Abstract
Introduction:
The use of novel oral nicotine delivery devices and compositions for human consumption and for animal research studies has been increasing in the last several years.
Methods:
Studies were undertaken to examine whether the systemic administration of methoxsalen, an inhibitor of human CYP2A6 and mouse CYP2A5, would modulate nicotine pharmacokinetics and pharmacological effects (antinociception in the tail-flick, and hot-plate tests, and hypothermia) in male ICR mouse after acute oral nicotine administration.
Results:
Administration of intra peritoneal (ip) methoxsalen significantly increased nicotine’s Cmax, prolonged the plasma half-life (fourfold decrease) of nicotine, and increased its area under the curve (AUC) compared with ip vehicle treatment. Methoxsalen pretreatment prolonged the duration of nicotine-induced antinociception and hypothermia (15mg/kg, po) for periods up to 6- and 24-hr postnicotine administration, respectively. Additionally, methoxsalen potentiated nicotine-induced antinociception and hypothermia as evidenced by leftward shifts in nicotine’s dose–response curve. Furthermore, this prolongation of nicotine’s effects after methoxsalen was associated with a parallel prolongation of nicotine plasma levels in mice. These data strongly suggest that variation in the rates of nicotine metabolic inactivation substantially alter pharmacological effects of nicotine given orally.
Conclusion:
We have shown that the pharmacological effects of inhibiting nicotine’s metabolism after oral administration in mice are profound. Our results suggest that inhibiting nicotine metabolism can be used to dramatically enhance nicotine’s bioavailability and its resulting pharmacology, which further supports this inhibitory approach for clinical development of an oral nicotine replacement therapy.
INTRODUCTION
Nicotine administration is known to result in several physiological and pharmacological effects and to produce subjective feelings of reward and pleasure in humans and animals. The use of novel oral nicotine delivery devices and compositions for human consumption and for animal research studies has been increasing in the last several years. Orally delivered nicotine products may provide a convenient route of administration allowing the drug to be absorbed from the intestine rather than buccally, as is the case with currently available oral nicotine replacement therapies (NRTs) products (i.e., nicotine gum). Two new NRT products have been proposed for the oral delivery of nicotine: the Straw and nicotine drops (for review, see Buchhalter, Fant, & Henningfield, 2008). The Straw is a single-use drinking straw containing loose nicotine bitartrate particles that are ingested with the first sip of a beverage. Dosing repeatedly with the Straw achieved nicotine plasma levels in humans that were comparable with, or greater than, those of other NRT products (D’Orlando & Fox, 2004). Similarly, nicotine drops appear to facilitate smoking cessation when evaluated in an open-label treatment trial in smokers (Westman, Tomlin, Perkins, & Rose, 2001). A steadily growing number of animal studies report the use of the oral route, generally in drinking water, to administer the nicotine (Rowell, Hurst, Marlowe, & Bennett, 1983). Most of these studies were used to explore various aspects of nicotine pharmacological effects and dependence such as colitis (AlSharari et al., 2012), withdrawal (Gäddnäs, Pietilä, & Ahtee, 2000; Grabus et al., 2005), and self-administration (Collins, Pogun, Nesil, & Kanit, 2012). In general, rodents are exposed to a choice between nicotine-containing solutions and water or to a forced-drinking procedure of nicotine solutions. Interestingly, nicotine oral self-administration in male mice is associated with the amount of nicotine metabolizing enzyme CYP2A5 as well as the rate at which nicotine is metabolized in vitro (Siu, Wildenauer, & Tyndale, 2006).
Oral nicotine is absorbed readily in the stomach and intestine and most, but not all, of this nicotine is subject to metabolism in the liver during the first pass; in humans, only 20%–40% of the nicotine survives metabolism by CYP2A6 in the liver during its entry into the systemic circulation (Compton et al., 1997; Zins et al., 1997). Therefore, compared with other routes of administration, the rate of nicotine getting to the brain is slower and the quantities are lower and more variable. In this study, we investigated the effects of an inhibitor of nicotine metabolism on orally delivered nicotine examining both the altered pharmacokinetics and the resulting effects on nicotine pharmacodynamics in the mouse. We hypothesized that inhibition of mouse CYP2A5, the orthologue of human CYP2A6, should both increase oral nicotine’s peak levels (Cmax) due to a reduction in first-pass effect, and decrease nicotine’s subsequent systemic clearance together substantially enhancing oral nicotine bioavailability. This would allow gastrically tolerable doses of nicotine to be administered, reducing a smoker’s need to consume nicotine via smoking and the concurrent exposure to other constituents of tobacco smoke. In addition, an oral formulation, particularly if the variation in pharmacokinetics was reduced by inhibition of CYP2A6, would likely improve pharmacokinetic variation, bioavailability and compliance with NRT.
The mouse is a better-suited model for the study of nicotine metabolism and behaviors, compared with rats, since the predominant enzyme responsible for the metabolism of nicotine in rats belong to the CYP2B family (Nakayama, Okuda, Nakashima, Imaoka, & Funae, 1993; Schoedel, Sellers, & Tyndale, 2001). In contrast to rats, human CYP2A6 and mouse CYP2A5 (orthologues) are the main enzymes involved in nicotine metabolism (Messina, Tyndale, & Sellers, 1997; Murphy, Raulinaitis, & Brown, 2005); they share close structural (84% amino acid sequence similarity) and functional (CYP2A5 metabolizes nicotine with similar efficiency to human CYP2A6) similarity (Murphy et al., 2005; Siu et al., 2006). In addition a similarly large portion of nicotine is metabolized to cotinine in humans and mice, relative to much less in the rat, making the mouse a good model for the pharmacological effects of manipulating nicotine metabolism in vivo. We have recently reported that methoxsalen, a specific and relatively selective inhibitor of human CYP2A6 (Zhang, Kilicarslan, Tyndale, & Sellers, 2001), inhibited CYP2A5-mediated nicotine metabolism in vitro in the ICR mice (Damaj, Siu, Sellers, Tyndale, & Martin, 2007). Furthermore, the inhibition was potent, as seen in human inhibition studies, with a Ki of 0.32 µM. Accordingly, studies were undertaken to examine whether methoxsalen would modulate acute nicotine pharmacokinetics and pharmacological effects (antinociception and hypothermia) after oral administration in the mouse. In addition, cotinine plasma levels after methoxsalen pretreatment were also measured because it is the major product of nicotine metabolism at CYP2A6/CYP2A5.
MATERIALS AND METHODS
Animals
Male adult ICR mice (20–25g) obtained from Harlan Laboratories were used throughout the study. Animals were housed in an AALAC approved facility in groups of five and had free access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt] and mecamylamine hydrochloride were purchased from Sigma-Aldrich. Methoxsalen was purchased from Sigma Chemical Company. All drugs except for methoxsalen were dissolved in physiological saline (0.9% sodium chloride) and injected at a total volume of 1ml/100g body weight unless noted otherwise. Methoxsalen was dissolved in a mixture of 1:1:18 (1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc, Inc.), and 18 volumes distilled water) and administered intraperitoneal (ip). All doses are expressed as the free base of the drug. Mecamylamine was injected subcutaneously (sc) and nicotine was given orally by gavage.
Nicotine and Cotinine LC/MS/MS Analysis
Specimen Extraction
To a 200 μl aliquot of plasma, 50 μl of internal standard containing 50ng of nicotine-d4 and cotinine-d3 in methanol was added with mixing. Then 100 μl of 5M ammonium hydroxide was added to each sample followed by 2ml of methylene chloride. The samples were mixed for 2min and centrifuged for 5min at 3,000rpm at a temperature of 4 °C. The organic layer was transferred to a clean test tube. The aqueous phase was extracted twice more with 2ml of methylene chloride. The organic phases were combined and 500 μl of 25mM HCl in methanol was added. Samples then were evaporated to dryness under a gentle stream of nitrogen. They were reconstituted with 100 μl of mobile phase and placed in auto-sample (LC/MS/MS) vials for analysis.
Instrumental Analysis
The LC/MS/MS system used was an Applied Bio systems 3200 Qtrap with a turbo V source for TurbolonSpray with a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation was performed using a Hypersil Gold, 3mm × 50mm, 5 micron (Thermo Scientific). The mobile phase contained 10mM ammonium formate; methanol (10:90vol/vol) and was delivered at a flow rate of 0.5ml/min. The acquisition mode used was multiple reaction monitoring in a positive mode. Transition ions monitored for nicotine (163 > 130; 163 > 117), nicotine-d4 (167 > 134), cotinine (177 > 80; 177 > 98), and cotinine-d3 (180 > 80). The total chromatographic separation time for each extract injection was 2min. A calibration curve ranging from 12.5 to 500ng/ml was constructed for each compound based on linear regression using the peak area ratios of the drug to its deuterated internal standard. Cotinine-d3 also was used as the internal standard for 3-hydroxycotinine.
Plasma Nicotine and Cotinine Levels Measurement
To determine plasma nicotine and cotinine levels, blood samples were drawn by cardiac puncture at 5, 15, 30, 45, 60, 120, and 180 and 360min after oral nicotine administration (15mg/kg, po). Animals were pretreated with ip vehicle or methoxsalen 15min before nicotine administration. Immediately, afterward the plasma samples were prepared by centrifugation at 3,000× g for 10min and frozen at –20 °C until analysis. To measure total nicotine and cotinine levels (free and glucuronides), the samples were incubated with β-glucuronidase at a final concentration of 5mg/ml in 0.2M acetate buffer, pH 5.0, at 37 °C overnight. After incubation the samples were processed and analyzed for nicotine and metabolite levels by LC/MS/MS as described above.
Behavioral Tests
Tail-Flick Test
The antinociceptive effect of drugs was assessed by the tail-flick assay. A control response (2- to 4-s latency) was determined for each mouse before treatment, and test latency was determined after drug administration. To minimize tissue damage, a maximum latency of 10 s was imposed. Antinociceptive response was calculated as percentage of maximum possible effect (%MPE), where %MPE = [(test value − control value)/(cutoff (10 s) − control value)] × 100.
Hot-Plate Test
Mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus) as a measure of antinociception. The hot plate was a rectangular heated surface surrounded by Plexiglas and maintained at 55 °C. The device was connected to a manually operated timer that recorded the amount of time the mouse spent on the heated surface before showing signs of nociception (e.g., jumping and paw licks). Two control latencies at least 10min apart were determined for each mouse. The normal latency (reaction time) of 8–12 s was assessed with a saline injection. To avoid tissue damage, the hot plate automatically disengaged after 40 s. Groups of 8–12 mice were used for each dose and treatment condition. Antinociceptive response was calculated as %MPE, where %MPE = [(test value − control)/ (cutoff time (40 s) − control) × 100]. The reaction time was scored when the animal jumped or licked its paws.
Body Temperature
Rectal temperature was measured by a thermistor probe (inserted 24mm) and digital thermometer (Yellow Springs Instrument Co.). Readings were taken just before and at 5min, 0.5, 1, 2, 4, 6, and 24hr after oral nicotine administration. The difference in rectal temperature before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21 to 24 °C from day to day.
Nicotine time-course evaluation was conducted in mice (n = 6–8) pretreated with vehicle or methoxsalen (15mg/kg, ip) and 15min later they were given nicotine (15mg/kg, po). Mice were then evaluated for antinociception and hypothermia at 5min, 0.5, 1, 2, 4, 6, and 24hr after oral nicotine administration. In a separate group of animals, nicotine dose–response curves were evaluated in mice after pretreatment with vehicle or methoxsalen (15mg/kg, ip). Mice were challenged 15min later with different doses of nicotine (po) and evaluated for antinociception in the tail-flick and hot-plate tests (5min after nicotine for vehicle-treated group and 30min for methoxsalen-treated group) and hypothermia (30min after nicotine- or vehicle-treated group and 120min for methoxsalen-treated group). Groups of six to eight animals were used for each dose and each treatment. Finally, to determine whether effects of methoxsalen on nicotine are mediated by nicotinic receptors, animals were pretreated with either saline or mecamylamine (2mg/kg, sc) followed by methoxsalen (15mg/kg, ip). Thirty minute later, mice received nicotine at a dose of 15mg/kg (po) and were then tested at 5 and 30min after injection for antinociception and hypothermia, respectively.
Statistical Analysis
Assessment of in vivo nicotine and cotinine plasma levels for the entire time course from individual animals was not possible due to limited blood volume; therefore, each timepoint represents data from four to six 6 individual mice. Due to this restriction to the experimental design, statistical parameters (i.e., half-life) were estimated by resampling methods using the PKR and Test software (H. L. Kaplan). Statistical analysis of all analgesic and behavioral studies was performed using either t test or analysis of variance (ANOVA) with Tukey’s test post hoc test when appropriate. All differences were considered significant if at p < .05. ED50 values with 95% CL for behavioral data were calculated by unweighted least-squares linear regression as described by Tallarida and Murray (1987).
RESULTS
Effects of Methoxsalen on Nicotine and Cotinine Plasma Levels After In Vivo Treatment
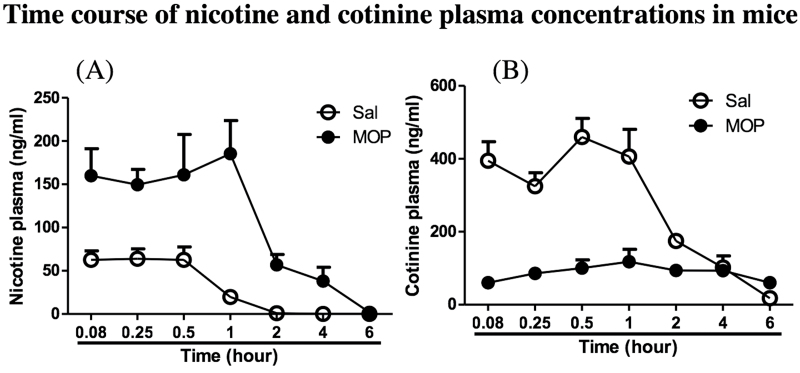
We tested the effect of methoxsalen (15mg/kg, ip) on in vivo plasma nicotine and cotinine levels after oral administration of nicotine at 15mg/kg in mice pretreated with vehicle. Intra peritoneal injection of methoxsalen significantly increased nicotine’s Cmax, prolonged the plasma nicotine half-life (fourfold increase), decreased its clearance (sixfold decrease), and increased its AUC compared with vehicle treatment (Figure 1A and Table 1A). The plasma levels of the primary CYP2A5-mediated metabolite of nicotine, cotinine, were also reduced as a result of inhibition by methoxsalen (Figure 1B). Indeed, injection of methoxsalen significantly decreased cotinine’s Cmax (fourfold decrease), decreased its AUC (2.5-fold decrease), prolonged its plasma half-life, and increased its clearance (2.5-fold increase) compared with vehicle control (Figure 1B and Table 1B).
Figure 1.
Time course of nicotine (A) and cotinine (B) plasma concentration in mice pretreated with methoxsalen. Nicotine was administered orally (15mg/kg) 15min after pretreatment with ip ( ) vehicle or (
) vehicle or ( ) methoxsalen. Each timepoint represents the mean ± SEM of four to six animals. For the vehicle pretreatment, all values for nicotine plasma levels at 4 and 6hr were below the limits of detection.
) methoxsalen. Each timepoint represents the mean ± SEM of four to six animals. For the vehicle pretreatment, all values for nicotine plasma levels at 4 and 6hr were below the limits of detection.
Table 1.
Pharmacokinetic Parameters of (A) Oral Nicotine and (B) Cotinine in Adult Male ICR Mouse After Pretreatment With <ethoxsalen
| Cmax (ng/ml) | AUC (ng · min/ml) | T1/2 (min) | CL/F (ml/min) | |
|---|---|---|---|---|
| A | ||||
| Saline/nicotine | 64±12.6 | 60.8±24.7 | 26.5±9.6 | 102.8±3.5 |
| Methoxsalen/nicotine | 185±10.5a | 373.7±24.7a | 105.8±21.3a | 16.7±3.3a |
| B | ||||
| Saline/nicotine | 460±43.8 | 957±55 | 117.3±39 | 6.5±0.8 |
| Methoxsalen/nicotine | 118±40a | 385±55a | >1,050 | 16.2±0.8a |
Note. AUC = area under the curve; CL = confidence level. Values are shown as mean ± SD (n = 4–6 per group).
a p < .05 compared with saline/nicotine group.
Effects of Methoxsalen on Nicotine Acute Pharmacological Effects (Antinociception and Hypothermia): Time-Course, Potency, and Blockade Studies
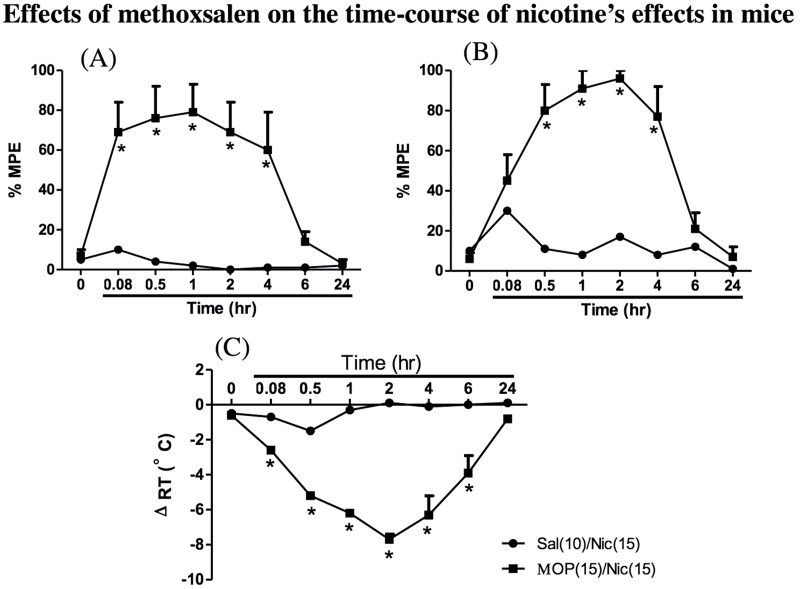
Methoxsalen (15mg/kg) given ip was evaluated for its ability to enhance nicotine-induced antinociception (tail-flick and hot-plate procedures) and hypothermia after oral administration (15mg/kg) of the drug. We first examined the impact of methoxsalen on the time course of nicotine pharmacological effects. Mice were given methoxsalen and 15min later they received nicotine and then tested at different times for antinociception and hypothermia responses. At this dose, no significant effect of nicotine (15mg/kg, po) alone was observed in the tail-flick (Figure 2A) and hot-plate (Figure 2B) tests at any of the timepoints tested. Additionally, no significant change was observed in body temperature after nicotine oral administration (Figure 2C). When animals were pretreated with methoxsalen (15mg/kg, ip), the effects of nicotine in both analgesic tests were significantly prolonged; nicotine-induced antinociception did not disappear completely till 6hr after nicotine administration in mice (Figure 2A and B). On the other hand, the effects of nicotine on body temperature were still significant until the 24-hr timepoint (Figure 2C). Interestingly, the plasma nicotine concentrations achieved during the first 30min in the vehicle-treated group (Figure 1) are comparable or even larger than the nicotine concentrations at 2–4hr when mice are pretreated with methoxysalen, whereas the efficacy differences in the three pharmacological measures between these groups are quite pronounced especially nicotine-induced hypothermia (Figure 2C).
Figure 2.
Effects of methoxsalen on the time course of nicotine’s effects in (A) the tail-flick test, (B) the hot-plate assay, and (C) body temperature in mice. Animals were pretreated with either methoxsalen (15mg/kg, ip) (closed square) or vehicle (closed circles) and 15min later received oral nicotine at a dose of 15mg/kg. Mice were tested at different times after injection. Each point represents the mean ± SE of 8–12 mice. *p < .05 compared with control.
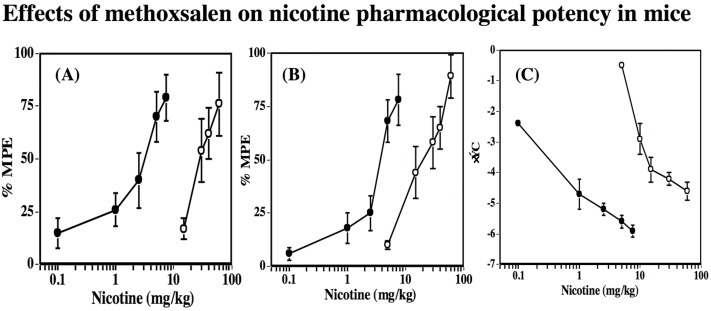
Based on the nicotine time-course results, subsequent studies examining the effects of methoxsalen on nicotine pharmacological potency were conducted. Mice pretreated with methoxsalen (15mg/kg, ip) were challenged 15min later with different doses of nicotine (po) and evaluated for antinociception in the tail-flick and hot-plate tests (5min after nicotine for vehicle-treated group and 30min for methoxsalen-treated group) and hypothermia (30min after nicotine for vehicle-treated group and 120min for methoxsalen-treated group). Nicotine produced a dose–responsive increase in the tail-flick latency with an ED50 (±CL) of 29.5 (22–39.5) mg/kg as can be seen in Figure 3A and Table 2. Methoxsalen pretreatment potentiated nicotine-induced antinociception in the tail-flick test as evidenced by leftward shifts in nicotine’s dose–response curve and the ED50 value of nicotine was shifted nearly 14-fold (ED50 = 2.1 [1.0–4.8] mg/kg). Similarly, methoxsalen pretreatment potentiated nicotine-induced antinociception in the hot-plate test and shifted nicotine dose–response curve to the left (Figure 3B) with a sevenfold decrease in the ED50 value of nicotine (ED50 = 21 [16–29] vs. 3.0 [1.4–6.4] mg/kg) (Table 2). Furthermore, as shown in Figure 3C, the magnitude of potentiation of nicotine-induced hypothermia was the largest with an 88-fold decrease in the ED50 value of nicotine (ED50 = 15 [12–21] vs. 0.17 [0.1–0.6] mg/kg) (Table 2). However, the range of in potency shifts across the three pharmacological measures (7–88-fold shifts) is more variable than the observed increase in plasma levels in nicotine after methoxsalen pretreatment (Figure 1).
Figure 3.
Effects of methoxsalen on nicotine-induced antinociception and hypothermia dose–response curves after po administration in mice. Vehicle ( ) or methoxsalen (15mg/kg, ip) (
) or methoxsalen (15mg/kg, ip) ( ) was administered ip. 15min before various doses of oral nicotine (0.5, 1, 2.5, 5, 10, 15, 20, 30, and 50mg/kg) and mice were tested in (A) the tail-flick test, (B) the hot-plate test, and (C) hypothermia. Each point represents the mean ± SE of 8–12 mice.
) was administered ip. 15min before various doses of oral nicotine (0.5, 1, 2.5, 5, 10, 15, 20, 30, and 50mg/kg) and mice were tested in (A) the tail-flick test, (B) the hot-plate test, and (C) hypothermia. Each point represents the mean ± SE of 8–12 mice.
Table 2.
Effects of Methoxsalen on Nicotine Potency in the Tail-Flick Test, Hot-Plate Test, and Body Temperature After Oral Administration in Mice
| Pharmacological response | Saline (ED50 mg/kg ± CL) | 8-MOP (ED50 mg/kg ± CL) | Potency ratio |
|---|---|---|---|
| Tail flick | 29.5 (22–39.5) | 2.1 (1.0–4.8) | 14 |
| Hot plate | 21 (16–29) | 3.0 (1.4–6.4) | 7 |
| Body temperature | 15 (12–21) | 0.17 (0.1–0.6) | 88 |
Note. CL = confidence level; MOP = methoxsalen at 15mg/kg, ip. ED50 values (± 95% CL) were calculated from the dose–response and expressed as mg/kg. Each dose group included six to eight animals.
Finally, the antinociceptive and hypothermic effects of methoxsalen/nicotine combination were significantly blocked by a pretreatment with mecamylamine, a noncompetitive nicotinic antagonist, at 2mg/kg (sc) (Table 3).
Table 3.
Blockade of Methoxsalen’s Effects on Nicotine Acute Pharmacological Responses After Oral Administration in Mice
| Treatment (mg/kg) | Tail-flick test % MPE | Hot-plate test % MPE | Hypothermia (∆˚C) (mean ± SEM) |
|---|---|---|---|
| Saline/vehicle/saline | 6±2 | 7±3 | −0.1±0.3 |
| Saline/methoxsalen (15)/saline | 8±3 | 11±6 | −1.5±0.3* |
| Meca (2)/methoxsalen (15)/saline | 7±5 | 12±7 | −1.2±0.6 |
| Meca (2)/vehicle/saline | 8±3 | 6±4 | −0.5±0.3 |
| Meca (2)/vehicle /nicotine (15) | 1±1 | 6±3 | −0.5±0.1 |
| Saline/vehicle /nicotine (15) | 5±1 | 10±5 | −1.6±0.2* |
| Saline/methoxsalen (15)/nicotine (2.5) | 87±9*,# | 65±8*,# | −5.8±0.2*,# |
| Meca (2)/methoxsalen (15)/nicotine (2.5) | 7±3 | 14±6 | −1.9±0.3* |
Note. Animals were pretreated with either saline or mecamylamine (2mg/kg, sc) followed by methoxsalen (15mg/kg, ip). Thirty minute later, mice received nicotine at a dose of 15mg/kg (po) and were then tested at 5 and 30min after injection for antinociception and hypothermia, respectively. Methoxsalen (15) = methoxsalen at 15mg/kg, ip; meca (2) = mecamylamine at 2mg/kg, sc; nicotine (15) = nicotine at 15mg/kg, po. Each point represents the mean ± SE of 8–12 mice.
*p < .05 compared with Saline/Vehicle/Saline.
# p < .05 compared with Saline/methoxsalen (15)/Saline.
DISCUSSION
This study was undertaken to examine whether methoxsalen, an inhibitor of human CYP2A6, would modulate acute nicotine pharmacokinetics and pharmacological effects (antinociception and hypothermia) after oral administration in the ICR mouse. Our results demonstrated that administration of methoxsalen significantly augmented nicotine’s Cmax, prolonged the plasma half-life resulting in an increase in nicotine AUC compared with vehicle treatment. Methoxsalen also enhanced the potency of pharmacological effects of oral nicotine (i.e., body temperature and analgesia). In addition, it prolonged the duration of nicotine-induced antinociception and hypothermia for periods up to 24-hr postnicotine administration. Furthermore, this prolongation in effects of nicotine after methoxsalen was associated with a parallel prolongation of nicotine plasma levels in mice. These data strongly suggest that variation in the rates of nicotine metabolic inactivation substantially alter pharmacological effects of nicotine when nicotine is given orally.
As predicted, the inhibition by methoxsalen of nicotine’s first-pass metabolism and subsequent clearance had a significant impact on nicotine pharmacokinetics. Methoxsalen pretreatment tripled the Cmax of nicotine, increased its elimination half-life by fourfold, and increased its AUC by more than sixfold (Table 1). Consistent with the route of delivery, this is a much larger impact on nicotine pharmacokinetics than previously seen when nicotine was given by sc administration (Damaj et al., 2007). Following the sc route, the inhibitor did not alter nicotine’s Cmax, but it did double its elimination half-life resulting in the smaller, relative to its effects on oral nicotine, doubling of its AUC (Damaj et al., 2007). This is not surprising since contrary to oral nicotine administration, the sc route by-passes the first-pass metabolism effect. The inhibition of oral nicotine metabolism by methoxsalen in the ICR mice is consistent with its ability to inhibit CYP2A5-mediated nicotine metabolism in vitro in liver microsomes (Damaj et al., 2007).
Our results confirmed that methoxsalen did indeed inhibit the conversion of nicotine to cotinine in vivo. Pretreatment with methoxsalen increased the AUC sixfolds and prolonged the nicotine plasma levels. These levels remained above 10ng/ml in the vehicle pretreatment for approximately 1hr, but with pretreatment with methoxsalen the nicotine plasma levels were still above 10ng/ml at 6hr. Similar to the marked effects of CYP2A5 inhibition on nicotine metabolism, there were dramatic enhancement in the pharmacological effects of nicotine (body temperature and analgesia) after methoxsalen treatment (Table 2). Furthermore, the duration of methoxsalen-induced potentiation of nicotine’s antinociceptive and hypothermic lasted close to 6 and 24hr, respectively, after drug pretreatment. These results showed that a mismatch do exist between the plasma concentrations of nicotine (in particular after the time point of 2hr) and pharmacological effects of the drugs after methoxsalen pretreatment. This mismatch was particularly evident in the hypothermia measure (Figure 2C). While many factors could underlie the fact that nicotine plasma concentration was not a good indicator of pharmacological responses after methoxsalen, it is likely related to a mismatch between the plasma and tissue concentrations of the drug leading to its “delayed” tissue distribution. Furthermore, this prolongation in effects of nicotine after methoxsalen was blocked by pretreatment with mecamylamine, a nonselective nicotinic antagonist (Table 3), indicating the mediation of nicotine’s effects by nicotinic receptors (and not by an off-target effect of methoxsalen).
Although the study of nicotine’s acute effects is important to the understanding of nicotine dependence, one must also consider responses after chronic exposure of the drug in animals. Animal models studying the different aspects of dependence such as tolerance, withdrawal, and reward involve a chronic/repeated exposure of the animals to nicotine. Assessing the impact of nicotine metabolism in these behavioral models will enhance our understanding of the molecular mechanisms involved in nicotine dependence and will facilitate the development of new strategies for smoking cessation therapies.
These data strongly suggest that variation in the rates of nicotine metabolic inactivation substantially alter pharmacological effects of nicotine when nicotine is given orally. For example, in mice with very rapid rates of nicotine metabolism, small differences in levels or function of CYP2A5 between different mouse strains may result in substantially differing nicotine pharmacological effects. Thus, pharmacokinetic differences likely contribute to differences in effects seen between studies employing the use of different mouse strains (Locklear, McDonald, Smith, & Fryxell, 2012; Wilking et al., 2012).
Similarly, changes in the rate of nicotine metabolism could have some implications on the use of nicotine for smoking cessation. Oral nicotine replacement is not routinely used in treatment because after oral ingestion less than 30% of the nicotine survives metabolism by the liver, during its entry into the systemic circulation (Benowitz, Jacob, Denaro, & Jenkins, 1991; Benowitz, Zevin, & Jacob, 1997; Svensson, 1987; Zins et al., 1997). Doses of nicotine higher than 4mg cannot be given because of nausea and diarrhea due to irritation of the gastrointestinal tract (Henningfield & Keenan, 1993). Therefore, because inhibition of CYP2A6 should facilitate oral low dose nicotine’s entry into and persistence in the systemic circulation, as well as delaying the clearance of inhaled nicotine, it should allow gastrically tolerable doses of nicotine to reduce a smoker’s intake of smoked nicotine and exposure to other constituents of tobacco smoke. Furthermore, there is substantial interethnic variation in nicotine C-oxidation primarily due to variation in the frequencies of CYP2A6 alleles; loss/reduce of function CYP2A6 alleles are more prevalent in Asians and Africans than amog Caucasians (Ho et al., 2009; Malaiyandi, Sellers, & Tyndale, 2005; Mwenifumbo & Tyndale, 2009). This is consistent with Asian Americans and African Americans metabolizing nicotine and cotinine more slowly than Caucasians (Benowitz et al., 2002). Within any of these ethnic groups, reduction in CYP2A6-mediated nicotine metabolism results in an altered smoking behaviors including lower smoking levels and greater rates of cessation (reviewed in Ray et al., 2009). However most people in these ethnic groups are not full poor metabolizers, with both alleles being fully null, rather they range from slow to intermediate, to normal, to fast nicotine metabolizers (Ho et al., 2009; Malaiyandi et al., 2005; Mwenifumbo & Tyndale, 2009). Thus, as people with the slowest rates of nicotine metabolism have the best quit rates in both placebo and nicotine replacement therapies (Ho et al., 2009; Lerman et al., 2006, 2010; Patterson et al., 2008; Ray et al., 2009), it is possible that slowing nicotine metabolism using inhibitors, along with nicotine, will combine the two effects observed in placebo and NRT treatment arms to increase the smoking cessation rates (Sellers et al., 2000; Tyndale et al., 1999).
In conclusion, we have shown that the pharmacological effects of inhibiting nicotine’s metabolism after oral administration are profound, suggesting the differences in resulting effects of nicotine that will occur between individuals and also between animal strains that differ in levels of CYP2A-mediated nicotine metabolism. Finally, our results suggest that inhibiting nicotine metabolism can be used to dramatically enhance nicotine’s bioavailability and its resulting pharmacology, which further supports the approach for clinical development of an oral NRT. The development or identification of potent, selective, and safe CYP2A6 inhibitors could be of great therapeutic utility for the treatment of nicotine dependence. Since the CYP2A6 gene is polymorphic, it is suggested that the inhibition of the enzyme will not have adverse effects ((Fernandez-Salguero et al., 1995). In addition, because very few therapeutically used drugs are metabolized by CYP2A6, the risk of clinically important drug interactions is also low (Ono et al., 1996).
FUNDING
This work was supported by National Institute on Drug Abuse (DA-05274 to MID; DA020830 to RFT); the Endowed Chair in Addiction for the Department of Psychiatry (RFT); Canadian Institutes of Health Research (MOP86471 and TMH109787); Centre for Addiction and Mental Health and the Centre for Addiction and Mental Health Foundation; the Canada Foundation for Innovation (20289 and 16014) and the Ontario Ministry of Research and Innovation.
DECLARATION OF INTERESTS
RFT has participated in one-day advisory meetings for Novartis and McNeil. SDA, ECKS, and MID declare no interest of conflict.
ACKNOWLEDGMENT
The authors greatly appreciate the technical assistance of Tie Han.
REFERENCES
- AlSharari S. D., Akbarali H. I., Abdullah R. A., Shahab O., Auttachoat W., Ferreira G. A., Damaj M. I. (2012). Novel insights on the effect of nicotine in a murine colitis model. Journal Pharmacology Experimental Therapeutics, 344, 207–217.10.1124/jpet.112.198796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P, III, Denaro C., Jenkins R. (1991). Stable isotope studies of nicotine kinetics and bioavailability. Clinical Pharmacology Therapeutics, 49, 270–277.10.1038/clpt.1991.28 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Pérez-Stable E. J., Herrera B., Jacob P., III (2002). Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. Journal National Cancer Institute, 94, 108–115 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Zevin S., Jacob P., III (1997). Sources of variability in nicotine and cotinine levels with use of nicotine nasal spray, transdermal nicotine, and cigarette smoking. British Journal Clinical Pharmacology, 43, 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter A. R., Fant R. V., Henningfield J. E. (2008). Novel pharmacological approaches for treating tobacco dependence and withdrawal: Current status. Drugs, 68, 1067–1088 [DOI] [PubMed] [Google Scholar]
- Collins A. C., Pogun S., Nesil T., Kanit L. (2012). Oral nicotine self-administration in rodents. Journal Addiction Research Therapy, S2.pii: 004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton R. F., Sandborn W. J., Lawson G. M., Sheets A. J., Mays D. C., Zins B. J., Green J. (1997). A dose-ranging pharmacokinetic study of nicotine tartrate following single-dose delayed-release oral and intravenous administration. Alimentary Pharmacology Therapeutics, 11, 865–874.10.1046/j.1365-2036.1997.00236.x [DOI] [PubMed] [Google Scholar]
- Damaj M. I., Siu E. C., Sellers E. M., Tyndale R. F., Martin B. R. (2007). Inhibition of nicotine metabolism by methoxysalen: Pharmacokinetic and pharmacological studies in mice. Journal Pharmacology Experimental Therapeutics, 320, 250–257.10.1124/jpet.106.111237 [DOI] [PubMed] [Google Scholar]
- D’Orlando K. J., Fox B. S. (2004). Tolerability and pharmacokinetics of single and repeated doses of nicotine with The Straw, a novel nicotine replacement product. Nicotine Tobacco Research, 6, 63–70.10.1080/14622200310001656876 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P., Hoffman S. M., Cholerton S., Mohrenweiser H., Raunio H., Rauto A. (1995). A genetic polymorphism in coumarin 7-hydroxylation: Sequence of the human CYP2A genes and identification of variation CYP2A6 alleles. American Journal of Human Genetics, 57, 651–660 [PMC free article] [PubMed] [Google Scholar]
- Gäddnäs H., Pietilä K., Ahtee L. (2000). Effects of chronic oral nicotine treatment and its withdrawal on locomotor activity and brain monoamines in mice. Behavioral Brain Research, 113, 65–72 [DOI] [PubMed] [Google Scholar]
- Grabus S. D., Martin B. R., Batman A. M., Tyndale R. F., Sellers E., Damaj M.I. (2005). Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl), 178, 183–192.org/10.1007/s00213-004-2007-3 [DOI] [PubMed] [Google Scholar]
- Henningfield J. E., Keenan R. M. (1993). Nicotine delivery kinetics and abuse liability. Journal Consulting Clinical Psychology, 61, 743–750 [DOI] [PubMed] [Google Scholar]
- Ho M. K., Mwenifumbo J. C., Al Koudsi N., Okuyemi K. S., Ahluwalia J. S., Benowitz N. L., Tyndale R. F. (2009). Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clinical Pharmacology and Therapeutics, 85, 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C., Jepson C., Wileyto E. P., Patterson F., Schnoll R., Mroziewicz M., Benowitz N., Tyndale R. F. (2010). Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clinical Pharmacology and Therapeutics, 87, 553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C., Tyndale R. F., Patterson F., Wileyto E. P., Pinto A., Benowitz N. (2006). Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical Pharmacology and Therapeutics, 79, 600–608 [DOI] [PubMed] [Google Scholar]
- Locklear L. L., McDonald C. G., Smith R. F., Fryxell K. J. (2012). Adult mice voluntarily progress to nicotine dependence in an oral self-selection assay. Neuropharmacology, 63, 582–592.10.1016/j.neuropharm.2012.04.037 [DOI] [PubMed] [Google Scholar]
- Malaiyandi V., Sellers E. M., Tyndale R. F. (2005). Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clinical Pharmacology Therapeutics, 77, 145–158 [DOI] [PubMed] [Google Scholar]
- Messina E. S., Tyndale R. F., Sellers E. M. (1997). A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. Journal Pharmacology Experimental Therapeutics, 282, 1608–1614 [PubMed] [Google Scholar]
- Murphy S. E., Raulinaitis V., Brown K. M. (2005). Nicotine 5′-oxidation and methyl oxidation by P450 2A enzymes. Drug Metabolism Disposition, 33, 1166–1173.10.1124/dmd.105.004549 [DOI] [PubMed] [Google Scholar]
- Mwenifumbo J. C., Tyndale R. F. (2009). Molecular genetics of nicotine metabolism. Handbook Experimental Pharmacology, 192, 235–259 [DOI] [PubMed] [Google Scholar]
- Nakayama H., Okuda H., Nakashima T., Imaoka S., Funae Y. (1993). Nicotine metabolism by rat hepatic cytochrome P450s. Biochemical Pharmacology, 45, 2554–2556.10.1016/0006-2952(93)90238-R [DOI] [PubMed] [Google Scholar]
- Ono S., Hatanaka T., Hotta H., Satoh T., Gonzalez F. J., Tsutsui M. (1996). Specificity of substrate and inhibitor probes for cytochrome P450s: Evaluation of in vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica, 26, 681–693 [DOI] [PubMed] [Google Scholar]
- Patterson F., Schnoll R. A., Wileyto E. P., Pinto A, Epstein L. H., Shields P. G., Hawk L. W., Tyndale R. F., Benowitz N., Lerman C. (2008). Toward personalized therapy for smoking cessation: A randomized placebo-controlled trial of bupropion. Clinical Pharmacology and Therapeutics, 84, 320–325 [DOI] [PubMed] [Google Scholar]
- Ray R., Tyndale R. F., Lerman C. (2009). Nicotine dependence pharmacogenetics: Role of genetic variation in nicotine metabolizing enzymes. Journal of Neurogenetics, 23, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell P. P., Hurst H. E., Marlowe C., Bennett B. D. (1983). Oral administration of nicotine: Its uptake and distribution after chronic administration to mice. Journal of Pharmacological Methods, 9, 249–261 [DOI] [PubMed] [Google Scholar]
- Schoedel K. A., Sellers E. M., Tyndale R.F. (2001). Induction of CYP2B1/2 and nicotine metabolism by ethanol in rat liver but not rat brain. Biochemical Pharmacology, 62, 1025–1036.10.1016/S0006-2952(01)00744-4 [DOI] [PubMed] [Google Scholar]
- Sellers E. M., Kaplan H. L., Tyndale R.F. (2000). Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clinical Pharmacology Therapeutics, 68, 35–43.10.1067/mcp.2000.107651 [DOI] [PubMed] [Google Scholar]
- Siu E. C., Wildenauer D. B., Tyndale R. F. (2006). Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology (Berl), 184, 401–408.10.1007/s00213-006-0306-6 [DOI] [PubMed] [Google Scholar]
- Svensson C. K. (1987). Clinical pharmacokinetics of nicotine. Clinical Pharmacokinetics, 12, 30–40 [DOI] [PubMed] [Google Scholar]
- Tyndale R. F., Li Y., Kaplan H. L., Seller E. M. (1999). Inhibition of nicotine’s metabolism: A potential new treatment for tobacco dependence. Clinical Pharmacology Therapeutics, 65, 145.10.1016/S0009-9236(99)80112-X [Google Scholar]
- Tallarida R.J., Murray R.B. (1987). Manual of pharmacological calculations with computer programs. New York: Springer-Verlag; [Google Scholar]
- Westman E. C., Tomlin K. F., Perkins C. E., Rose J.E. (2001). Oral nicotine solution for smoking cessation: A pilot tolerability study. Nicotine Tobacco Research, 3, 391–396.10.1080/14622200126973 [DOI] [PubMed] [Google Scholar]
- Wilking J. A., Hesterberg K. G., Nguyen V. H., Cyboron A.P., Hua A.Y., Stitzel J.A. (2012). Comparison of nicotine oral consumption and baseline anxiety measures in adolescent and adult C57BL/6J and C3H/Ibg mice. Behavioral Brain Research, 233, 280–287.10.1016/j.bbr.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Kilicarslan T., Tyndale R. F., Sellers E. M. (2001). Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metabolism Disposition, 29, 897–902 http://dmd.aspetjournals.org/content/29/6/897 [PubMed] [Google Scholar]
- Zins B. J., Sandborn W. J., Mays D. C., Lawson G. M., McKinney J. A., Tremaine W. J., Lipsky J. J. (1997). Pharmacokinetics of nicotine tartrate after single-dose liquid enema, oral, and intravenous administration. Journal Clinical Pharmacology, 7, 426–436 Retrieved from www.ncbi.nlm.nih.gov/pubmed/9156375 [DOI] [PubMed] [Google Scholar]