Abstract
Revision lumbar spine surgeries are technically challenging with inconstant outcome results. This article discusses the preoperative, intraoperative, as well as postoperative management in these difficult patients. Successful intervention requires a detailed history and physical examination and carefully chosen diagnostic tests. Preoperative planning is paramount in these cases. The decision-making process should address the timing of the surgery, surgical approach, level of interbody fusion required, correction of sagittal imbalance, type of osteotomy, location of the osteotomy, and the end of the construct. Surgeons should be prepared to manage associated complications such as dural tear and massive blood loss. The use of autograft and/or biologic graft is necessary to help in achieving a successful fusion. Postoperative management includes prophylactic antibiotic, anticoagulation, nutritional support, and brace.
Keywords: revision, lumbar spine, preoperative evaluation, intraoperative strategies, postoperative management
Preoperative Evaluation
Careful assessment to determine the exact cause of symptoms and the effect on the patients' emotional and functional state is paramount in revision back surgery. Patients should undergo a detailed history and physical examination to rule out nonspinal causes for their current symptoms and to identify their pain generator. Such an approach can help with the preoperative planning, avoid any unexpected intraoperative findings, and improve the outcome after surgery.
History
Review of medical records, reports of previous surgeries, and radiographs can identify causes of failed back surgery. Assessment of the medical history, medications to rule out narcotics abuse, review of systems, and social history including work history, motivation for return to work, and involvement in litigation can identify comorbidities and predict functional outcome of revision surgery. History of smoking increases the risk of pseudarthrosis after fusion surgery. Mechanical back pain after a short period of pain relief following spine fusion may be indicative of a pseudarthrosis. Nonmechanical back pain may indicate infection especially in the presence of constitutional symptoms such as fever, chills, and weight loss. Differentiation between neurogenic and vascular claudication can be determined from the history (stationary bike and shopping cart sign). Depression is common in this group of patients especially in patients with multiple previous surgeries. Red flags for depression can be identified from the patient's history such as feelings of despair, sleep disturbance, loss of appetite, loss of sexual desire, irritability, and inability to make decisions. Addressing these psychosocial factors before revision surgery improves outcomes.1
Physical Examination
The followings should be included in the physical examination:
Neurological: Examination should include sensory, motor power, deep tendon reflexes, nerve root tension signs, and gait pattern.
Sagittal balance: Clinical evaluation of sagittal balance starts with an examination of the patient while standing with the knees fully extended to eliminate any compensatory knee flexion that may mask a severe sagittal plane deformity. The patient is then examined in the sitting position; if the trunk appears with good balance in relation to the pelvis, then a hip flexion contracture may be the cause of sagittal imbalance, and this can be demonstrated with the Thomas test. If forward displacement of the cervical spine in relation to the pelvis remains in the sitting position, the patient is then assessed in supine position. When the deformity is localized to the lumbar spine, the patient will be able to lie with their shoulders on the table. If the head and upper thoracic spine remains elevated from the table, a fixed deformity in the cervical and or thoracic spine is likely.
Peripheral pulses: Patients should be evaluated to rule out a vascular origin of the symptoms. Prior to revision surgery, the patient should be referred to a vascular surgeon if there is any concern regarding peripheral circulation.
Hip joints: Deformity and range of motion should be evaluated to rule out the hip joint as the main pain generator.
Pain: patients should be evaluated for Waddell signs such as superficial or nonanatomic tenderness, overreaction to stimuli, or reports of pain during evaluations that are designed not to be painful. More than two Waddell findings strongly predicts poor outcome, regardless of spinal pathology.2
Imaging
The following image studies should be performed:
Flexion-extension radiographs: In a patient with a previous fusion, plain radiograph and fine-cut computed tomography (CT) should be assessed for evidence of pseudarthrosis such as screw loosening or hardware failure. Lucencies around the hardware or subsidence have been found to have a poor correlation with findings at the time of open revision. Pseudarthrosis is likely when motion is present on flexion-extension radiographs. However, controversy exists regarding the amount of motion that is compatible with a solid fusion. Criteria range from no motion to 5 degrees of motion.3 Adjacent segments should also be assessed for occult instability such as an unstable spondylolisthesis and degenerative changes.
Radiological assessment of sagittal balance: Proper radiographic evaluation is essential to assess segmental, regional, and global sagittal balance. Plain radiographs include upright anteroposterior and lateral full-length scoliotic views, long 17- by 36-inch cassette of the entire spine taken with the shoulders at 45-degree forward flexion and the hips and knees fully extended. Full extension of the hips and knees are important to eliminate any compensatory flexion that may mask a severe deformity. Currently, the most used radiographic method to assess sagittal balance is the standing full-length lateral radiograph measuring the horizontal distance between a C7 plumb line and the posterosuperior aspect of the sacrum at the L5–S1 disk space, also known as sagittal vertical axis. Positive sagittal imbalance is defined as an anterior deviation of the C7 plumb line.4
Pelvis plain radiography: This can assess for hip joint pathology, such as osteoarthritis, osteonecrosis, or unrecognized stress fractures that may be the source of groin and thigh pain.
CT to assess bony anatomy including any bony defect after previous decompression surgery: The CT will accurately assess any laminectomy defect that should be carefully watched during the surgical approach to avoid dural violation. CT also can demonstrate any iatrogenic pars defect or the extent of a facetectomy that may account for the patient's current back pain (Fig. 1). CT with fine-section coronal and sagittal reconstructions is the best imaging study to determine posterolateral and interbody fusion status.
CT myelography: This is useful for patients in whom the magnetic resonance imaging (MRI) scan is difficult to evaluate, such as in patients with previous instrumentation due to metal artifact or in scoliosis due to the deformity. CT myelography is also useful when MRI is contraindicated, such as in a patient with pacemaker.
MRI with and without gadolinium enhancement5: Enhancement with gadolinium results in increased signal in vascularized tissues, especially epidural scar. Comparing enhanced and nonenhanced sequences can accurately distinguish epidural scar from a nonenhancing recurrent disk herniation. In addition, gadolinium enhancement in the intervertebral disk and vertebral bodies may demonstrate the presence of postoperative infection.
Figure 1.
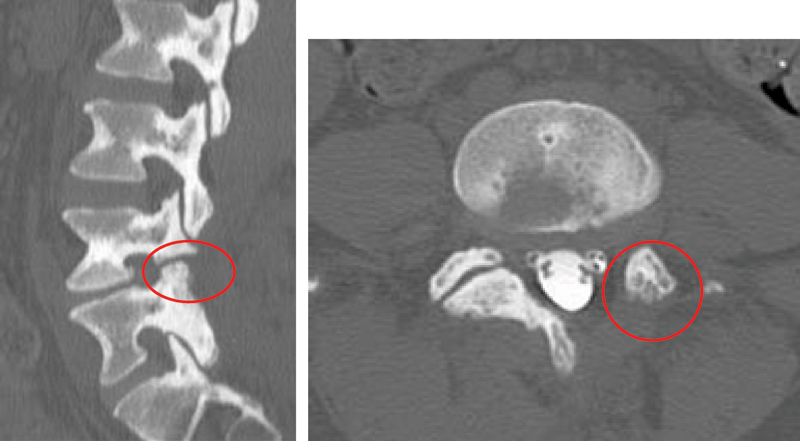
A 36-year-old woman presented with low back pain post-microdiscectomy. Computed tomography, sagittal and axial cuts, showed iatrogenic pars defect and complete facetectomy on the left side. Patient back pain improved after instrumented fusion.
Laboratory Tests
Erythrocyte Sedimentation Rate and C-Reactive Protein
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are obtained prior to revision surgeries to rule out occult infection especially in a patient with a suspected pseudarthrosis. Patient presenting with a history of infection, wound drainage, or constitutional symptoms should be assessed for infection. Early or midterm onset of severe low back pain after discectomy may indicate discitis. ESR and CRP levels are usually elevated, although they are not specific for infection. CRP returns to a normal level sooner than the ESR, usually in 14 days, and may be useful in determining response to treatment.
Albumin and Transferrin
Patients who require lumbar spine revision surgery, especially those who had previous multiple surgeries, and patients with infection are often inadequately nourished and should be monitored with sampled albumin and transferring levels. If these indices are low, then the patient will require nutritional supplements to help with the healing process.
Electrodiagnostic Studies
Physical examination findings are sometimes difficult to interpret in failed back surgeries, especially in patients with second gain issues such as active workers' compensation claims or involvement in litigation. In such cases more objective diagnostic tests such as electromyograms and nerve conduction velocity can be valuable, although they may not be predictive of outcomes following further surgery. Electromyograms and nerve conduction velocity studies may be helpful to assess the severity and location of nerve injury and to differentiate between radiculopathy and peripheral neuropathy.
Diagnostic Block
Selective nerve root blocks are helpful to confirm the exact localization of neural abnormalities and perhaps to predict outcomes of surgery.
Diagnostic facet or pars defect blocks are useful to identify the presence of a pain generator at the same or adjacent level of previous surgery (Fig. 2).
Discography is controversial, and the resultant pain provocation is even less well understood in patients with prior surgery. Discography can be used to identify painful transitional motion segments. Discography has identified painful disk segments after posterior fusion with good clinical outcomes following interbody fusion.
Figure 2.
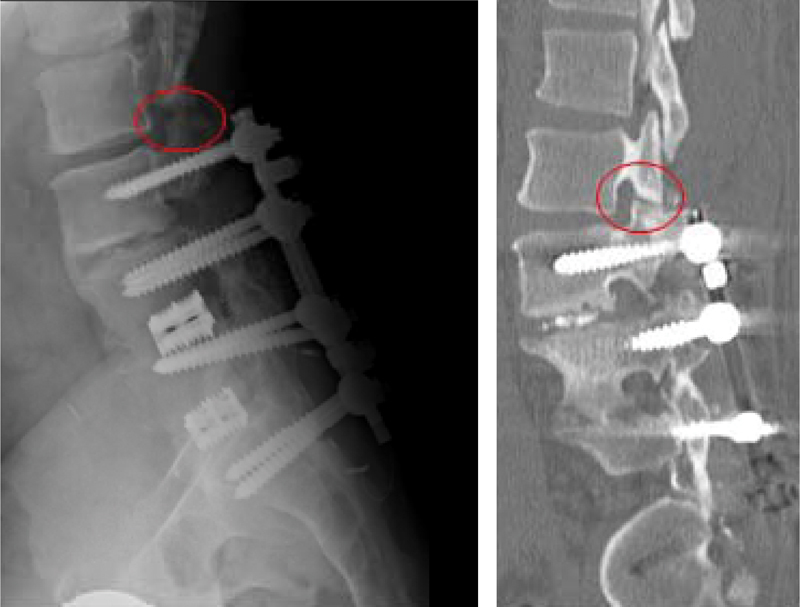
A 49-year-old man presented with low back pain after multiple lumbar spine surgeries. Computed tomography (CT) scan, sagittal cut, showed L2 pars defect. Patient had relief of his back pain for few days after CT-guided local injection of the pars defect. This confirmed that the patient pain generator was the L2 pars defect. The patient was then managed with extension of the instrument fusion to L2.
Operative Tips
Intraoperative Cultures
Ruling out infection before revision lumbar spine surgery, especially in the presence of instrumentation, is an important step to improve outcomes and decrease complications. The authors recommend taking a minimum of three intraoperative cultures in all revision surgeries if an infection is suspected. If only one culture is taken and the result comes back positive, one cannot rule out the possibility of contamination. In addition to intraoperative cultures, ruling out an occult infection can be achieved though a systematic approach that starts with the history and physical examination, looking for any history of postoperative wound infection, persistent drainage or a prolonged course of antibiotic after the previous spine surgery, fever, chills, and nonmechanical back pain. Elevated preoperative ESR and CRP levels, although nonspecific, may yield suspicion for a deep or occult wound infection. In such a case, the surgeon should counsel the patient during the preoperative visit about the possibility of an occult wound infection; if proven intraoperatively through wound inspection and positive intraoperative gram stain results, then a staged procedure with the first stage involving irrigation and debridement and removal of hardware may be necessary. In this scenario, the patient should then undergo culture-specific intravenous antibiotics pending the intraoperative culture. The revision procedure can then be performed in 1 to 3 weeks.
Antibiotic
The current recommendations for the use of prophylactic antibiotics in revision spine surgery are the following.6 7 8 9
Choice of Antimicrobial Agent
Cephalosporin (cefazolin, cefuroxime) is first choice.
In presence of β-lactam allergy, use clindamycin or vancomycin.
Timing of Administration
Provided there is no concern for occult infection (normal preoperative ESR and CRP), start cefazolin, cefuroxime, clindamycin up to 60 minutes before incision and complete by the time of incision, and start vancomycin up to 120 minutes before incision and complete by the time of incision.
If there is concern for occult infection such as a history of previous postoperative wound infection or elevated preoperative ESR and CRP, start antibiotics after taking intraoperative cultures.
Dosing
Cefazolin dose is 1 to 2 g (2 g for patient weighing >86 kg).
Cefuroxime dose is 1.5 g.
Vancomycin and clindamycin doses are based on patient mass.
Duration of Antimicrobial Use
Single preoperative dose is used.
Redose antimicrobial intraoperatively every 4 hours for a prolonged procedure or significant blood loss. Swoboda et al8 noted that blood loss correlated with the change in tissue antibiotic concentrations for cefazolin and recommended additional doses of cefazolin when the operation approaches 4 hours or blood loss exceeds 1500 mL.
Postoperatively antibiotics should be discontinued within 24 hours after wound closure as continuing of antibiotic prophylaxis longer than 24 hours after wound closure has not been proven to be beneficial; indeed, it may contribute to the development of antimicrobial resistance.
Continuing prophylactic antibiotics for the duration that drains and catheters are in place has not been shown to reduce surgical site infections rates.
Surgical Approach
Approach to lumbar spine revision surgery can be from the back (posterior), front (anterior) and back, or all from the front. This depends on the pathology and the nature of the revision surgery as well as surgeon preference.
Back (Posterior)
The majority of lumbar spine revision surgeries are performed from the back. With recent advances in instrumentation and techniques, there is a trend toward performing all aspects of the revision from a posterior approach. The indications for a posterior approach include surgery for recurrent or residual disk herniation following microdiscectomy, pseudarthrosis, adjacent segment degenerative disk disease or spondylolisthesis, lumbar flat back syndrome, failed lumbar disk arthroplasty, and infection following previous lumbar spine surgery that was done though a posterior approach.
Front and Back
Some surgeons may choose this approach in patients with previous laminectomy and no significant neural compression to avoid dissection through scarred dura. The anterior interbody fusion is performed under the same anesthesia or as a staged procedure followed by posterior instrumentation and posterolateral fusion.
Front
Barrick et al10 conducted a retrospective study that included 20 patients who underwent an anterior interbody fusion following a prior posterolateral spinal fusion. All had low back pain, a solid posterolateral spinal fusion, and a painful disk(s) on discography within the posterolateral spinal fusion level(s). They reported that 89% were satisfied with their results and concluded that low back pain that continues or recurs after an apparently solid posterolateral spinal fusion may be caused by painful disk(s) at motion segment(s) within the fusion. A solid posterolateral spinal fusion may not protect the residual disk(s) from injury. Anterior interbody fusion can provide significant improvements in pain and function and a high degree of patient satisfaction in this clinical setting. Based on this study, some surgeons may elect to perform an anterior interbody fusion in patients with previous posterolateral fusion who have continued back pain in the presence of positive discography in motion segment(s) within the fusion.
With the development of lumbar disk arthroplasty, some surgeons may elect to perform lumbar disk arthroplasty for adjacent segment degenerative disk disease caudal or cephalad to a previous instrumented fusion.
Instrumentation
Segmental spinal instrumentation improves deformity correction and allows for early postoperative mobilization.11 12 Lumbar pedicle screw instrumentation, as well as thoracic pedicle screw instrumentation, has become the standard.
Pelvic fixation may be required in some patients. In adults with spinal deformity, fusion across the L5–S1 junction is recommended in the presence of lumbosacral pathology, such as postlaminectomy defects, lumbar spinal stenosis, oblique take-off of L5, and severe L5–S1 degenerative disk disease.1 3 To avoid complications leading to failure of S1 pedicle screws, multiple solutions for load-sharing have been used, including bilateral iliac screw fixation and interbody fusion. Interbody graft placement increases compression stiffness and helps to restore sagittal alignment. Lack of rigid sacral fixation, such as with bilateral bicortical S1 screws, anterior column support, or bilateral iliac screws, has been associated with a significantly increased risk of pseudarthrosis and, thus, poor patient satisfaction.11 13
Sagittal Balance
Sagittal balance of the spine is becoming one of the most important factors in the assessment of spinal deformity. The literature is clear that patients' satisfaction after lumbar spine surgery correlates with restoration of the sagittal balance.14 15
Preoperative planning is paramount in these cases. Sagittal imbalance can be corrected through circumferential fusion or spinal osteotomy. The decision-making process should address the timing of the surgery, surgical approach, level of interbody fusion required, type of osteotomy, location of the osteotomy, and extent of the fusion. Bridwell16 classified spinal deformities into three categories based on curve flexibility: (1) totally flexible, (2) partially correcting through mobile segments, and (3) fixed deformity with no correction in the recumbent position. The flexibility of the deformity is the most important criterion to consider when deciding which surgical approach will best restore sagittal balance. In the patient with flexible and principally disk-based deformity, it may be possible to restore sagittal balance with anterior-posterior or posterior-only surgery. Sagittal balance is improved by structurally grafting the anterior column through an anterior or a posterior approach. Cages, structural allograft, and structural autograft may be used. The posterior column is then addressed with laminectomies when there is evidence of stenosis, facetectomies, and fusion with instrumentation (Fig. 3). In patients with fixed deformity, a posterior-column shortening procedure may be performed to restore sagittal balance. Options include the Smith-Petersen osteotomy, pedicle subtraction osteotomy, and vertebral column resection. The amount of correction needed determines the surgical procedure.15
Figure 3.
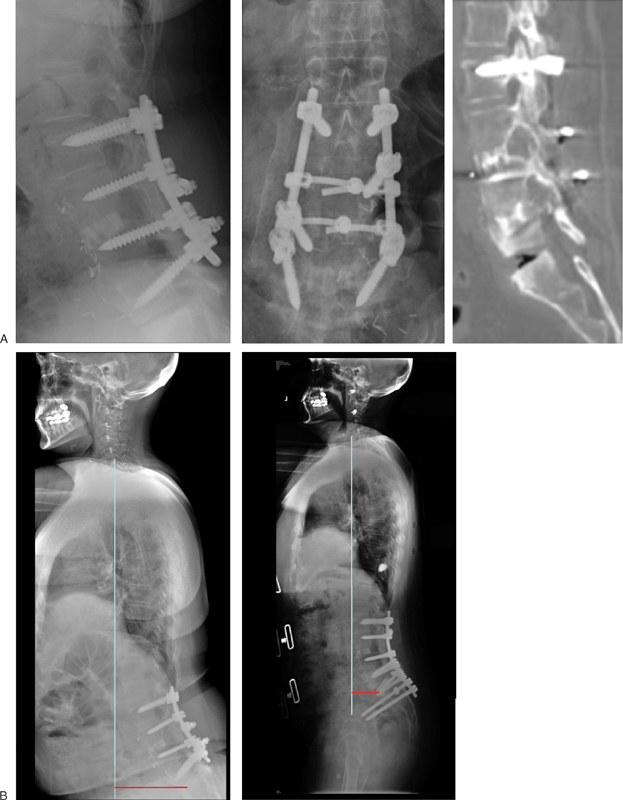
(A) A 61-year-old woman presented with low back pain and L5 radiculopathy after two previous lumbar spine instrumented fusion. Clinical and radiological assessment showed lumbar flat back syndrome and L5–S1 degenerative lumbar disc. (B) Patient was managed with L5–S1 transforaminal lumbar interbody fusion, with extension of the instrumented fusion to the ilium. Patient back pain and sagittal imbalance improved after the surgery. The sagittal vertical axis improved from 8 cm preoperative to 2 cm postoperative.
Anteroposterior surgery is the most common procedure used for adults with fixed deformity; however, recent advances in surgical techniques and instrumentation have led to more frequent use of purely posterior approaches. The posterior approach allows for greater correction due to two major advances in the surgical method: osteotomy techniques and pedicle screw instrumentation in the thoracolumbar spine, both of which allow for greater curve correction with fewer levels of fixation. The two most commonly used osteotomies for correction of sagittal imbalance are the Smith-Petersen osteotomy and pedicle subtraction osteotomy.
Smith-Petersen osteotomy can achieve ~10 degrees of correction in the sagittal plane at each spinal level at which it is performed. The osteotomies are usually performed at two or more levels. Smith-Petersen osteotomy corrects sagittal imbalance through posterior column shortening and anterior column lengthening. It requires a mobile disk space, otherwise the osteotomy will not close. It may result in coronal decompensation toward the concavity in the coronal plane if done in a patient with scoliosis. This osteotomy is beneficial for patients who have a degenerative imbalance in the sagittal plane. The Smith-Petersen osteotomy is a relatively safe procedure, although rupture of the great vessels has been reported following anterior-column lengthening.17
Pedicle subtraction osteotomy can achieve ~30 degrees of correction in the sagittal plane. It corrects sagittal imbalance through posterior column shortening. It can be done in a coronal deformity without risking decompensation. The ideal candidate has substantial sagittal imbalance >10 cm or a sharp, angular kyphosis or has had a circumferential fusion along multiple segments.11 16 18 19 Posterior segmental pedicle screw instrumentation is used to maintain the correction. Instrumentation of at least three vertebral levels above and below the osteotomy is preferred.20 Pedicle subtraction osteotomy is associated with an 11% neurological deficit in which 2.8% are permanent.
For the patient requiring 10 to 20 degrees of lordosis or 4 to 7 cm of correction of the C7 plumb line, it is more appropriate to perform a limited number of Smith-Petersen osteotomies than one pedicle subtraction osteotomy, unless the fixed deformity is fused anteriorly.16 21 Regardless of the technique employed, restoring sagittal balance and achieving a fused spine are crucial to success. The risk of pseudarthrosis has been associated with patient age >55 years, longer fusions (>12 vertebrae), thoracolumbar kyphosis >20 degrees, osteoarthritis of the hip joint, positive sagittal balance ≥5 cm at 8 weeks postoperatively, and incomplete sacropelvic fixation.11 22
Dural Tear
The incidence of durotomy during spine surgery is estimated to range from 0.3 to 13% in an index procedure and up to 17% in revision surgery. Nearly 7% of these tears are unnoticed at the time of surgery.23 Dural entry is much more common in revision procedures because of adhesions in the epidural space and dural scarring and fibrosis. Prevention is the most important aspect of treatment. Careful preoperative planning and meticulous, systematic surgical technique during such procedures can minimize its prevalence. It is recommended to begin the dissection in areas of unscarred tissue and proceed toward the potentially scarred regions.24
If the leak is observed intraoperatively, it should be repaired with sutures, fibrin glue, fascial grafts, or a combination. When dural repair is combined with a tight fascial closure, a negative leak with intraoperative Valsalva maneuver after the repair and bed rest for 24 to 48 hours, no increased long-term morbidity should be expected. Wang et al23 concluded from their study of 88 consecutive dural tears that incidental durotomy, when treated appropriately, does not result in a substantial difference in long-term morbidity, nor does it increase the infection or neurologic sequelae. The authors also concluded that a subfascial drain is not contraindicated in patients at risk for a hematoma with a repaired dural tear.
When dural tear occurs in areas not directly reparable with sutures, such as in the nerve root axilla, the tear may be enveloped with a thrombin-soaked bioabsorbable gelatin sponge or fibrin glue. Subsequent watertight fascial and skin closure is obtained. A lumbar subarachnoid drain is placed several segments from the tear and allowed to drain up to 10 mL per hour for ~5 days. During this time, patients may or may not be placed on bed rest. Antibiotics for gram-positive coverage are administered during this period with cerebrospinal fluid samples drawn every day for analysis. On day 6, the drain is removed and a skin suture placed at the drain exit.25 26
Postoperatively, detection of a dural tear may be more difficult. Clinical history, physical examination, imaging studies, and laboratory tests all may be necessary to identify a cerebrospinal fluid leak. A subcutaneous or subfascial fluid collection or frank wound drainage may be early clinical findings. When the diagnosis is in question, immunofixation electrophoresis for β-2 transferrin can be done. β-2 transferrin is a protein produced by cerebral neuraminidase and is found only in cerebrospinal fluid and perilymph.27 Immediate reoperation has been the time-honored traditional treatment and is the gold standard. However, to minimize the risks of reoperation, other noninvasive modalities, such as subarachnoid drainage, epidural blood patch, and percutaneous fibrin glue, have been employed. Should these methods fail after a reasonable trial period, surgery should be done.
Blood Loss
Intraoperative blood loss is a commonly encountered problem in revision spine surgeries. Significant hemorrhage and associated comorbidities in spine surgery have not yet been clearly identified. Such blood loss often leads to blood, platelet, and factor transfusions. Although blood screening has improved the safety considerably over the years, there are still known risks of transfusion including potential transfusion reactions and alloimmunization as well as infectious risks such as hepatitis, HIV, and cytomegalovirus. Furthermore, the costs of blood replacement must be considered. Several preoperative and intraoperative techniques are currently available to reduce blood loss and transfusion requirements such as preoperative autologous blood donation, cell saver, recombinant factor VIIa, and perioperative antifibrinolytic agents such as aprotinin, tranexamic acid, and epsilon-aminocaproic acid.
From the currently available studies, one can conclude that for revision spine surgeries in patients with a normal coagulation profile, preoperative blood donation is not beneficial.28 Recombinant factor VIIa reduces blood loss and requirement for transfusion, and its use is not associated with increased morbidity or mortality.29 The efficacy and cost-effectiveness of intraoperative cell saver usage remains unclear in elective spinal surgery. However, most of the recently published studies showed that cell saver use is not able to decrease the need for blood transfusion and is associated with a significantly higher blood loss.30 31 Studies have shown that antifibrinolytic agents (aprotinin, tranexamic acid, epsilon-aminocaproic acid) reduce blood loss in spine surgery.32 33 34 However, a recently published study raised concern regarding increased morbidity with the use of aprotinin in coronary heart surgery.35
Summary
Careful preoperative patient evaluation is paramount in successful lumbar spine revision surgery. These include history, examination, appropriate use of imaging, electrodiagnostic studies, and diagnostic block. Preoperative laboratory tests and intraoperative cultures are important to rule out any subtle infection. Preoperative surgical planning is essential including approach, level of instrumentation, and maintaining/regaining the sagittal balance. The surgeon should be prepared to manage possible intraoperative complications such as dural tear, and massive blood loss.
Disclosures
Hossein Elgafy, None
Alexander R. Vaccaro, None
Jens R. Chapman, None
Marcel F. Dvorak, Consulting: Medtronic; Royalties: Medtronic; Research Support: Medtronic, Synthes, DePuy; Fellowship Support: Medtronic, AOSpine North America
References
- 1.Guyer R D, Patterson M, Ohnmeiss D D. Failed back surgery syndrome: diagnostic evaluation. J Am Acad Orthop Surg. 2006;14:534–543. doi: 10.5435/00124635-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Waddell G, McCulloch J A, Kummel E, Venner R M. Nonorganic physical signs in low-back pain. Spine. 1980;5:117–125. doi: 10.1097/00007632-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Raizman N M, O'Brien J R, Poehling-Monaghan K L, Yu W D. Pseudarthrosis of the spine. J Am Acad Orthop Surg. 2009;17:494–503. doi: 10.5435/00124635-200908000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cho K J Bridwell K H Lenke L G Berra A Baldus C Comparison of Smith-Petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance Spine 2005302030–2037.; discussion 2038 [DOI] [PubMed] [Google Scholar]
- 5.Ross J S, Masaryk T J, Schrader M, Gentili A, Bohlman H, Modic M T. MR imaging of the postoperative lumbar spine: assessment with gadopentetate dimeglumine. AJR Am J Roentgenol. 1990;155:867–872. doi: 10.2214/ajr.155.4.2119123. [DOI] [PubMed] [Google Scholar]
- 6.Barker FG II Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis Neurosurgery 200251391–400.; discussion 400–401 [PubMed] [Google Scholar]
- 7.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16:283–293. doi: 10.5435/00124635-200805000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Swoboda S M Merz C Kostuik J Trentler B Lipsett P A Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch Surg 19961311165–1171.; discussion 1171–1172 [DOI] [PubMed] [Google Scholar]
- 9.Bratzler D W Houck P M; Surgical Infection Prevention Guidelines Writers Workgroup; American Academy of Orthopaedic Surgeons; American Association of Critical Care Nurses; American Association of Nurse Anesthetists; American College of Surgeons; American College of Osteopathic Surgeons; American Geriatrics Society; American Society of Anesthesiologists; American Society of Colon and Rectal Surgeons; American Society of Health-System Pharmacists; American Society of PeriAnesthesia Nurses; Ascension Health; Association of periOperative Registered Nurses; Association for Professionals in Infection Control and Epidemiology; Infectious Diseases Society of America; Medical Letter; Premier; Society for Healthcare Epidemiology of America; Society of Thoracic Surgeons; Surgical Infection Society. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project Clin Infect Dis 2004381706–1715. [DOI] [PubMed] [Google Scholar]
- 10.Barrick W T, Schofferman J A, Reynolds J B. et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25:853–857. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y J, Bridwell K H, Lenke L G, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31:2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 12.Bess R S, Lenke L G, Bridwell K H, Cheh G, Mandel S, Sides B. Comparison of thoracic pedicle screw to hook instrumentation for the treatment of adult spinal deformity. Spine. 2007;32:555–561. doi: 10.1097/01.brs.0000256445.31653.0e. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya K, Bridwell K H, Kuklo T R, Lenke L G, Baldus C. Minimum 5-year analysis of L5–S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine. 2006;31:303–308. doi: 10.1097/01.brs.0000197193.81296.f1. [DOI] [PubMed] [Google Scholar]
- 14.Berven S H Deviren V Smith J A Hu S H Bradford D S Management of fixed sagittal plane deformity: outcome of combined anterior and posterior surgery Spine 2003281710–1715.; discussion 1716 [DOI] [PubMed] [Google Scholar]
- 15.Joseph SA Jr, Moreno A P, Brandoff J, Casden A C, Kuflik P, Neuwirth M G. Sagittal plane deformity in the adult patient. J Am Acad Orthop Surg. 2009;17:378–388. doi: 10.5435/00124635-200906000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Bridwell K H. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine. 2006;31(19, Suppl):S171–S178. doi: 10.1097/01.brs.0000231963.72810.38. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Petersen M N, Larson C B, Aufranc O E. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6–9. [PubMed] [Google Scholar]
- 18.Booth K C, Bridwell K H, Lenke L G, Baldus C R, Blanke K M. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance) Spine. 1999;24:1712–1720. doi: 10.1097/00007632-199908150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Bridwell K H, Lewis S J, Rinella A, Lenke L G, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. Surgical technique. J Bone Joint Surg Am. 2004;86–A(Suppl 1):44–50. doi: 10.2106/00004623-200403001-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kim K T, Lee S H, Suk K S, Lee J H, Im Y J. Spinal pseudarthrosis in advanced ankylosing spondylitis with sagittal plane deformity: clinical characteristics and outcome analysis. Spine. 2007;32:1641–1647. doi: 10.1097/BRS.0b013e318074c3ce. [DOI] [PubMed] [Google Scholar]
- 21.Bridwell K H, Lewis S J, Edwards C. et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine. 2003;28:2093–2101. doi: 10.1097/01.BRS.0000090891.60232.70. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y J, Bridwell K H, Lenke L G, Rinella A S, Edwards C II. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine. 2005;30:468–474. doi: 10.1097/01.brs.0000153392.74639.ea. [DOI] [PubMed] [Google Scholar]
- 23.Wang J C, Bohlman H H, Riew K D. Dural tears secondary to operations on the lumbar spine. Management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg Am. 1998;80:1728–1732. doi: 10.2106/00004623-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bosacco S J, Gardner M J, Guille J T. Evaluation and treatment of dural tears in lumbar spine surgery: a review. Clin Orthop Relat Res. 2001;(389):238–247. doi: 10.1097/00003086-200108000-00033. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro S A, Scully T. Closed continuous drainage of cerebrospinal fluid via a lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;30:241–245. doi: 10.1227/00006123-199202000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Kitchel S H, Eismont F J, Green B A. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg Am. 1989;71:984–987. [PubMed] [Google Scholar]
- 27.Ryall R G, Peacock M K, Simpson D A. Usefulness of beta 2-transferrin assay in the detection of cerebrospinal fluid leaks following head injury. J Neurosurg. 1992;77:737–739. doi: 10.3171/jns.1992.77.5.0737. [DOI] [PubMed] [Google Scholar]
- 28.Brookfield K F, Brown M D, Henriques S M, Buttacavoli F A, Seitz A P. Allogeneic transfusion after predonation of blood for elective spine surgery. Clin Orthop Relat Res. 2008;466:1949–1953. doi: 10.1007/s11999-008-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachs B, Delacy D, Green J. et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial. Spine. 2007;32:2285–2293. doi: 10.1097/BRS.0b013e3181557d45. [DOI] [PubMed] [Google Scholar]
- 30.Reitman C A Watters WC III Sassard W R The Cell Saver in adult lumbar fusion surgery: a cost-benefit outcomes study Spine 2004291580–1583.; discussion 1584 [DOI] [PubMed] [Google Scholar]
- 31.Gause P R, Siska P A, Westrick E R, Zavatsky J, Irrgang J J, Kang J D. Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine. 2008;33:571–575. doi: 10.1097/BRS.0b013e3181657cc1. [DOI] [PubMed] [Google Scholar]
- 32.Kokoszka A, Kuflik P, Bitan F, Casden A, Neuwirth M. Evidence-based review of the role of aprotinin in blood conservation during orthopaedic surgery. J Bone Joint Surg Am. 2005;87:1129–1136. doi: 10.2106/JBJS.D.02240. [DOI] [PubMed] [Google Scholar]
- 33.Tayyab N A, Mariller M M, Rivlin M. et al. Efficacy of aprotinin as a blood conservation technique for adult deformity spinal surgery: a retrospective study. Spine. 2008;33:1775–1781. doi: 10.1097/BRS.0b013e31817b87c4. [DOI] [PubMed] [Google Scholar]
- 34.Thompson G H, Florentino-Pineda I, Poe-Kochert C, Armstrong D G, Son-Hing J P. The role of Amicar in same-day anterior and posterior spinal fusion for idiopathic scoliosis. Spine. 2008;33:2237–2242. doi: 10.1097/BRS.0b013e31817bd889. [DOI] [PubMed] [Google Scholar]
- 35.Mangano D T Tudor I C Dietzel C; Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery N Engl J Med 2006354353–365. [DOI] [PubMed] [Google Scholar]


