Abstract
Primary spine tumors are rare, accounting for only 4% of all tumors of the spine. A minority of the more common primary benign lesions will require surgical treatment, and most amenable malignant lesions will proceed to attempted resection. The rarity of malignant primary lesions has resulted in a paucity of historical data regarding optimal surgical and adjuvant treatment and, although we now derive benefit from standardized guidelines of overall care, management of each neoplasm often proceeds on a case-by-case basis, taking into account the individual characteristics of patient operability, tumor resectability, and biological potential. This article aims to provide an overview of diagnostic techniques, staging algorithms and the authors' experience of surgical treatment alternatives that have been employed in the care of selected benign and malignant lesions. Although broadly a review of contemporary management, it is hoped that the case illustrations given will serve as additional “arrows in the quiver” of the treating surgeon.
Keywords: spinal tumors, primary tumors
Primary neoplasms of the spine are rare. Although hamartomas (the group of non-neoplastic, ectopic cellular rests including hemangiomas and fibrous dysplasia) are considerably more common, few will ultimately require surgical treatment. Of all the primary musculoskeletal neoplasms, themselves rare conditions, primary spinal tumors represent 5% of all primary bone tumors, and the latter occur in 0.8 to 8.0 per 100,000 population.1 2 4 A minority of the benign lesions will require operation. The more frequently encountered benign spinal tumors in surgical practice are osteoid osteoma and osteoblastoma, neural sheath tumors, aneurysmal bone cyst, benign fibrous tumor, giant cell tumor, and aggressive hemangioma. Malignant primary spinal tumors are even less common. In the United States there are ∼600 new cases of primary spinal malignancy identified each year.5 If identified at an amenable stage, with careful planning, most will be treated by attempts at resection. Primary musculoskeletal spinal malignancy includes chordoma, chondrosarcoma, osteogenic sarcoma (OGS), Ewing's sarcoma, and malignant peripheral nerve sheath tumor as well as plasmacytoma.
Over a period of ∼20 years, surgical capability in the realm of spinal tumor resection has been considerably enhanced by advances in anesthesia allowing for access to all spinal regions through multiple options, by improved collaboration with medical and radiation oncologist colleagues enabling multidisciplinary care pathways, and with the advent of magnetic resonance imaging (MRI), which allows more thorough staging and preoperative planning. Although resection strategy will ultimately be determined on a case-by-case basis, there are several constants in the planning of surgery. All vertebral resection surgery has an inexorable resection margin at the borders of the neural canal. Extirpation of tumors of the spinal column must, therefore, commonly divide the encircling ring around the neural elements, substantially complicating the compartmental model of musculoskeletal tumor management as described by Enneking.12 Despite these limitations, the same biological principle of “adequate” excision to avoid local tumor recurrence holds true as reported by Weinstein, Boriani, and Biagini in their spinal adaptation of the work of Enneking.2 7 As in any musculoskeletal site, adequate excision of a benign lesion, such as an osteoid osteoma, may be intralesional. In the case of primary malignancy of the spine, however, extirpation requires wide excision with often complex reconstruction over multiple vertebral levels. The location of the spinal neurological structures often calls for a balance between the aggression of complete tumor excision and the expediency of neural preservation. It is this balance that individualizes the surgery of spinal malignancy and from which has been borne the numerous more specialized resection techniques reported here. In general, spinal en bloc resection techniques are challenging procedures with a high rate of complication. For this reason, along with a high requirement for resources and the need for multidisciplinary care, referral to a specialized service in spinal oncology should be strongly considered.
Tumoral Evaluation
Clinical Assessment
Initial assessment of the patient with a primary spinal tumor requires meticulous application of common clinical tools including a detailed history and clinical examination. Clinical presentation usually relates to pain, which may be due to either mass effect or neurological compression. Rarely do patients present with painless neurological deterioration. A careful clinical history is mandatory in evaluating the important aspects of the pain character, frequency, duration, site, radiation, as well as aggravating and alleviating factors. Typical examples include the exquisite responsiveness of osteoid osteoma to the analgesic effects of salicylates and the nocturnal, nagging character of the pain of malignancy. To differentiate the pain of spinal neoplasia from that of the vastly more common degenerative conditions, the concept of historical “red flags” has evolved. Malignancy in the spine (primary or secondary) is typically associated with pain that is uncharacteristic, unremitting, and unsociable. The pain is uncharacteristic in that there is frequently no history of prior similar symptoms, whereas degenerative conditions commonly cause symptoms that pursue a relapsing and remitting course. The pain is unremitting, tending not to be alleviated by rest or simple analgesia in contradistinction to the more common degenerative processes, and is typically “unsociable” in its tendency to cause nocturnal discomfort. Collateral historical features include recent weight loss, malaise, tumoral fever, and, infrequently, autonomic sphincteric disturbance, which may be due to either neurological compression within the spinal canal or local mass effect in the case of, for example, sacral chordoma.
Biopsy
Despite radiological appearance, diagnosis needs to be confirmed by biopsy. Even the most typical radiological appearance lacks the scientific veracity of histological diagnosis, and the latter is mandatory in successful tumor management (Fig. 1). Biopsy may be percutaneous, open, or excisional.
Figure 1.
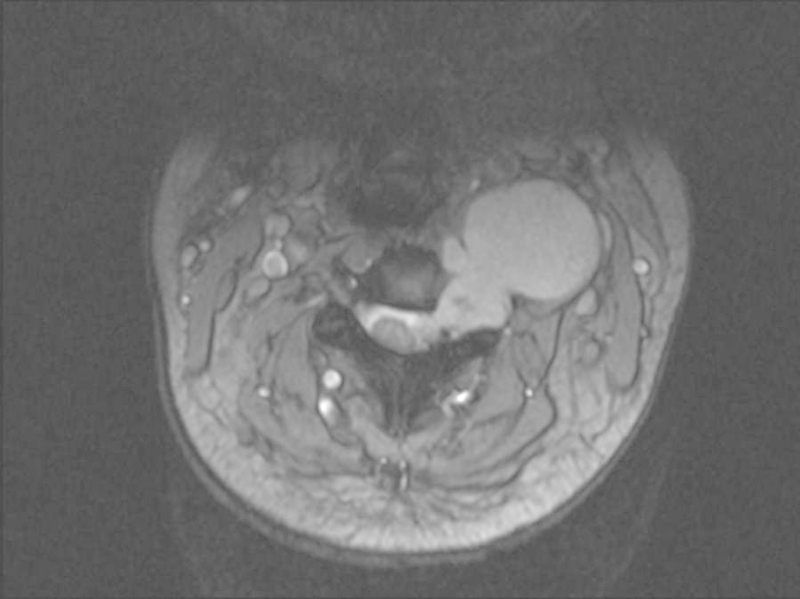
This radiological appearance was considered likely to be a neural sheath tumor. Biopsy revealed chordoma.
Biopsy procedures must be performed in accordance with standardized principles to maximize diagnostic accuracy while avoiding the potential jeopardy of tumor spread. Open and percutaneous techniques are governed by similar principles. The biopsy site must be placed in line with the wound planned for definitive treatment to allow later excision. The approach that offers the shortest route and violates the fewest anatomic compartments should be taken. Tissue sampling must be representative and sufficient to enable diagnosis. The pathologist should be consulted prior to biopsy to ensure this is achieved. Hemostasis must be meticulous as an expanding hematoma may contaminate adjacent fascial planes. In the case of open biopsy, surgical drains need be placed in line with the incision to allow for excision of the drainage tract. Although surgeon-supervised, radiologically guided needle biopsy may be emerging as the preferred technique to minimize tumor contamination,8 open biopsy may still be preferable in some pathology services due to preservation of in situ histological architecture of the tumor and/or its capsule with tissue block sampling allowing pathologists to identify patterns of cellular orientation and vascularity more completely.
Local Staging
The aim of local staging is to define the anatomic structures affected by the tumor. This assists in formulating prognosis as well as in operative planning and pooling of research data. Particular attention is paid to neurological, vascular, and osseous relationships.
The descriptive staging system of Weinstein-Boriani-Biagini expands on the work of Enneking and relates tumor involvement anatomically to the vertebra in one or more of 12 zones (with zone 12 at the spinous process), which radiate like the spokes of a wheel centered on the spinal canal.2 Tumors in zones 4 to 7 are appropriate for en bloc resection. Tumors from zones 2 to 5 may be resected by anterior and posterior combined approach.
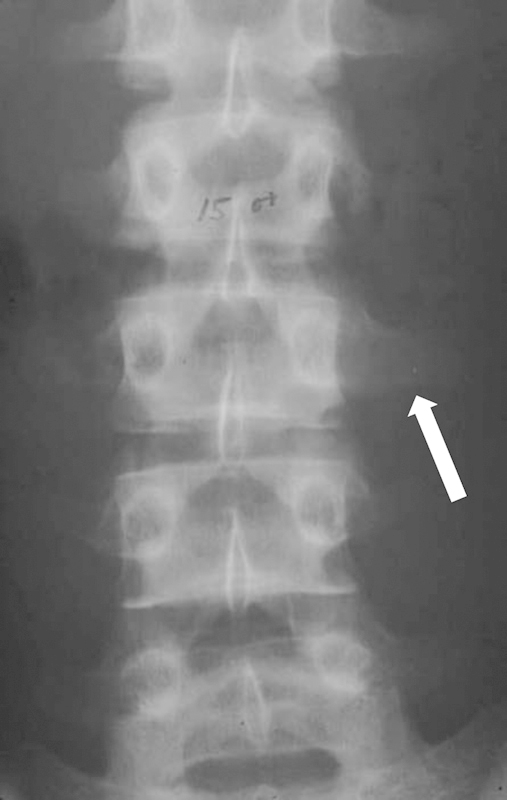
Radiological evaluation is critical in staging and in preoperative planning. In terms of the evaluation of the identified lesion, plain radiographs will indicate overt osteolysis and postural abnormalities including sagittal imbalance. In selected cases, pedicular osteolysis may be evident on anteroposterior view of the thoracolumbar spine as the so-called “owl's eye” sign with loss of the typical elliptical appearance of the pedicle on the affected side (Fig. 2). Computed tomography (CT) demonstrates bony tumoral content and extent and MRI depicts the local environment of the lesion, including soft tissue infiltration or expansion, tumor extent and internal consistency, level of hydration, and potential for neurological compromise. On occasion, angiography may be required, particularly when resection of cervical lesions is contemplated, to assess the course and patency of the vertebral arteries.
Figure 2.
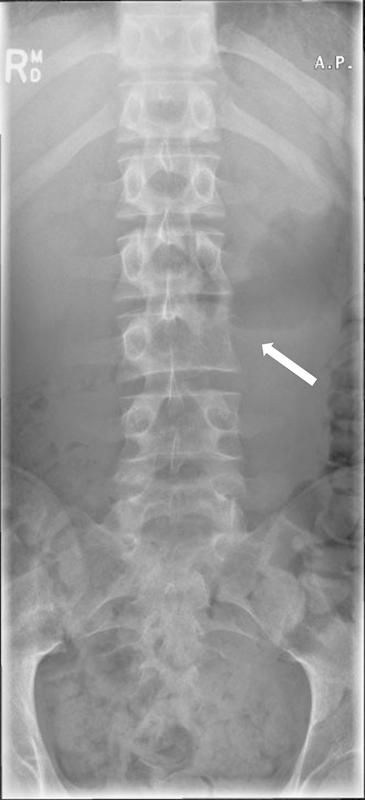
The “owl eye sign” (arrow) in a patient with a left L3 Ewing's sarcoma.
A more recent advance in operative planning includes the commercially available construction of an anatomic biomodel of the tumor and resection region,9 10 particularly in cases where the lesion is in a characteristically inaccessible location such as the craniocervical junction or the atlantoaxial articulation. Biomodeling has been employed in the operative treatment of congenital spinal deformity with some success and allows the surgeon to develop an accurate three-dimensional appreciation of the lesion and its anatomic relations.8
Systemic Staging
Tumoral staging is critical to management strategy and proceeds along the lines described by Enneking with respect to musculoskeletal neoplasia.11 12 To this end, bone scan is an important tool in establishing the solitary nature of the lesion. Where the condition is associated with a dominance of local osteoclasis over osteoid formation, skeletal survey may be employed. Identification of both visceral and bony metastases is also pursued via CT scanning of head, chest, and abdomen, as well as by positron emission tomography (PET) with or without combination with CT imaging. PET-CT, a particularly exciting emerging modality of imaging, exploits the glucose affinity of tumor cells. Cellular avidity is reflective of metabolic activity in a linear relationship, which allows visualization of the biological activity of a particular tumor, its response to conventional treatment, and its susceptibility to evolving targeted therapies.13 Adjunctive clinical examination is also critical to tumor diagnosis and staging and must include a breast examination and rectal palpation. Appropriate blood screening is directed at tumor diagnosis and physiological impact. Tumor-specific investigations include serum alkaline phosphatase, carcinomatous antigens, serum electrophoretic pattern, prostate-specific antigen (PSA), erythrocyte sedimentation rate, and C-reactive protein. Systemic evaluation includes serum electrolytes as well as liver, thyroid, and renal function tests. Bone marrow aspirate and trephine should also be considered for some tumors, particularly Ewing's sarcoma. Osteoporosis is a global skeletal condition that may have a significant effect on the surgeon's reconstructive options following successful resection. When dual-energy X-ray absorptiometry reveals a T-score of less than −2.0, reconstructive efforts may become limited. Finally, but not least importantly, the general health of the patient is a strong determinant of his or her response to what is often major surgery, and comorbidities must be accurately identified and minimized by multidisciplinary consultation and investigation.14 Of late, greater emphasis has been ascribed to patient performance status in the perioperative surgical planning of spinal malignancy. Two validated instruments of performance status in common usage in the medical oncology fields are the 5-point Eastern Cooperative Oncology Group (ECOG) score15 and the percentage-based Karnofsky score.3 Both are useful measures of the impact of patient comorbidity on activities of daily living.
Treatment Strategy
Neoadjuvant Therapy
At the completion of local and systemic staging, neoadjuvant therapy may be considered. Tumor size reduction with neoadjuvant chemotherapy may be dramatic, depending on the histological origins of the tumor. Chemotherapy prior to surgery is often used in both Ewing's sarcoma and OGS. Despite its well-demonstrated effects in these malignancies, chemotherapy has numerous musculoskeletal effects that adversely affect future surgical treatment. Foremost among these is a profound effect on bone mineralization (Fig. 3), whereby spinal stabilization may be difficult to achieve with standard techniques of instrumentation. Neoadjuvant therapy may also compromise the patient's general physical condition as a significant perioperative comorbidity, particularly with respect to long-standing immunosuppression. Long-term chemotherapy may cause peripheral neuropathy in relation to altered vitamin B metabolism among other causes, and, when present, these symptoms need be differentiated from signs of evolving neurological compromise within the spine.
Figure 3.
Advanced osteoporosis postchemotherapy causing “fish spine” in a 16-year-old male.
Surgical Strategy
When planning the surgical treatment of primary spinal tumors, particularly in cases of malignancy, the value of a multidisciplinary approach to care cannot be overestimated. The team approach to medical and surgical treatment enables the formulation of a coordinated perioperative care plan and promotes a spirit of cooperation among treating surgeons of multiple specialties, which may include musculoskeletal oncology, vascular surgery, otolaryngology, urology, gastrointestinal surgery, and plastic and reconstructive surgery. Co-opting a colleague with experience in sarcoma management often greatly helps at the time of tumor resection whereby the colleague's “policing” influence will expertly direct the spinal surgeon away from the plane of the tumor, substantially reducing the risk of breach of the resection margin. Combined surgery with oncology surgical colleagues is also of particular benefit in tackling tumors such as sacral chordoma where adequate resection often requires extension into the ilia from both anterior and posterior approaches.
Typically, benign lesions are manageable by intralesional (e.g., osteoid osteoma) or marginal excision. Those lesions with a tendency for local recurrence (e.g., benign fibrous lesions, aggressive osteoblastoma, and giant cell tumor) are best excised with a wide margin. For the most part, resectable malignant lesions require a wide/radical margin by en bloc excision.
Local staging and biological potential of the tumor will guide surgical approach. A useful guide to deciding on the required approach has been described by Tomita et al (Figs. 4, 5).43 Options to be considered are posterior, anterior, and combined approaches. In general, a posterior approach alone may be considered when the anterior margin of the tumor is contained within the vertebral body. Involvement of anterior structures beyond the vertebrae may necessitate a combined approach: posteriorly to remove involved posterior elements and to obtain stability and anteriorly to dissect vital structures free of the tumor with a clear margin. Simultaneous, combined anteroposterior surgery using a tri-star incision may be feasible for extensive or multiple-level resection. The patient is typically supported in the lateral position, allowing tumor manipulation from both anterior and posterior directions simultaneously. The latter is particularly useful in the en bloc excision of unilaterally disposed malignant lesions where the operative specimen may be lifted safely away from the thecal sac after contralateral osteotomy, although placement of pedicular instrumentation with the patient in the lateral position can be somewhat challenging and may be assisted by image guidance techniques.
Figure 4.
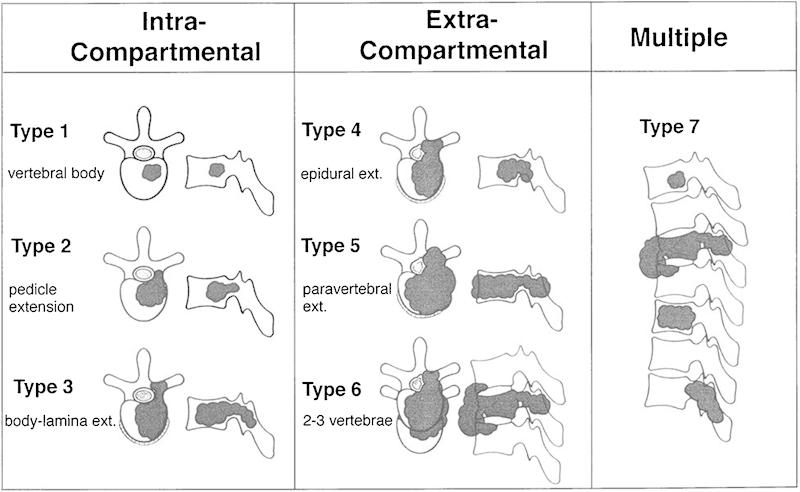
Tomita's surgical classification of spinal tumors.
Figure 5.
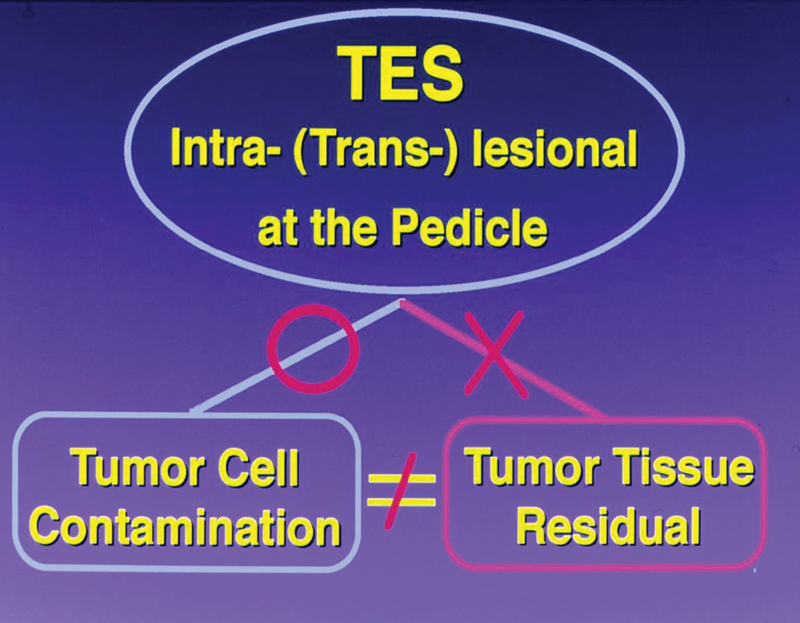
Tomita's view of transpedicular tumor division in the technique of total en bloc spondylectomy (TES).
Anterior-only approaches may be utilized for anteriorly situated intracompartmental tumors in a setting where either marginal or intralesional excision is adequate.
Neurological sacrifice, where necessary, must be anticipated and disclosed to the patient preoperatively. Histological subtypes and tumor staging will guide the surgeon in the appropriate level of “aggression” to be employed in obtaining a satisfactory margin of clearance, being mindful of the balance between life, limb, and function. The same may be said for the involvement of viscera including the ureters, major vessels, and gastrointestinal structures.
Adjuvant Therapy
For primary malignant tumors of the spine, adjuvant treatment after primary surgical management is recommended. The most common adjuvant treatments are chemotherapy and/or radiotherapy. The choice of these two modalities is determined by several factors including risk of distant and local relapse, surgical margins, and the tumor histology, which may indicate the relative chemo- and radiosensitivity. Perioperative chemotherapy is the standard for both Ewing's sarcoma and osteosarcoma.
With recent advances in the planning and delivery of radiotherapy, it is now possible to deliver higher doses to the surgical bed to sterilize microscopic (and potentially macroscopic) disease while selectively sparing the critical adjacent neural structures and preventing radiation myelopathy. This can be done using standard “fractionated” treatment, which is typically undertaken over a course of 5 to 6 weeks. The delivery of these treatments is due to the availability of advanced radiotherapy techniques including intensity-modulated radiotherapy (IMRT), tomotherapy, Cyberknife (Accuray, Sunnyvale, CA), and proton beam radiotherapy.
IMRT and tomotherapy optimize the delivery of radiation to irregularly shaped volumes via modulation of the treatment beam and have the ability to produce concavities within the treated volume as required when treating a spinal tumor.16 17 Cyberknife is a robotic system that delivers many independently targeted treatment beams with high precision, which when summed together enable treatment of complex spinal targets.18 Proton beam therapy shows promise in many sites but particularly spine malignancy, owing to the physical characteristics of the proton beam with near-zero dose deep to the target for each proton beam path.19 These advanced techniques ensure millimeter accuracy of radiotherapy delivery by utilizing volumetric and stereoscopic imaging (image-guided radiotherapy or IGRT).
More recently, the emergence of stereotactic spine radiotherapy delivers doses that are biologically much higher than previously achievable in between one and five treatments.8 20 Its usefulness in metastatic disease to the spine is well documented but benefit in primary spinal malignancy is unresolved. In general, the likelihood is that external beam radiation can delay local recurrence after a resection with a positive surgical margin; however, it is unlikely to prevent it.
Benign Spinal Tumors
Osteoid Osteoma—Intralesional Excision without Reconstruction
Osteoid osteoma and osteoblastoma are the most common symptomatic benign spinal tumors. Osteoid osteoma accounts for 11% of all primary bone tumors, and between 10% and 20% of these occur in the spine. Occurring almost exclusively within the posterior spinal elements, peak incidence is between childhood and early adult life. The tumors are differentiated based in size, histological appearance being identical, with an osteoid osteoma having an osteoblastic nidus of less than 10 mm in diameter. Osteoblastomas have a less characteristic pain pattern and are less responsive to salicylates. Forty percent of osteoblastomas occur in the spine and up to 40% occur within the vertebral body. Despite the histological similarity to osteoid osteoma, osteoblastomas have a greater tendency to biological aggression with local recurrence so that so-called “aggressive” osteoblastomas are best treated by en bloc resection.8
Clinical features of osteoid osteoma are typical with older children or adolescents commonly presenting with a painful postural scoliosis (60 to 70%), which is readily relieved by aspirin. Radiological appearances are also characteristic with the lesion often detectable on close inspection of plain radiographs. In children and adolescents, the transverse process immediately adjacent to the lesion is often hypertrophic, possibly as a result of local osseous hyperemia (Fig. 6). The intense osteoblastic activity of the lesion is markedly avid to radioisotope.21
Figure 6.
Osteoid osteoma left L3 pedicle with hypertrophic transverse process (arrow).
Radiofrequency ablation is a popular technique in the management of long bone osteoid osteoma; however, its use is limited in periosteal lesions and those close to neurological elements. Surgical excision remains a useful technique in the spine.
Intralesional resection of the osteoblastic nidus is facilitated by intraoperative radioisotope localization using a portable gamma probe.22 Approximately 2 hours preoperatively, the patient is administered a standard dosage of Tc-99m. At the commencement of surgery, the probe is used to record a background reading of radioactivity from the sternum. After patient positioning and exposure of the affected vertebra, the probe will usually record intense activity over the nidus, at ∼2 to 3 times the activity of adjacent bone. Following curettage of the nidus, the radioactivity of the affected area should reduce to near reference levels. Intralesional excision is, for the most part, immediately and completely effective in relieving symptoms.
Aggressive Hemangioma—Intralesional Excision T11 with Realignment/Reconstruction
Vertebral body hemangioma is a common incidental finding, occurring in 10 to 12% of routine autopsy subjects and in spinal plain film series. On rare occasions, a so-called “aggressive” hemangioma may enlarge, extending beyond the compartment of the vertebral body where either neural compression or pathological fracture of the vertebra may ensue. In these cases, treatment may be required either for symptoms of direct nerve root or spinal cord compression or for progressive kyphosis secondary to vertebral body collapse. Angiography may or may not identify a key arterial nutrient vessel, depending on the histological subtype of the lesion. When the characteristic arteriolar blush from a singular feeding vessel is reproduced on angiography, embolization may be curative to the pain of nerve compression due to mass effect. The low rate of recurrence associated with these lesions allows definitive treatment by intralesional resection when operation is required (i.e., in cases of neurological deficit of nerve root or spinal cord or in addressing a progressive pathological fracture). Treatment methods that have been employed in our center range from radiologically guided vertebroplasty23 to open vertebroplasty with stabilization. In the following case example, the patient presented with progressive, painful kyphosis with evolving spinal cord compression. The low risk of recurrence allowed intralesional resection followed by reconstruction using the “closing-opening wedge” osteotomy technique described by Kawahara et al.24 The biological behavior of “aggressive” hemangioma differs substantially from aneurysmal bone cyst. Although both may be considered non-neoplastic conditions, the latter has a greater tendency toward rapid expansion and local recurrence following intralesional excision.
Case 1
Summary
A 53-year-old woman presented with 3 months of back pain following a mechanical fall. Pain was unable to be controlled with analgesia, and she experienced 2 weeks of altered sensation in her legs that was worsening and 1 week of evolving weakness in her legs and loss of control of bowel and bladder function. On examination she had a marked thoracolumbar kyphosis, her neurological status was T10 American Spinal Injury Association Standard Neurological Classification of Spinal Cord Injury (ASIA) category C.
Imaging workup included CT and MRI (Fig. 7) for both local and systemic staging as well as a protocol battery of blood tests. Following a detailed explanation of her condition and the possible treatment options, she elected to have the lesion treated surgically. An open biopsy confirmed the nature of the lesion as a hemangioma.
Figure 7.
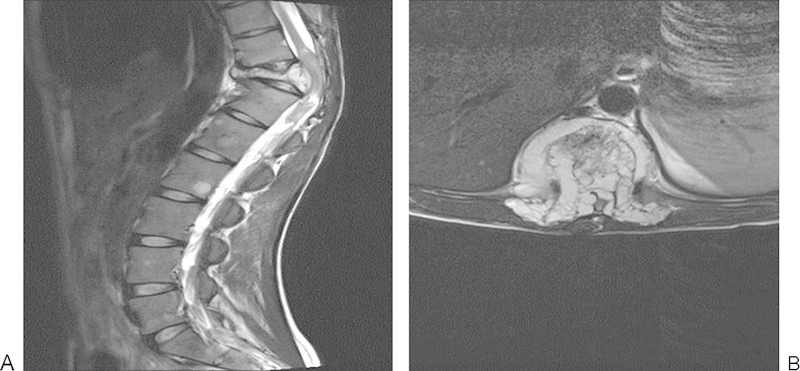
T11 hemangioma with pathological fracture.
At 3-year follow-up the patient has a pleasing recovery to motor level L3 ASIA category D. The construct remains sound (Fig. 8).
Figure 8.
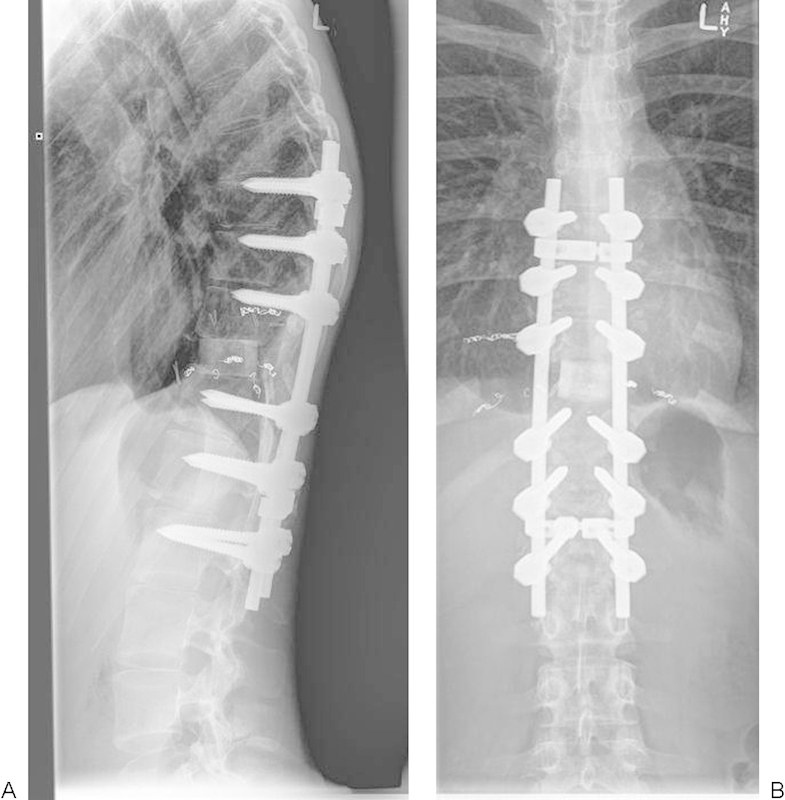
Postoperative images (case 1).
Technical Note
Despite variable reports regarding its efficacy,25 we have found preoperative embolization to be a reliable adjunct to surgical treatment of hemangioma of the spine.
In this case, the patient was positioned prone on the operating table with a central bed break to facilitate correction of sagittal alignment using the “closing-opening wedge” correction technique.25 As depicted in Fig. 9, adequate exposure necessitated resection of two rib heads to the rib angles bilaterally in the same manner as may be employed in performing total en bloc spondylectomy. After a three-segment laminectomy, both pedicles of the involved vertebral body were amputated. The segmental vascular bundle on each side of the affected vertebra must be isolated and divided as a specific step as these vessels cross the plane of access to the anterior vertebral body.
Figure 9.
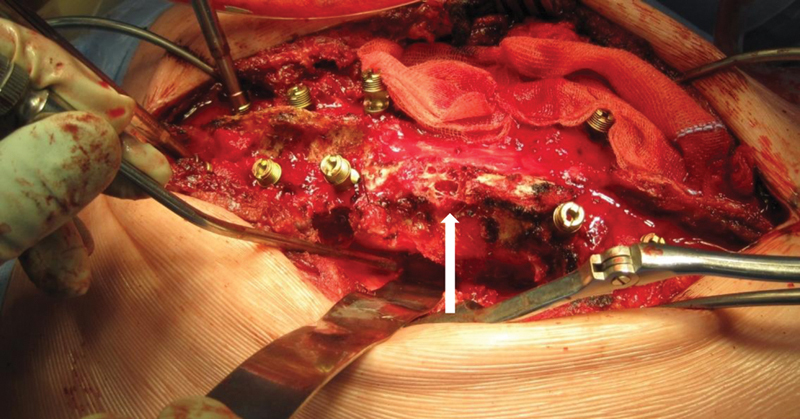
Intraoperative view T10 hemangioma showing typical cavernous appearance of the involved bone (arrow).
Following successful embolization, the hemangioma can be resected piecemeal with a minimum of bleeding (Fig. 9). The intervertebral discs above and below the involved vertebra were resected and the vertebral body was cleared to the level of the anterior longitudinal ligament. After complete resection, the sagittal deformity was corrected according to the principles described by Kawahara et al,24 to avoid spinal cord shortening and potential neurological compromise. In a modification of this technique, we prefer to divide the anterior longitudinal ligament from posteriorly to facilitate lengthening of the anterior column.
Fibrous Dysplasia—Transoral Intralesional Resection C2 Odontoid Process with Complex Reconstruction
Fibrous dysplasia is a rare disorder characterized by replacement of the normal cancellous bone by fibrous tissue. Lesions may be isolated or widespread. Isolated lesions of the cervical spine are rare with only 13 previously reported cases. Polyostotic lesions more commonly involve the spine and scoliosis due to spinal fibrous dysplasia and are probably more common than previously thought.26 Pathological fractures of the mobile spine are common and radical intralesional resection, bone grafting, and stabilization are the mainstays of treatment.
Case 2
Summary
A 34-year-old man presented with axial neck pain and occipital headache 6 weeks following a rugby tackle. He displayed no neurological symptoms, but he required regular paracetamol and struggled to work due to constant nagging pain. Examination revealed a symmetrically reduced range of rotation and a severely reduced range of flexion such that he could bring his chin to within four fingerbreadths from his chest. CT demonstrated a C2 pathological fracture with underlying fibrous dysplasia (Fig. 10).
Figure 10.
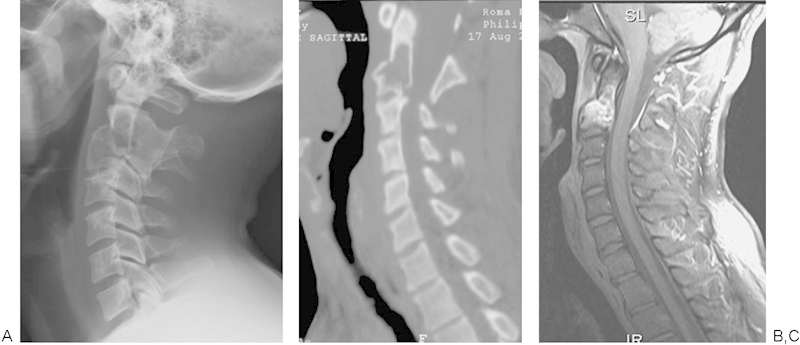
C2 pathological fracture through fibrous dysplasia. Note the congenital fusion of C2 and C3. Congenital fusion is a common association of cervical fibrous dysplasia.
Technical Note
Transoral resection of the odontoid process and C2 vertebral body is an exacting procedure requiring meticulous preoperative planning. Due to the vulnerability of the pharyngeal wall repair in the immediate postoperative period, we advise that patients undergo preoperative insertion of a percutaneous endoscopic gastrostomy (PEG) and insertion of a tracheostomy at the time of surgery.
The patient was positioned supine in a Mayfield head clamp in exaggerated atlantooccipital extension. The assistance of an ear, nose, and throat colleague can be most helpful in creating a bloodless soft palate by adrenaline injection into the region of the palatine arteries and also in providing guidance in the use of the McIvor retractor system, which is used regularly in otolaryngology.
Rather than dividing the soft palate, the uvula was retracted with the use of a stay suture.
The posterior pharyngeal wall, a deceptively substantial layer, is ∼10 mm in thickness. This approach allows exposure extending from the C1 anterior arch to the upper endplate of C4.
After piecemeal excision of the lesion, a devascularized fibular graft was harvested to bridge the tumor bed from the C1 anterior arch to C4. This bridging technique was employed due to the tendency of the lesion to resorb even the most robust bone graft reconstruction. The upper end of the graft was fashioned into a “saddle” shape to optimize contact with C1. The lower end of the fibular strut was secured with an interference screw in the C4 vertebral body, and the patient was immobilized in a postoperative halothoracic vest until evidence of bony union was demonstrated on postoperative reconstructed CT (Fig. 11).
Figure 11.
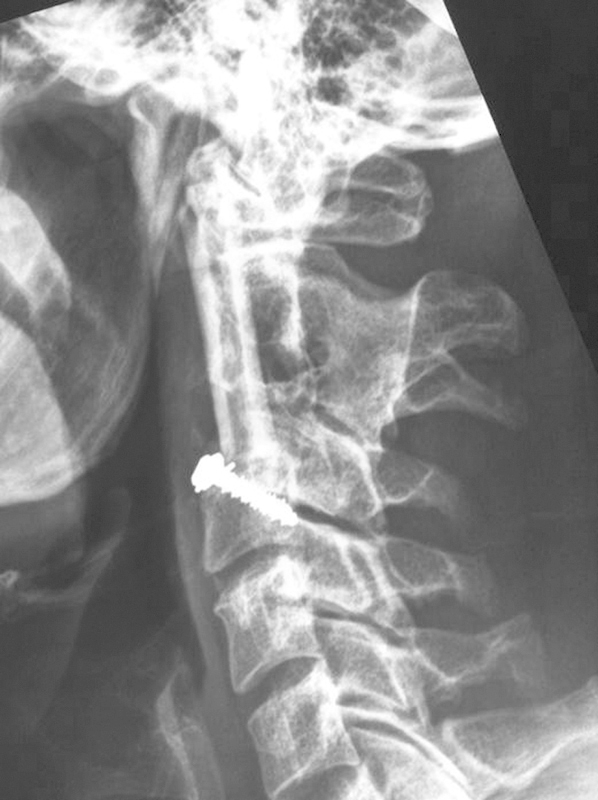
Postoperative reconstruction after excision of C2 fibrous dysplasia.
Particular vigilance is required in managing the frequent complication of dehiscence of the posterior pharyngeal wall. Although the wound appearance can be alarmingly suggestive of infection, our experience has been that, if bypassed by PEG and/or temporary tracheostomy, the wound heals eventually over a period of what can be many weeks. Gradual introduction of oral feeding is guided by repeated contrast swallowing studies, which should not demonstrate contrast communication with the reconstruction.
Benign Solitary Fibrous Tumor—Wide Resection Paravertebral Tissue/Laminae C4–T2 with Reconstruction
Solitary fibrous tumors are very rare lesions previously known as hemangiopericytomas. Few cases of spinal occurrence have been reported.27 Most lesions relate to serosal membranes, the first being described in relation to the pleura. There is one reported case of a solitary fibrous tumor causing pathological fracture of a cervical vertebral body and requiring extensive and repeated surgery.28
The lesions tend to be well circumscribed, at least partially encapsulated, and of unpredictable biological potential. Ten to 15% of lesions behave aggressively and must be differentiated from the malignant variant as determined by clinical disease progression and histomorphology with more than four mitoses per high-power field. Following successful marginal resection, most do not recur; however, there is a reported tendency to dedifferentiation with repeated excisional surgery, and for these patients, adjuvant radiotherapy should be considered.
Case 3 demonstrates treatment of a large benign fibrous tumor involving the paravertebral musculature and posterior laminar surfaces across four or more segments of the cervical spine. The technical note highlights a resection technique that may be of value in resecting potentially aggressive lesions involving primarily paravertebral soft tissue, but where a satisfactory margin requires laminar resection without breech of the tumor capsule.
Case 3
History
A 37-year-old man presented with 14 months of an insidious onset and slowly progressive axial neck pain. His partner had noticed a lump in the month prior to presentation. He had no neurological symptoms. Regular analgesia in the form of paracetamol and tramadol provided limited relief. Examination revealed a large mass deep to fascia. Neurology was normal. Imaging studies revealed a lesion located in the left paraspinal muscles adjacent to the lamina (Fig. 12). Following routine local and systemic staging, a CT-guided core needle biopsy was performed. Histological diagnosis and multidisciplinary musculoskeletal oncology panel review resulted in an en bloc excision with a wide margin being performed.
Figure 12.
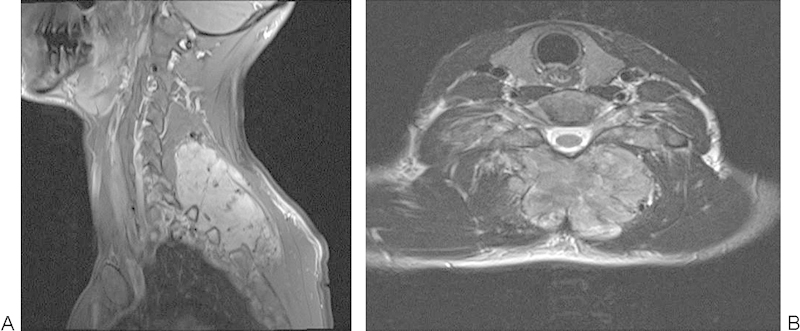
Large posterior cervical solitary fibrous tumor in a 37-year-old man.
Technical Note
Owing to the unpredictable biological behavior of this rare lesion, particularly given the recent history of rapid expansion in this case, a technique of wide resection was employed.
The patient was positioned prone with head secured in a Mayfield head clamp. Initial dissection exposed the paravertebral muscles, which were divided above and below the lesion with a cuff of normal muscle tissue. As the lesion extended into the left first and second intercostal spaces to the level of the pleura, multiple rib resections became necessary with dissection and preservation of the left T1 nerve root.
The tumor extended onto the dorsal surfaces of several contiguous cervical laminae, so that a wide margin around the deep extent of the lesion necessitated removal of these laminae. To facilitate this, a complete laminectomy was performed at the level immediately subjacent to the resection to access the spinal canal deep to the tumor. In sequence then, working from inferiorly to superiorly, the pedicles of each vertebra were transacted from within the spinal canal using a 5-mm osteotome, enabling the tumor to be gradually elevated from the spine level by level (Fig. 13).
Figure 13.
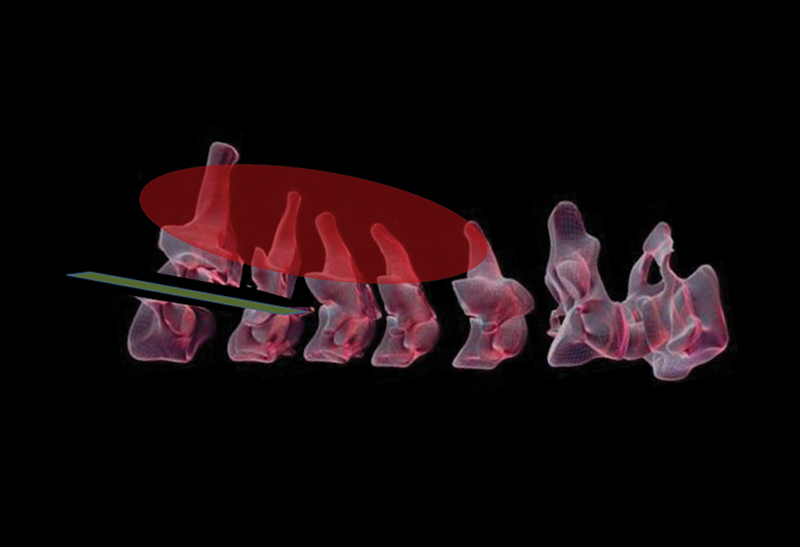
Schematic representation of technique of pedicular division to facilitate wide excision of tumor extending to the dorsal surface of the cervical laminae.
The dynamic balance of the head was defunctioned after such extensive cervical muscle resection, and a long posterior construct utilizing pedicle screws over the cervical segments where the lateral masses had been resected (Fig. 14) was required.
Figure 14.
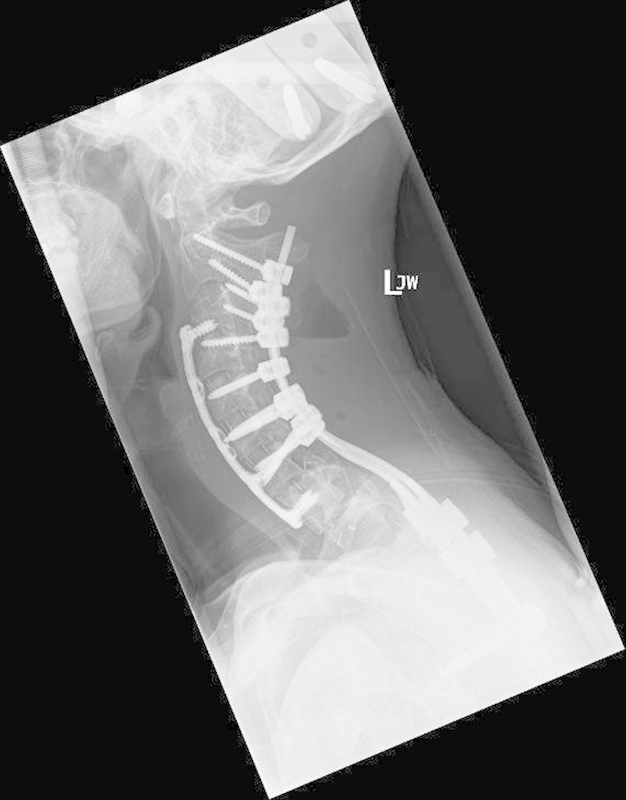
Postoperative image after resection of cervical benign fibrous tumor.
Malignant Spinal Tumors
Malignant primary bone tumors occur with rarity in the spine, representing only 1% of spinal malignancies. The most commonly encountered malignant lesions are osteosarcoma, Ewing's sarcoma, chordoma, and chondrosarcoma. The Surveillance Epidemiology and End Result (SEER) Database has recently been studied by Mukherjee et al,29 whose review spanned a 30-year period (1973–2003) and 1892 cases of aggressive primary spine neoplasia. Survival rates improved for Ewing's tumor, chordoma, and chondrosarcoma over this period, a change ascribed to improvements in surgical and adjuvant therapy. As in all musculoskeletal malignancy, prognosis depends on tumor staging at diagnosis and the absence of local recurrence following surgical resection.
Chordoma—Wide Resection C3 via Lateral Approach
A tumor of notochordal remnants, chordoma accounts for 1 to 4% of malignant bone tumors. Thirty-five percent of lesions occur in the sacrum and 50% in the region of the clivus. The remaining 15% occur in the mobile spine where the lesions are overwhelmingly located in the midline. Seventy-five percent of patients have lower back pain for greater than 6 months and 20% have rectal dysfunction. The majority (if not all) of sacral lesions are palpable by rectal examination. Patients presenting with coccydynia may warrant further screening (e.g., by bone scan). MRI is the investigation of choice and often demonstrates amorphous calcification internal to the lesion. Although considered to be a lower-grade malignancy, inadequate resection leads to rapid and often unmanageable local recurrence and, rarely, metastatic disease. Propensity for local aggression is such that biopsy tract seeding, wound margin, and intradural extension have all been described. In their series of 48 cases of chordoma affecting the mobile spine treated over a 50-year period, Boriani et al reported that all patients treated with intralesional resection with or without radiotherapy experienced local tumor recurrence within 2 years, sometimes surviving long periods with local disease.44 By contrast, 12 of 18 patients treated by en bloc resection were disease-free at an average of 8 years' follow-up. These results contrast with those of Moojen et al,30 who reviewed treatment of 15 patients with sacrococcygeal chordoma. All 10 patients treated by extralesional resection without adjuvant radiotherapy had local tumor recurrence at a mean of 2 years following surgery. Those treated with radiotherapy for local recurrence survived an average of 7 years. Only one of five patients treated with adjuvant radiotherapy and intralesional resection had local recurrence at 11 years.
The role of surgical treatment of chordoma was reinforced by Jawad and colleagues in their study of the SEER database of the National Cancer Institute.31 In their review of 962 patients, they identified age < 59 years, Hispanic ethnicity, size < 8 cm, and surgical resection as positive prognostic indicators. Although failing to reach statistical significance, those patients who received radiotherapy survived a median 60 months in comparison with 35 months for those who did not undergo either surgery or radiotherapy.
The role of radiotherapy for spinal chordoma is poorly documented; however, for skull base lesions in patients with gross, recurrent, or microscopic residual disease, adjuvant high-dose radiotherapy improves local control. As chordoma is relatively radio-resistant, the doses required to sterilize this disease was, until recently, not achievable in the spine. However, with the techniques previously described adjuvant radiotherapy to the spine should be considered in patients with a high risk of local recurrence after definitive surgery. Future directions in the treatment of chordoma involve the use of proton beam therapy, which may have some superiority over traditional photon therapy.32
The illustrative case demonstrates a rare unilateral chordoma of the mobile spine managed by wide resection via a lateral approach to the upper cervical spine.
Case 4
Summary
A 29-year-old woman presented with a 9-month history of an enlarging mass in the left side of her neck. She reported 1 month of rapid increase in size and pain at night worsening over 3 months, radiating into the occiput as well as progressive difficulty swallowing. On examination she had a nontender deep mass at the apex of her left posterior triangle. Central and peripheral neurological examinations were normal (Fig. 15).
Figure 15.
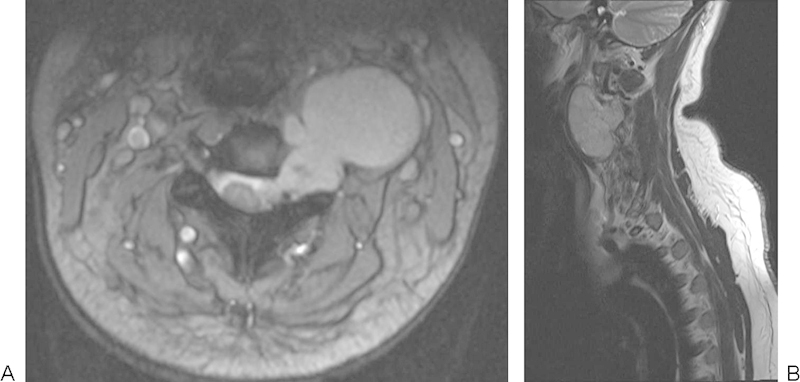
Large chordoma emanating from the left side of C3–4 intervertebral foramen.
Technical Note
The unusual lateral disposition of this typically midline lesion gave the impression of a neural sheath tumor on initial radiological imaging. This underscores the importance of obtaining a histological diagnosis preoperatively.
One of the significant preoperative issues to be established was the location and patency of the left vertebral artery. Temporary angiographic occlusion can help determine the neurological effects of ligation of the vessel prior to definitive surgery.
At surgery, the patient was positioned in the right lateral position with head tilted laterally to the right and immobilized in a Mayfield clamp. A formal approach to the middle third of the left vertebral artery was performed using the extensile exposure described by Henry33 in which the sternomastoid muscle is detached from its origin, affording access to the lateral mass of the C1 vertebra superiorly. This approach allowed access to both the anterior and posterior bony extent of the tumor within the C4 vertebra.
Ewing's Sarcoma—Simultaneous, Combined en bloc Resection L3
Ewing's sarcoma is derived from neuroectodermal cell lineage and represents the second most common malignant bone tumor of childhood after osteosarcoma. It has a peak incidence in the 10- to 15-year-old (adolescent) age group and most commonly occurs in the extremities. Ewing's family tumors are high grade and considered a systemic disease from the outset so that intensive multiagent chemotherapy regimens are used perioperatively.
As this lesion is considered to have micrometastases in essentially all cases at presentation, the advent of effective chemotherapy has revolutionized the treatment of both Ewing's sarcoma and of OGS prior to which 2-year tumor survival was negligible.
Surgery is considered the treatment of choice for primary disease where the lesion can be resected with negative margins (debulking surgery should be avoided), without excessive morbidity, and with the expectation of a reasonable functional result. Effective neoadjuvant therapy will commonly markedly reduce the primary lesion in size, with MRI demonstration of a characteristic “rind” of pseudocapsule surrounding the residual lesion. Histological analysis of the “kill” rate of cellular necrosis in the resected specimen provides valuable prognostic information and is one of the major advantages of systemic neoadjuvant treatment.
Neoadjuvant or definitive radiotherapy is used for borderline or unresectable disease, with adjuvant radiotherapy considered in cases of a marginal resection. Neoadjuvant chemotherapy is mandatory and most commonly involves three- or four-drug regimens consisting of vincristine, cyclophosphamide, and Adriamycin with or without Actinomycin-D.
In the recent review of the Rizzoli Institute, 42 patients with Ewing's sarcoma were divided into two groups: 26 who were treated by radiotherapy alone and 17 by surgery followed by lower-dose radiotherapy. There was no difference in overall survival between the groups, which was 42% and 32% at 5 and 10 years, respectively.34 Furthermore, spinal Ewing's disease had a significantly poorer outlook than disease of the appendicular skeleton, with those lesions occurring in the nonmobile spine carrying the worst prognosis of all. In a recent meta-analysis of multidisciplinary care in both Ewing sarcoma and OGS, there was sufficient evidence to support a strong recommendation that both lesions should receive neoadjuvant chemotherapy to improve local control and overall survival. There was only weak evidence for enhanced survival after en bloc resection of either lesion.35
Case 5
Summary
The patient was a 16-year-old male with 3 months' back pain, weight loss, and fevers. He displayed left-sided paraspinal muscle spasm and grade II power of hip flexors and knee extensors on the left side. His medical history was unremarkable. He underwent radiotherapy followed by 14 cycles of etoposide and ifosfamide, during which he developed ifosfamide encephalopathy, systemic Candida albicans infection, and osteoporosis (Figs. 16, 17), although with drastic reduction in tumor volume.
Figure 16.
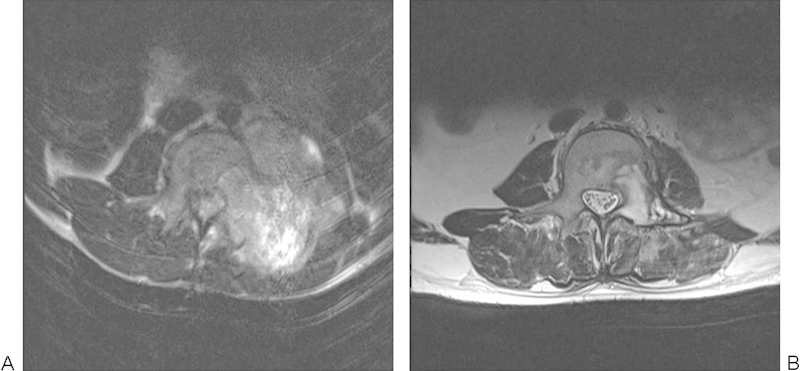
Marked reduction in Ewing's tumor volume following neoadjuvant chemotherapy.
Figure 17.
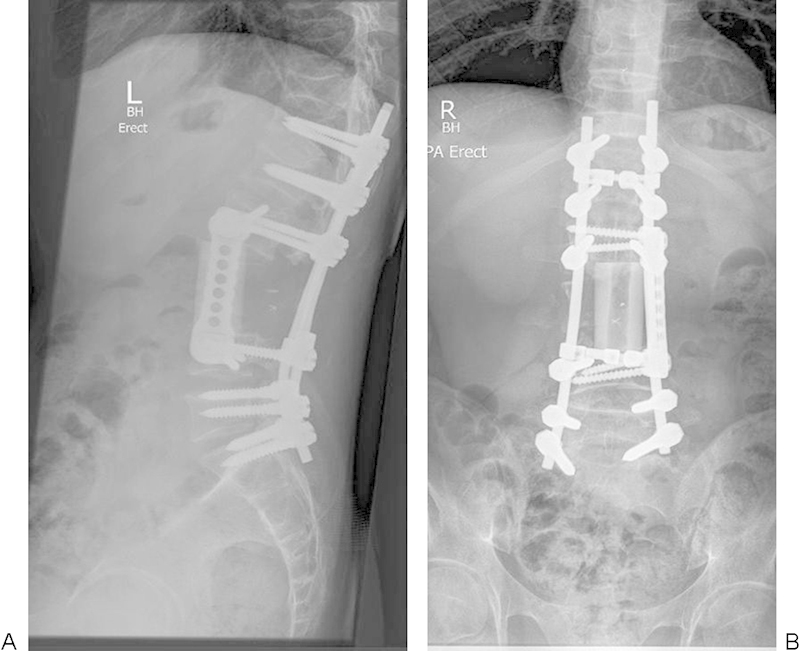
Reconstruction following en bloc excision of L3 Ewing's sarcoma.
Technical Note
In this case, prolonged neoadjuvant chemotherapy and radiotherapy led to marked osteoporosis, which posed considerable difficulty in reconstruction. Despite the recent report suggesting little prognostic benefit to resection of spinal Ewing's sarcoma, in our view where the lesion is accessible without necessity for extensive neural sacrifice, our center remains in favor of extirpative resection.
En bloc resection of middle and lower lumbar vertebrae is technically difficult via the posterior approach alone for two specific reasons. First, the lumbar nerve roots at these levels are usually unable to be sacrificed without causing significant functional implications and, second, the attachment of the psoas muscles complicates mobilization of the vertebral body from a purely dorsal exposure. For these reasons, a simultaneous, combined approach to L3 was performed with the patient in the right lateral position over a table break. A tri-star incision extended from the left loin to the midline, and the left paravertebral musculature was divided to facilitate tumor mobilization. Thus, bimanual tumor mass manipulation was possible, facilitating mobilization of the lesion and division of soft tissue restraints. The left L3 nerve root was intimately related to the tumor and was sacrificed, causing remarkably little functional deficit to the left lower limb in this patient.
For anterior column reconstruction, we prefer to use banked structural allograft for its biological healing capacity and low cost (Fig. 17). Biological reconstructive materials also allow superior postoperative monitoring of both the state of bony union36 and local tumor recurrence.
Chondrosarcoma—Multiple-Level Total en bloc Spondylectomy with Reconstruction
Chondrosarcoma is a malignant cartilage-forming bone neoplasm that accounts for 10% of all primary bone tumors. Typically low grade, these neoplasms arise de novo, and less than 10% of all chondrosarcomas occur in the spine. Most occur in the thoracic spine, and patients typically present in middle age with back pain and/or neurological symptom, with men affected more than women. The recommended treatment for spinal chondrosarcoma is complete en bloc resection of the tumor. These lesions have a typically wide spectrum of biological activity and are commonly of lower grade with repeated resection for local recurrence being required often over a very prolonged time course.
In a review of the management of 69 cases of chondrosarcoma, Bergh et al reported that negative prognostic indicators included tumor size, internal necrosis, positive surgical resection margins, and prior surgery.5
Case 6
Summary
A 60-year-old man presented with 6 months' duration of worsening left chest wall pain and weight loss. Pain was typically worse at night. There was a history of well-controlled hypertension. On examination, he had altered sensation on the left chest wall below the nipple and above the xiphisternum. Motor examination was unremarkable (Figs. 18, 19).
Figure 18.
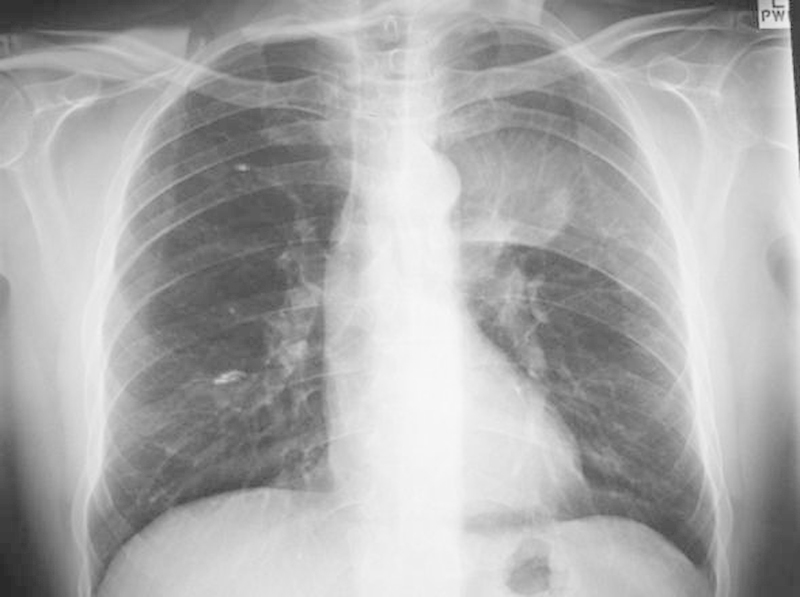
Large left thoracic mass lesion.
Figure 19.
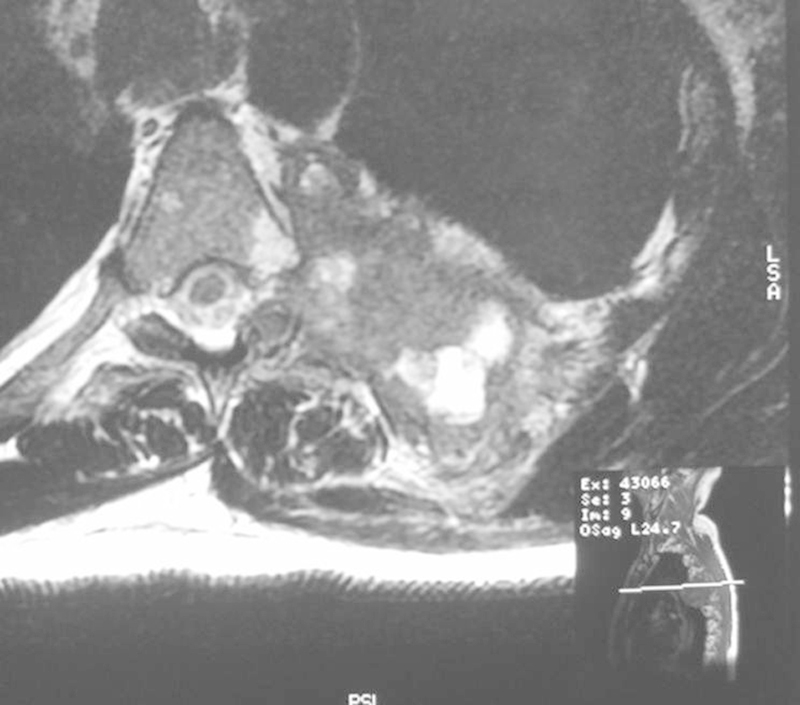
Large chondrosarcoma involving the left fifth rib and posterior chest wall.
Technical Note
Multiple-level, total en bloc spondylectomy is a lengthy and demanding procedure requiring multidisciplinary surgical input and well-established high-dependency perioperative services. The patient needs to be aware that recovery from such extensive operation may take at least 12 months and reoperation in dealing with frequently encountered complications is a possibility.
The guidance of an assisting musculoskeletal oncology surgeon is critical in avoiding breech of the tumor. In this case, three contiguous thoracic vertebral segments and five attached ribs were excised to achieve clearance of a high-grade chondrosarcoma (Fig. 20).
Figure 20.
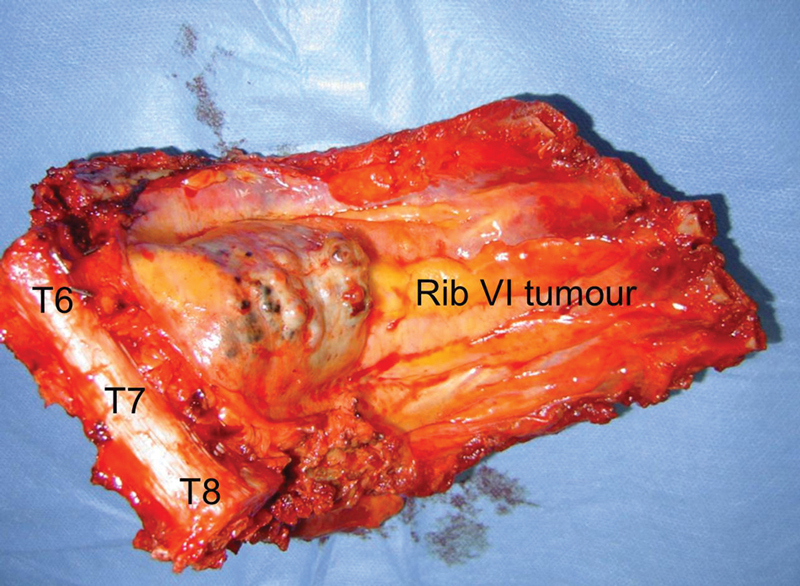
Resected specimen involving T6–8 vertebral bodies and ribs 5–9.
Osteogenic Sarcoma
The spine is a rare site of OGS, accounting for fewer than 3% of all cases. OGS is perhaps the most challenging of spinal malignancies to treat in view of its high-grade and generally lethal nature. Although existing as a spectrum of biological potential in the appendicular skeleton that encompasses many grades of osteoid forming lesions, OGS has a tendency to greater biological potential in the spine, where the few available series report a uniformly poor prognosis. This is reflected by the review of 22 patients with sarcoma of the mobile spine by Ozaki et al.37 Of 22 patients, 12 underwent surgery, 10 of whom had either of a marginal or intralesional resection. Median survival was 23 months. In their recent report, Schwab et al review 17 patients with OGS of the mobile spine.38 All patients received neoadjuvant high-dose methotrexate and Adriamycin. Of the nine patients who underwent en bloc resection, median overall survival was 77 months as compared with 17 months in those patients treated with intralesional resection. It should be noted that in the nine patients undergoing en bloc spondylectomy, five had positive resection margins. Of the four patients who had negative surgical margins after en bloc resection, median overall survival was 82 months. Although the small numbers involved require caution in data interpretation, it may be that successful en bloc resection in concert with neoadjuvant chemotherapy carries the greatest hope of improved survival.38
Late Complications of Multiple Segmental en bloc Resection of the Spine
Experience in complex multisegmental spinal resection for primary malignancy is limited to small case series and prospectively collected datasets in large centers,39 40 making detailed analysis of long-term postsurgical complications difficult to report. In our small series of cases, we have identified a consistent late tendency to major spinal imbalance (sagittal and/or coronal) occurring most commonly 12 months or more into the postoperative period. In our experience, this imbalance centers on the region of the spine that is superior or inferior to the reconstructed segment, implying that aggressive unilateral or bilateral resection of paraspinal musculature interrupts the global spinal dynamic “check rein” of soft tissue leading to decompensation over time. This phenomenon is illustrated in case 6. With increasingly aggressive resection of primary and recurrent spinal malignancy, lower rates of local recurrence, and improved survival, late complications of these more major procedures may increase in prevalence.41
Case 7
Summary
A 49-year-old man presented with an 8-month history of lumbar spinal pain and weight loss. MRI revealed a large right-sided lesions emanating from the L1 vertebra (Fig. 21). Biopsy proved OGS. The patient proceeded to single-stage multilevel total en bloc spondylectomy (Fig. 22).
Figure 21.
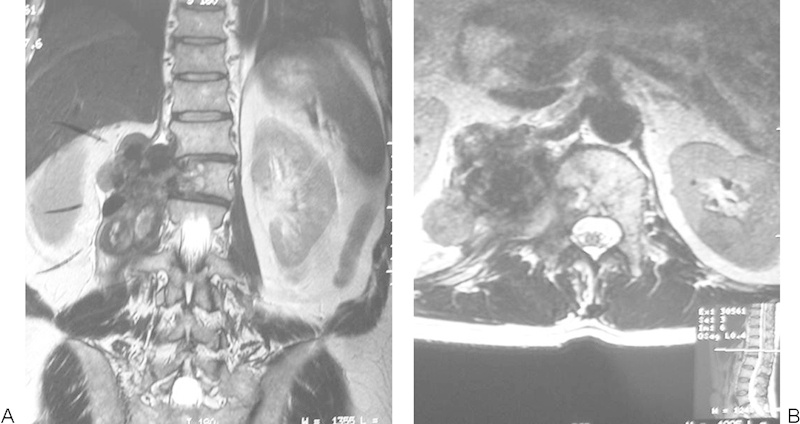
Extensive right paravertebral osteogenic sarcoma L2 vertebra.
Figure 22.
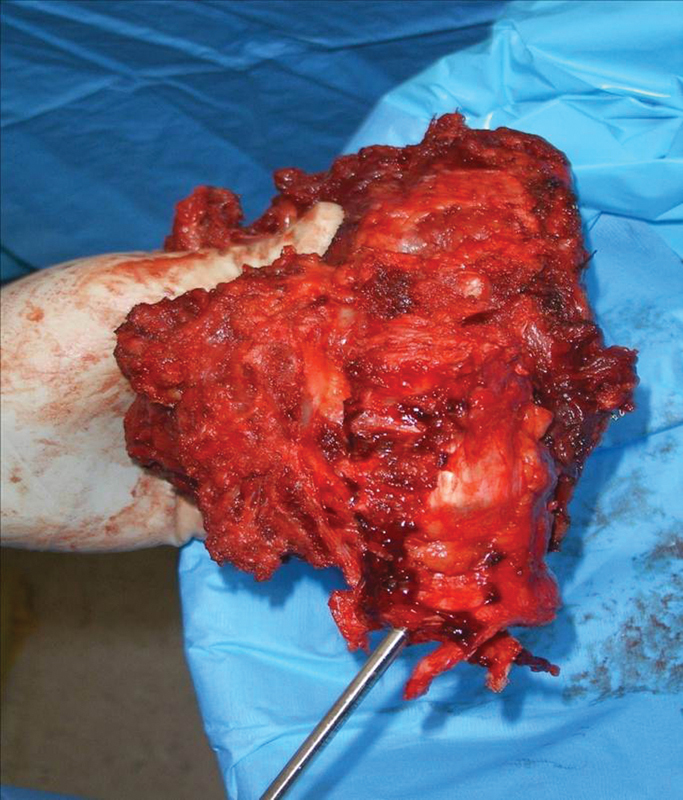
Resected specimen: osteogenic sarcoma L2 vertebra. Note extensive resection of right paravertebral muscle column.
Technical Note
At ∼12 months after extensive three-level resection of a large L1 level OGS, the patient rapidly lost both sagittal and coronal spinal balance leading to difficulty in mobilization and lower back pain (Fig. 23). Initial surgery had included right-sided paravertebral muscle column resection, interrupting the balancing “check rein” of paraspinal soft tissues. Note that decompensation occurred below the instrumented segment, implying failure of soft tissue supports outside the zone of the reconstruction. Using a corrective biplanar pedicle subtraction osteotomy technique, balance was restored and fixation was extended to the ilia (Fig. 24).
Figure 23.
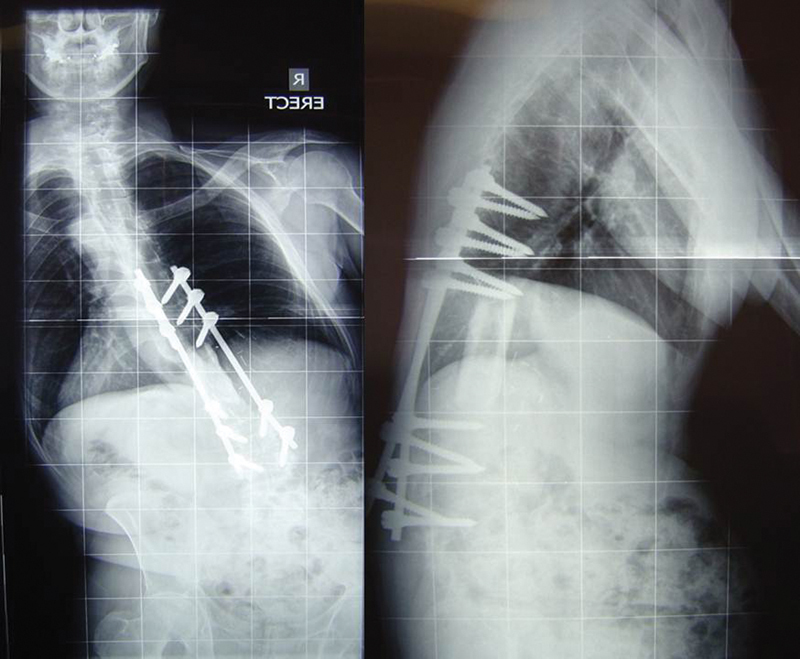
Loss of sagittal and coronal balance 12 months after massive resection of L1 vertebral osteogenic sarcoma. Note that the deformity occurred inferior to the resected/reconstructed segment.
Figure 24.
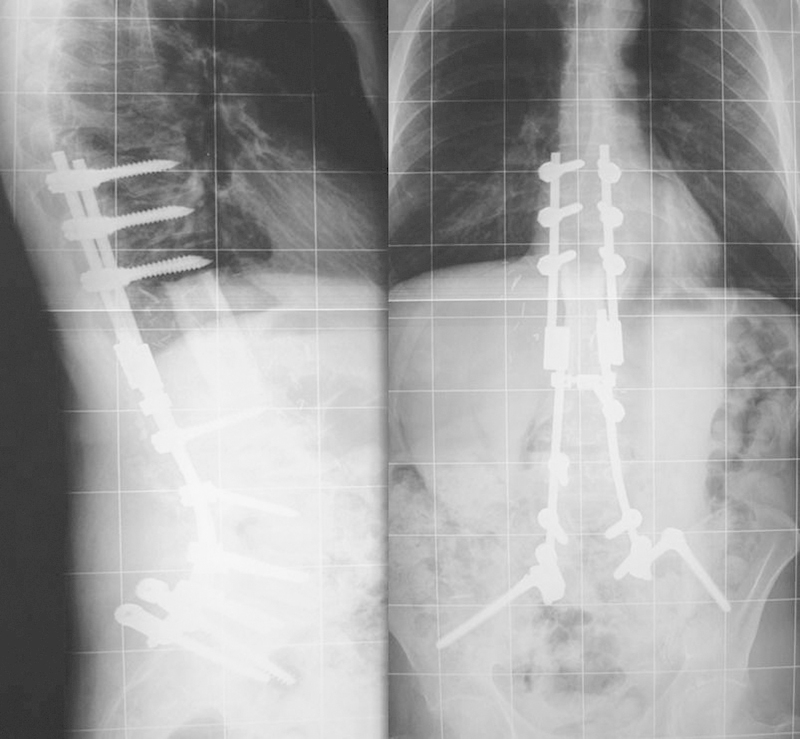
Rebalancing procedure in case 7.
Discussion
The technical examples provided above reflect growing support for the application of Enneking's principles of resection of musculoskeletal neoplasms to primary lesions of the spine as recently championed by Weinstein, Boriani, and Biagini.2 7 The individual cases detailed here represent a series of somewhat unusual anatomic locations or resection problems, and particular strategies were employed on a case-by-case basis. For a comprehensive summary of consensus guidelines in the general management of primary spinal tumors, the reader is referred to the recent Management Recommendations in Spinal Oncology as derived by the Spine Oncology Study Group.45
It has been previously reported that the biological behavior of primary spinal malignancy parallels that of sarcoma throughout the remainder of the musculoskeletal system, with local recurrence almost always associated with rapid demise of the patient.42 In stark contrast, the local recurrence rate after successful en bloc excision may be as low as 0%.6 To maximize survival in these rare and lethal tumors, there is a need for standardization of staging characteristics, outcome measurement, recording of perioperative complications, and survival figures. These aims will ultimately only become achievable by multicentric pooling of data, most probably in registry format.
It should be reinforced that the surgery of primary spinal neoplasms is frequently technically demanding and is also generally reliant on complex multidisciplinary infrastructure. The few cases encountered even in high-volume spinal services suggest that definitive treatment is best undertaken in one specialized tertiary or quaternary referral center per state/province (or even country) where what is often a limited caseload is able to concentrated. In technical terms, the complication rate of these procedures is high and the learning curve steep, with one study quoting an improvement in average operative time from 1132 minutes in the first 20 cases to 943 minutes in the last six cases.6
Despite the daunting prospect of performing extirpative resection, the temptation to undertake intralesional decompressive surgery in the face of presenting neurological compromise with an unknown tumor type must be resisted. When faced with a solitary spinal mass, the treating surgeon must define the histopathology of the lesion and stage its progress as far as possible before embarking on a surgical procedure, which may ultimately render a curable malignancy incurable, and referral might be considered to a quaternary service in spinal tumor care, many of which offer external consultation or case presentation in a multidisciplinary forum.
Footnotes
Disclosures Richard Williams, None Matthew Foote, None Hamish Deverall, None
References
- 1.Kelley S P, Ashford R U, Rao A S, Dickson R A. Primary bone tumours of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J. 2007;16:405–409. doi: 10.1007/s00586-006-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boriani S, Weinstein J N. Philadelphia, PA: Lippincott-Raven; 1997. Differential diagnosis and surgical treatment of primary benign and malignant neoplasms; pp. 951–987. [Google Scholar]
- 3.Karnofsky D A, Burchenal J H. New York, NY: Columbia University Press; 1949. The clinical evaluation of chemotherapeutic agents in cancer; p. 196. [Google Scholar]
- 4.Maddams J, Brewster D, Gavin A. et al. Cancer prevalence in the United Kingdom: estimates for 2008. Br J Cancer. 2009;101:541–547. doi: 10.1038/sj.bjc.6605148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergh P, Gunterberg B, Meis-Kindblom J M, Kindblom L G. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201–1212. doi: 10.1002/1097-0142(20010401)91:7<1201::aid-cncr1120>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Fisher C G, Keynan O, Boyd M C, Dvorak M F. The surgical management of primary tumors of the spine: initial results of an ongoing prospective cohort study. Spine. 2005;30:1899–1908. doi: 10.1097/01.brs.0000174114.90657.74. [DOI] [PubMed] [Google Scholar]
- 7.Boriani S, Biagini R, De Iure F. et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine. 1996;21:1927–1931. doi: 10.1097/00007632-199608150-00020. [DOI] [PubMed] [Google Scholar]
- 8.Fisher C G, Andersson G BJ, Weinstein J N. Spine focus issue. Summary of management recommendations in spine oncology. Spine. 2009;34(22, Suppl):S2–S6. doi: 10.1097/BRS.0b013e3181baae29. [DOI] [PubMed] [Google Scholar]
- 9.Izatt M T, Thorpe P L, Thompson R G. et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J. 2007;16:1507–1518. doi: 10.1007/s00586-006-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahsan F, Inglis T, Allison R, Inglis G S. Cervical chordoma managed with multidisciplinary surgical approach. ANZ J Surg. 2011;81:331–335. doi: 10.1111/j.1445-2197.2010.05575.x. [DOI] [PubMed] [Google Scholar]
- 11.Fisher C G, Saravanja D D, Dvorak M F. et al. Surgical management of primary bone tumors of the spine: validation of an approach to enhance cure and reduce local recurrence. Spine. 2011;36:830–836. doi: 10.1097/BRS.0b013e3181e502e5. [DOI] [PubMed] [Google Scholar]
- 12.Enneking W F, Spanier S S, Goodman M A. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 13.Nakamoto Y, Osman M, Wahl R L. Prevalence and patterns of bone metastases detected with positron emission tomography using F-18 FDG. Clin Nucl Med. 2003;28:302–307. doi: 10.1097/01.RLU.0000057556.54046.7A. [DOI] [PubMed] [Google Scholar]
- 14.Campbell P G, Yadla S, Nasser R, Malone J, Maltenfort M G, Ratliff J K. Patient comorbidity score predicting the incidence of perioperative complications: assessing the impact of comorbidities on complications in spine surgery. J Neurosurg Spine. 2012;16:37–43. doi: 10.3171/2011.9.SPINE11283. [DOI] [PubMed] [Google Scholar]
- 15.Oken M M, Creech R H, Tormey D C. et al. Toxicity and response criteria of the Eastern Cooperative Oncology group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 16.Terezakis S A, Lovelock D M, Bilsky M H, Hunt M A, Zatcky J, Yamada Y. Image-guided intensity-modulated photon radiotherapy using multifractionated regimen to paraspinal chordomas and rare sarcomas. Int J Radiat Oncol Biol Phys. 2007;69:1502–1508. doi: 10.1016/j.ijrobp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Bhide S A, Nutting C M. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilby W, Dooley J R, Kuduvalli G, Sayeh S, Maurer C R Jr. The CyberKnife Robotic Radiosurgery System in 2010. Technol Cancer Res Treat. 2010;9:433–452. doi: 10.1177/153303461000900502. [DOI] [PubMed] [Google Scholar]
- 19.DeLaney T F, Liebsch N J, Pedlow F X. et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–739. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg A K, Wang X S, Shiu A S. et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117:3509–3516. doi: 10.1002/cncr.25918. [DOI] [PubMed] [Google Scholar]
- 21.Harish S, Saifuddin A. Imaging features of spinal osteoid osteoma with emphasis on MRI findings. Eur Radiol. 2005;15:2396–2403. doi: 10.1007/s00330-005-2816-8. [DOI] [PubMed] [Google Scholar]
- 22.Osebold W R, Lester E L, Hurley J H, Vincent R L. Intraoperative use of the mobile gamma camera in localizing and excising osteoid osteomas of the spine. Spine. 1993;18:1816–1828. doi: 10.1097/00007632-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Galibert P, Deramond H, Rosat P, Le Gars D. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty] Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 24.Kawahara N, Tomita K, Baba H, Kobayashi T, Fujita T, Murakami H. Closing-opening wedge osteotomy to correct angular kyphotic deformity by a single posterior approach. Spine. 2001;26:391–402. doi: 10.1097/00007632-200102150-00016. [DOI] [PubMed] [Google Scholar]
- 25.Mendel E, Bourekas E, Gerszten P, Golan J D. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine. 2009;34(22, Suppl):S93–S100. doi: 10.1097/BRS.0b013e3181b77895. [DOI] [PubMed] [Google Scholar]
- 26.Leet A I, Magur E, Lee J S, Wientroub S, Robey P G, Collins M T. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86-A:531–537. [PubMed] [Google Scholar]
- 27.Anders J O, Aurich M, Lang T, Wagner A. Solitary fibrous tumor in the thigh: review of the literature. J Cancer Res Clin Oncol. 2006;132:69–75. doi: 10.1007/s00432-005-0055-7. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto K, Miyamoto K, Hosoe H. et al. Solitary fibrous tumor in the cervical spine with destructive vertebral involvement: a case report and review of the literature. Arch Orthop Trauma Surg. 2008;128:1111–1116. doi: 10.1007/s00402-007-0529-y. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee D, Chaichana K L, Gokaslan Z L, Aaronson O, Cheng J S, McGirt M J. Survival of patients with malignant primary osseous spinal neoplasms: results from the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2003. J Neurosurg Spine. 2011;14:143–150. doi: 10.3171/2010.10.SPINE10189. [DOI] [PubMed] [Google Scholar]
- 30.Moojen W A, Vleggeert-Lankamp C LA, Krol A DG, Dijkstra S PD. Long-term results: adjuvant radiotherapy in en bloc resection of sacrococcygeal chordoma is advisable. Spine. 2011;36:E656–E661. doi: 10.1097/BRS.0b013e3181f8d1f3. [DOI] [PubMed] [Google Scholar]
- 31.Jawad M U, Haleem A A, Scully S P. Malignant sarcoma of the pelvic bones: treatment outcomes and prognostic factors vary by histopathology. Cancer. 2011;117:1529–1541. doi: 10.1002/cncr.25684. [DOI] [PubMed] [Google Scholar]
- 32.Brada M, Pijls-Johannesma M, De Ruysscher D. Proton therapy in clinical practice: current clinical evidence. J Clin Oncol. 2007;25:965–970. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 33.Henry A K. Edinburgh: Churchill Livingstone; 1957. Extensile Exposure. 2nd ed; pp. 58–72. [Google Scholar]
- 34.Bacci G, Boriani S, Balladelli A. et al. Treatment of nonmetastatic Ewing's sarcoma family tumors of the spine and sacrum: the experience from a single institution. Eur Spine J. 2009;18:1091–1095. doi: 10.1007/s00586-009-0921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciubba D M, Okuno S H, Dekutoski M B, Gokaslan Z L. Ewing and osteogenic sarcoma evidence for multidisciplinary management. Spine. 2009;34:S58–S68. doi: 10.1097/BRS.0b013e3181ba6436. [DOI] [PubMed] [Google Scholar]
- 36.Tan G H, Goss B G, Thorpe P J, Williams R P. CT-based classification of long spinal allograft fusion. Eur Spine J. 2007;16:1875–1881. doi: 10.1007/s00586-007-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozaki T, Flege S, Liljenqvist U. et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069–1077. [PubMed] [Google Scholar]
- 38.Schwab J, Gasbarrini A, Bandiera S. et al. Osteosarcoma of the mobile spine. Spine. 2012;37:E381–E386. doi: 10.1097/BRS.0b013e31822fb1a7. [DOI] [PubMed] [Google Scholar]
- 39.Boriani S, Bandiera S, Donthineni R. et al. Morbidity of en bloc resections in the spine. Eur Spine J. 2010;19:231–241. doi: 10.1007/s00586-009-1137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine. 2002;27:1802–1806. doi: 10.1097/00007632-200208150-00021. [DOI] [PubMed] [Google Scholar]
- 41.Druschel C, Disch A C, Melcher I. et al. Surgical management of recurrent thoracolumbar spinal sarcoma with 4-level total en bloc spondylectomy: description of technique and report of two cases. Eur Spine J. 2012;21:1–9. doi: 10.1007/s00586-011-1859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talac R, Yaszemski M J, Currier B L. et al. Relationship between surgical margins and local recurrence in sarcomas of the spine. Clin Orthop Relat Res. 2002;(397):127–132. doi: 10.1097/00003086-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical Strategy for Spinal metastases. Spine. 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 44.Boriani S, Bandiera S, Biagini R. et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31(4):493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 45.Fisher C G, Andersson G B, Weinstein J N. Spine focus issue. Summary of management recommendations in spine oncology. Spine. 2009;34(22 Suppl):S2–S6. doi: 10.1097/BRS.0b013e3181baae29. [DOI] [PubMed] [Google Scholar]


