 mL. There were no dural tears or neurological injuries intraoperatively. Fracture of the spinous process was detected postoperatively in two patients, both of whom were asymptomatic. All patients could be followed up for at least 12 months. Their median Japanese Orthopaedic Association (JOA) score improved significantly from 16 points preoperatively to 27.5 points after the surgery (p < 0.001). The case series showed that the modifications of the technique improved the safety and ease of performance of the microendoscopic decompression surgery for LSCS.
Keywords: microendoscopic surgery, posterior decompression, minimally invasive surgery, lumbar spinal canal stenosis, kissing spine, spinous process
Introduction
The indications for microendoscopic spinal decompression surgery for lumbar lesions, which was first reported in 1997 by Foley and Smith1 as discectomy for lumbar disk herniation, has been used to treat lumbar spinal canal stenosis (LSCS). Bilateral decompression is possible via a unilateral approach, by slanting the microendoscope diagonally and angling the microendoscope to see over to the other side. The nerve roots on the contralateral side can be decompressed through the enlarged laminotomy from the side of the surgical approach. The technique has increased in popularity as a minimally invasive option for LSCS in Japan.
However, there is still room for improving this technique. First, resection of the facet joint on the side of the surgical approach is needed to reach the spinal canal when the distance between the spinous process and facet joint is small, especially at the upper lumbar levels. Second, flavectomy in the region of severe stenosis can be associated with a high risk of a dural tear. We therefore devised a modified microendoscopic decompression technique via the paramedian approach to overcome these potential problems. The purpose of this study was to investigate the clinical outcomes of using our technique for the treatment of patients with LSCS.
Methods
A total of 70 patients (46 men and 24 women; mean age at the time of surgery, 67.6 years; range 50 to 86 years) with LSCS underwent microendoscopic decompression surgery using the paramedian approach by a single surgeon (K.N.) between February 2008 and August 2010 at Sumiya Orthopaedic Hospital. All patients had been diagnosed as having LSCS and complained of leg symptoms, for which conservative treatment had proven ineffective. Patients with LSCS due to spondylolisthesis with segmental instability included in this series. Cases of discectomy were not included in the present study. There were no cases of reoperation. The number of decompressed intervertebral levels was one level in 19 patients, two levels in 46 patients, and three levels in 5 patients. Thus, a total of 126 stenotic levels were decompressed (Table 1).
Table 1. Patient Demographics (n = 70).
| Mean age (range) | 67.6 (50–86) |
| Sex | N |
| Male | 46 |
| Female | 24 |
| Number of operated levels | N |
| One | 19 |
| Two | 46 |
| Three | 5 |
| Level of stenosis | N |
| L2-3 | 15 |
| L3-4 | 61 |
| L4-5 | 50 |
| Comorbidity | N |
| Degenerative spondylolisthesis | 23 |
| Ossification of ligamentum flavum | 3 |
| Calcification of ligamentum flavum | 2 |
| Epidural lipomatosis | 1 |
The following items were investigated: (1) operating time, (2) intraoperative blood loss, (3) perioperative complications, and (4) surgical outcomes as evaluated utilizing the JOA score for low back pain (Table 2) before and after 12 months of follow-up. The JOA scores were analyzed using the Mann–Whitney U test. Probability values of <0.05 were considered to be statistically significant.
Table 2. The Japanese Orthopaedic Association (JOA) Score for Low Back Pain.
| Definition and Description | Score |
|---|---|
| Subjective symptoms (9 points) | |
| Low back pain | |
| None | 3 |
| Occasional mild pain | 2 |
| Frequent mild or occasional severe pain | 1 |
| Frequent or continuous severe pain | 0 |
| Leg pain and/or tingling | |
| None | 3 |
| Occasional mild pain | 2 |
| Frequent mild or occasional severe pain | 1 |
| Frequent or continuous severe pain | 0 |
| Gait | |
| Normal | 3 |
| Able to walk >500 m, w/pain, tingling, and/or muscle weakness | 2 |
| Unable to walk >500 m, due to leg pain, tingling, and/or muscle weakness | 1 |
| Unable to walk >100 m, due to leg pain, tingling, and/or muscle weakness | 0 |
| Clinical signs (6 points) | |
| Straight leg-raising test (including tight hamstring) | |
| Normal | 2 |
| 30–70° | 1 |
| <30° | 0 |
| Sensory disturbance | |
| None | 2 |
| Slight disturbance | 1 |
| Marked disturbance | 0 |
| Motor disturbance (manual muscle testing) | |
| None (grade 5) | 2 |
| Slight weakness (grade 4) | 1 |
| Marked weakness (grade 3-0) | 0 |
| Restriction of activities of daily living (14 points) | |
| Turning over while lying down | 0–2 |
| Standing | 0–2 |
| Washing face | 0–2 |
| Leaning forward | 0–2 |
| Sitting (1 hour) | 0–2 |
| Lifting or holding | 0–2 |
| Walking | 0–2 |
| (A score of 0 indicates a severe restriction; a score of 1, moderate restriction; and a score of 2, no restriction) | |
| Urinary bladder function (−6 points) | |
| Normal | 0 |
| Mild dysuria | −3 |
| Severe dysuria | −6 |
Surgical Procedures
The patient under general anesthesia was placed prone on a laminectomy frame. The operation levels were confirmed using an X-ray image intensifier and were marked on the skin with ink. The METRx system (Medtronic Sofamor Danek, Memphis, TN, USA) was used for the operation. A 16-mm paramedian skin incision was usually sufficient for decompression at as many as three levels. An additional skin incision was made if the skin did not move sufficiently in the craniocaudal direction to allow decompression at three levels. The 16-mm skin incision was made ~7 mm lateral to a spinous process. The muscle was sequentially dilated after fasciotomy, and a tubular retractor of 16-mm diameter was placed. When the width of the lamina was small, the tubular retractor could not be inserted down to the level of the lamina, due to interference from the spinous process and the facet joint. Muscles covering the lamina and ligamentum flavum were carefully resected, and the bony structure was exposed. The surgical level was reconfirmed using an X-ray image intensifier.
The midline of the spinal canal was confirmed first by resecting the base of the spinous process with a high speed drill. Then, the tubular retractor was pulled out halfway and slanted medially. The base of the spinous process, which obstructed the placement of the tubular retractor, was resected partially to secure sufficient working space (Fig. 1). Bone resection was performed carefully so as not to separate the spinous process from the lamina (Fig. 2). The tip of the tubular retractor was advanced to the midline of the lamina. The lamina was resected using a high-speed drill as far as the attachment of ligamentum flavum to the lamina. Once the ligament was detached from the bone, bleeding from the epidural space and dural pulsation through the ligament were seen. The ligamentum flavum was split from the midline like “French doors,” using a ball-tipped probe and resected (Fig. 3). The use of curved Kerrison rongeurs and a curved high-speed drill enabled the lateral recess to be enlarged while keeping the facet joint intact. The endpoint of decompression was the outer edges of the bilateral nerve roots. After hemostasis and lavage, a drain was placed at every operated level, and the incision was closed in layers with 2-0 Vicryl Plus (Ethicon, Inc., Somerville, NJ, USA) and Steri-Strips (3M Health Care, St. Paul, MN, USA).
Figure 1.

Illustration showing the placement of a tubular retractor for microendoscopic surgery via the paramedian approach (A) and the conventional approach (B) in the treatment of LSCS with hypertrophic facet joints in cross section. FJ, facet joint; SP, spinous process.
Figure 2.
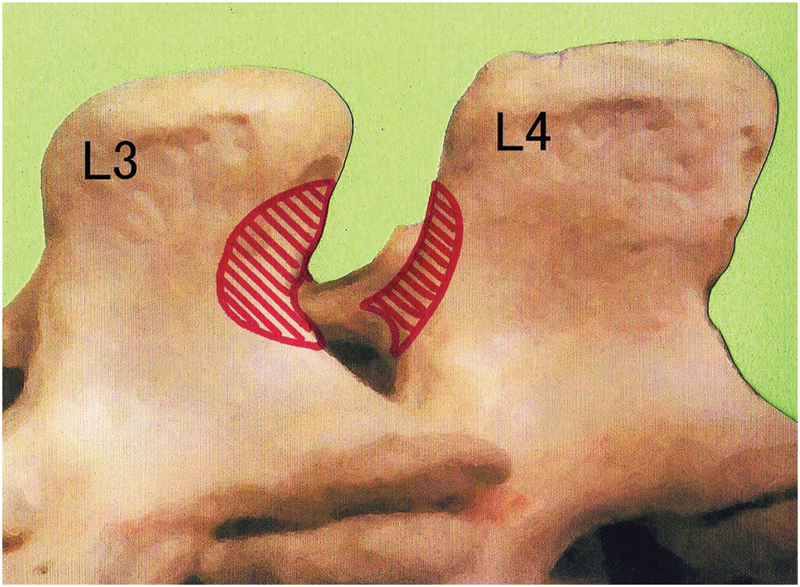
Illustration showing bone resection of the spinous processes (shaded area) for the midline placement of a tubular retractor at L3-4 in the left paramedian approach.
Figure 3.
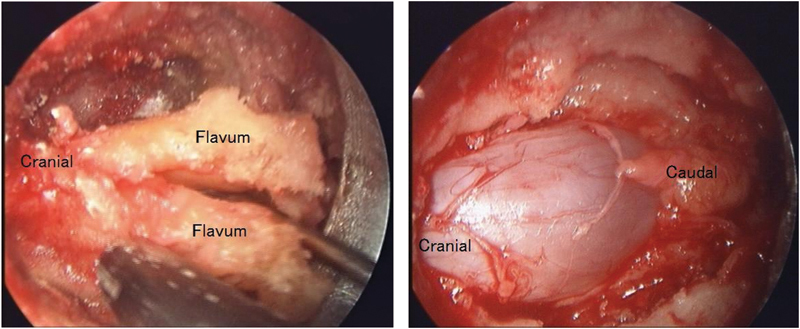
Intraoperative pictures of microendoscopic decompression surgery using the paramedian approach from the left at the L3-4 level. Left, ligamentum flavum split from the midline like “French doors.” Right, bilateral decompression of the dural tube.
Ambulation was allowed at 5 hours after the surgery without a brace. Rehabilitation was started from the day after the operation. Drains were pulled out 2 days after the operation. Most patients were discharged from the hospital within several days of the microendoscopic surgery (Fig. 4).
Figure 4.
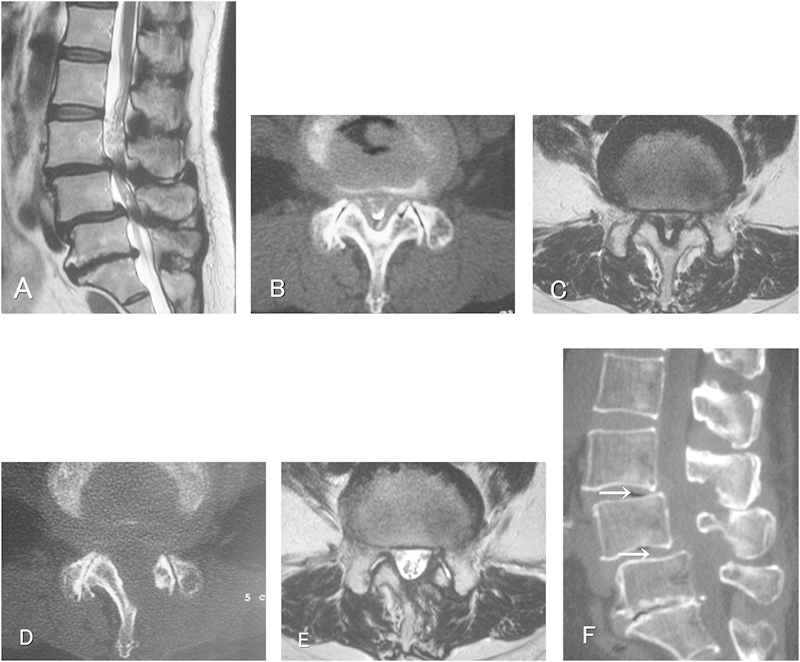
Images obtained in a 69-year-old woman who underwent two-level decompression. (A) Sagittal T2-weighed MR image showing central spinal canal stenosis at the L3-4 and L4-5 with degenerative spondylolisthesis. (B) Preoperative CT myelogram showing hypertrophic facet joints causing narrowing of the spinal canal at the L3-4 level. (C) Preoperative axial T2-weighed MR image. (D) Postoperative cross-sectional CT obtained at the L3-4 level, showing enlargement of the spinal canal in “trumpet” shape, with preserved facet joints. (E) Postoperative axial T2-weighed MR image showing a decompressed spinal canal at the L3-4 level. (F) Postoperative sagittal-sectional CT scan showing bone resection of the spinous processes at the operated levels (arrows). Intact kissing spines at the L3-4 level. CT, computed tomography; MR, magnetic resonance.
Results
For the microendoscopic decompression using the paramedian approach, the mean operating time per level was 77.0 minutes (range, 42.5 to 111.5 minutes), and the mean intraoperative blood loss per level was 15.0 mL (range, 0.5 to 85 mL). There were no dural tears or neurological injuries during the operation. Fracture of the spinous process was detected as a postoperative complication by computed tomography in two patients, both of whom were asymptomatic (Table 3). All patients were followed up for at least 12 months. The median of the JOA score of these patients improved significantly from 16 points (range, 8 to 24 points) preoperatively to 27.5 points (range, 19 to 29 points, p < 0.001) after the surgery. In many cases, the scores for low back pain, leg pain and/or tingling, gait, and restriction of activities of daily living improved after surgery, and their median values improved significantly (Table 4).
Table 3. Operative Results.
| Mean operative time per level, minute (range) | 77.0 (42.5–111.5) |
| Mean operative blood loss per level, mL (range) | 15.0 (0.5–85) |
| Complication | N |
| Dural tear | 0 |
| Neural injury | 0 |
| Fracture of spinous process | 2 |
| Infection | 0 |
| Reoperation | 0 |
Table 4. Clinical Outcomes as Indicated by the JOA Score for Low Back Pain.
| Ameliorated (N) | Unchanged (N) | Deteriorated (N) | Median of Score, Point | p Value | ||
|---|---|---|---|---|---|---|
| Preoperative | 12 Months after Operation | |||||
| Total JOA score | 68 | 1 | 1 | 16 | 27.5 | <0.001 |
| Low back pain | 45 | 21 | 4 | 2 | 3 | <0.001 |
| Leg pain and/or tingling | 55 | 12 | 3 | 1 | 3 | <0.001 |
| Gait | 62 | 7 | 1 | 0.5 | 3 | <0.001 |
| Straight leg-raising test | 5 | 65 | 0 | 2 | 2 | <0.05 |
| Sensory disturbance | 18 | 51 | 1 | 2 | 2 | <0.001 |
| Motor disturbance | 17 | 53 | 0 | 2 | 2 | <0.001 |
| Restriction of activities of daily living | 66 | 2 | 2 | 7 | 13.5 | <0.001 |
| Urinary bladder function | 5 | 64 | 1 | 0 | 0 | NS |
JOA, Japanese Orthopaedic Association; NS, not significant.
Discussion
In microendoscopic decompression surgery using the unilateral approach, the integrity of the facet joint on the side of the surgical approach often needs to be sacrificed to reach the spinal canal, because the lamina is short, especially at the higher lumbar levels. This may lead to fracture of the inferior articular process,2 which can result in iatrogenic low back pain. It is imperative to preserve the facet joint while decompressing the spinal canal to prevent this complication. Some procedures, such as lumbar spinous process-splitting laminectomy,3 muscle-preserving interlaminar decompression (MILD),4 and microendoscopic MILD5 have been developed to preserve the facet joint and the paraspinal muscles. The demerits of these procedures, however, is that the integrity of the facet joint can only be preserved at the expense of the spinous process (which is injured) while decompressing the spinal canal from the midline. It has been reported that the facet joints and the spinous processes play important roles in stabilizing the lumbar spine in some cases.6 It has also been reported that breakdown of the bony contact between spinous processes, the so-called “kissing spines,” often results in subsequent foraminal stenosis.7 Thus, we have devised a novel approach using the existing unilateral-approach decompression technique to advantage, to preserve “kissing spines.”
There are earlier reports of bilateral decompression being conducted via the unilateral approach by gouging a part of the spinous process.8 9 10 The purpose of cutting the base of spinous process in this technique was to obtain a larger surgical field of view for effecting decompression on the contralateral side of the spinal canal. Meanwhile, in our approach, the objective was to secure a working space for placing a tubular retractor in the midline for bilateral decompression in a “trumpet” shape. In other words, the major difference in our approach was to preserve the facet joint on the side of the surgical approach. Surgeons who are familiar with conventional microendoscopic laminectomy would likely be able to perform this method with ease, without acquiring additional special skills. However, surgical tools such as high-speed drills and Kerrison rongeurs with a curved shaft help facilitate the procedure.
Fracture of the spinous process as a postoperative complication was detected by computed tomography in two patients, both of whom were asymptomatic, about 1 month after the operation. The fractures took the form of a longitudinal fissure at the base of the spinous process, presumed to be caused while the tubular retractor was advanced to the midline. The fractures were, however, expected to heal easily because the soft tissues and periosteum around the fracture site had not been detached from the bone. Both of the fractures healed. Therefore, a fracture of the spinous process, which was the only complication noted in the case series, was not a critical issue. Yagi et al11 reported a series of microendoscopic midline decompressions for single-level degenerative spinal stenosis. The spinous process was osteotomized and separated from the lamina in their description of their technique. They reported observing bone union in all of their cases of single-level decompressions. In the case of multiple-level decompressions, as performed for many patients in our present series, the circumstances may be different. Union of spatially continuous osteotomized spinous processes would seem to be difficult to achieve.
Many surgeons would recommend a decompression and fusion to treat LSCS with degenerative spondylolisthesis. In the present case series, however, low back pain improved in 45 cases (64.3%) 1 year after microendoscopic decompression surgery, and the pain deteriorated in only 4 cases (5.7%). Minamide et al reported clinical outcomes of degenerative spondylolisthesis cases that had been treated with microendoscopic decompression surgery at 5 years follow-up.12 The mean percentage slip was 18.1% preoperatively and 16.8% at the last follow-up, and 4 out of 71 patients (5.6%) required additional fusion operations. As minimally invasive spinal surgery appears to cause less instability, some of the degenerative spondylolisthesis patients treated with fusion operation may be eligible for microendoscopic decompression surgery.
Another advantage of our new technique relates to the manner of flavectomy. The laminae on both sides should be resected adequately through the base of the spinous process before flavectomy. Ligamentum flavum is detached from the laminae at the cephalic and caudal ends. With the flavum now floating on the dural sac, dural pulsations can be observed through the ligament. Then, the ligamentum flavum is split from the midline using a ball-tipped probe like “French doors,” and resected. Even in patients with severe LSCS, the dural sac can be decompressed to some extent at the time of flavectomy, which enables safer surgical manipulation within the enlarged spinal canal.
It is known that there is a learning curve for the technical acquisition of microendoscopic discectomy (MED). We reported that surgeons encountered dural tears at the rate of 2.5 to 5.4% in their first 40 cases of MED.13 In Japan, the JOA approves board-certified microendoscopic spine surgeons who have passed the requisite practical examination, and the authors are accordingly qualified. We experienced 329 cases treated with microendoscopic decompression surgery in 2011, and there were only four cerebrospinal fluid (CSF) leaks (1.2%), all of which were salvaged without open conversion. There were no CSF leaks in our present 70 cases.
Our surgical approach described here can also be applied to discectomy for central disk herniations at the upper lumbar levels. Our technique makes it possible to preserve not only the facet joints on the side of surgical approach, but also the spinous processes while decompressing the lumbar spinal canal. This may lead to a reduction of postoperative low back pain and to the prevention of segmental instability at the operated levels over the long term. Moreover, the technique is also useful in LSCS patients with severe spinal deformities, in which the loss of interlaminar spaces and extremely hypertrophic facet joints often cause distortion of the anatomical landmarks. Even in such difficult cases, the base of the spinous process serves as the best landmark to find the spinal canal because the lesion to be decompressed inevitably lies just under the spinous process.
Conclusions
Microendoscopic decompression surgery using the paramedian approach was an effective and minimally invasive surgical technique for the treatment of LSCS. The integrity of the facet joints and spinous processes could be preserved after spinal decompression even in patients with severe LSCS, which would contribute to the segmental stability at the operated level over the long term. Moreover, the “French-door” flavectomy enabled safer surgical manipulation within the stenosed spinal canal. The results in our case series showed that the modified technique allowed safer and easier performance of the microendoscopic decompression surgery for LSCS.
Disclosures
Kazunori Nomura, None
Munehito Yoshida, None
References
- 1.Foley K T, Smith M M. Microendoscopic discectomy. Tech Neurosurg. 1997;3:301–307. [Google Scholar]
- 2.Yoshida M. Kyoto: Kimpodo; 2005. Microendoscopic Discectomy; pp. 63–83. [Google Scholar]
- 3.Watanabe K, Hosoya T, Shiraishi T, Matsumoto M, Chiba K, Toyama Y. Lumbar spinous process-splitting laminectomy for lumbar canal stenosis. Technical note. J Neurosurg Spine. 2005;3(5):405–408. doi: 10.3171/spi.2005.3.5.0405. [DOI] [PubMed] [Google Scholar]
- 4.Hatta Y, Shiraishi T, Sakamoto A. et al. Muscle-preserving interlaminar decompression for the lumbar spine: a minimally invasive new procedure for lumbar spinal canal stenosis. Spine. 2009;34(8):E276–E280. doi: 10.1097/BRS.0b013e318195d943. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa K, Mikami Y, Hatta Y, Hase H, Koyama K, Kubo T. New microendoscopic decompression method via midline interlaminar approach for lumbar canal stenosis: microendoscopic muscle preserving interlaminar decompression; ME-MILD. Surgical Technique for Spine and Spinal Nerves. 2006;8:105–108. [Google Scholar]
- 6.Murata H Hachiya Y Muramatsu K Onitake H Unilateral microscopic decompression for spinal canal stenosis in upper lumbar lesions Surgical Technique for Spine and Spinal Nerves 200810133–137. (Jpn) [Google Scholar]
- 7.Itoi A, Shimamura Y, Muta T, Kaneko K. Problems of the posterior lumbar approach technique that does not keep kissing spines. J Spine Res. 2010;1:1449–1456. [Google Scholar]
- 8.Nishijima Y. Unilateral microdecompression for lumbar spinal canal stenosis. Surgical Technique for Spine and Spinal Nerves. 2007;9:40–44. [Google Scholar]
- 9.Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg. 2002;97(2, Suppl):213–217. doi: 10.3171/spi.2002.97.2.0213. [DOI] [PubMed] [Google Scholar]
- 10.Poletti C E. Central lumbar stenosis caused by ligamentum flavum: unilateral laminotomy for bilateral ligamentectomy: preliminary report of two cases. Neurosurgery. 1995;37(2):343–347. doi: 10.1227/00006123-199508000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Yagi M, Okada E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293–299. doi: 10.3171/2009.1.SPINE08288. [DOI] [PubMed] [Google Scholar]
- 12.Minamide A, Yoshida M, Yamada H. et al. Clinical outcomes more than 5 years after microendoscopic decompression surgery for lumbar spinal stenosis including degenerative spondylolisthesis. Journal of Spine Research. 2012;3:478. [Google Scholar]
- 13.Nomura K, Yoshida M, Kawai M, Maio K, Nakao S. Microendoscopic discectomy as a minimally invasive surgery for lumbar disc herniation. Technical training and learning curve. Journal of the Japanese Society for Spine Surgery and Related Research. 2009;20:649–652. [Google Scholar]


