Abstract
Introduction:
Thirdhand tobacco smoke consists of substances remaining on the surfaces or in the dust of areas where people have smoked. While previous studies have demonstrated the presence of nicotine and various other constituents of tobacco smoke on surfaces in smokers’ homes, none has investigated the presence of tobacco-specific carcinogens.
Methods:
We used liquid chromatography-tandem mass spectrometry to analyze surface dust samples from both the homes of smokers and nonsmokers for the powerful tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Results:
We positively identified NNK on surfaces in 33 of 37 smokers’ homes (700±788 pg/100cm2 [range, not detected–3,500 pg/100cm2]), but only in 3 of 19 nonsmokers’ homes (235±176 pg/100cm2 in the homes where NNK was detected [range, not detected–435 pg/100cm2]). The differences in occurrence and levels of NNK in the homes of smokers and nonsmokers were significant (p < .0001).
Conclusions:
The powerful tobacco-specific lung carcinogen NNK is present on surfaces in most homes occupied by smokers. Potential renters or buyers of apartments or homes should be notified if previous residents were smokers in order to avoid unnecessary exposure of their families to a potent lung carcinogen.
INTRODUCTION
Lung cancer, a deadly disease generally detected too late for successful therapy, kills more than 150,000 people in the United States each year, and more than 1.3 million people worldwide (Siegel, Naishadham, & Jemal, 2012; World Health Organization, 2012). Without question, cigarette smoking is the main cause of this appalling death toll, as clearly demonstrated by hundreds of epidemiology studies carried out over the past 60 years (International Agency for Research on Cancer, 2004). Secondhand cigarette smoke, consisting mainly of sidestream smoke and exhaled smoke, contains all of the same carcinogens to which cigarette smokers are exposed. Secondhand smoke is also an accepted cause of lung cancer in nonsmokers (International Agency for Research on Cancer, 2004; U.S. Department of Health and Human Services, 2006). The risk for lung cancer in a nonsmoker exposed to secondhand smoke is, however, far less than that of a smoker because the carcinogen dose is generally less than 10% of that experienced by a smoker. Thirdhand tobacco smoke (also known as residual or aged tobacco smoke) consists of material remaining on surfaces and in dust in rooms or other areas where smoking has taken place. As detailed in a recent historical review by Burton (2011), thirdhand smoke has been defined with a “three Rs” description: aged tobacco smoke pollutants that remain on surfaces and in dust after tobacco has been smoked, are re-emitted back into the gas phase, or react with oxidants and other compounds in the environment to yield secondary pollutants (Burton, 2011). Despite a lack of human health studies on the long-term health effects of thirdhand smoke exposure, recent evidence recognizes residual tobacco smoke as a potential source of carcinogen and toxicant exposure (Matt et al., 2011; Sleiman et al., 2010; Winickoff et al., 2009). However, there are limited data in the literature on carcinogen and toxicant levels in rooms where cigarette smoking has taken place. Constituents of cigarette smoke reported to be present in thirdhand smoke in real-world settings include nicotine, 3-ethenylpyridine, and various polycyclic aromatic hydrocarbons (PAHs) (Hoh et al., 2012; Matt et al., 2011).
In this study, we investigated the presence of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; Figure 1) on surfaces in homes of smokers and nonsmokers. NNK is present in the mainstream and sidestream smoke of all tobacco products (International Agency for Research on Cancer, 2004, 2007). Mainstream smoke levels typically range from 10 to 200ng per cigarette, while amounts in sidestream smoke are from 50 to 100ng per cigarette (International Agency for Research on Cancer, 2004, 2007; Hecht, 2012). NNK is a potent lung carcinogen in laboratory animals, inducing mainly adenocarcinoma of the lung in all species tested independent of the route of administration (Hecht, 1998). In studies with F-344 rats, multiple low doses of NNK (1 ppm in the drinking water or 0.1mg/kg by subcutaneous injection) have produced significant incidences of lung tumors (Belinsky, Foley, White, Anderson, & Maronpot, 1990; Rivenson, Hoffmann, Prokopczyk, Amin, & Hecht, 1988). Of note, with respect to this study, lung tumors also have been produced in mice treated by application of NNK to the skin (LaVoie et al., 1987). While previous studies in model systems have demonstrated that reaction of surface-bound nicotine with gaseous nitrous acid can produce tobacco-specific nitrosamines including NNK (Sleiman et al., 2010), we are aware of no reports in the literature of NNK on surfaces in homes.
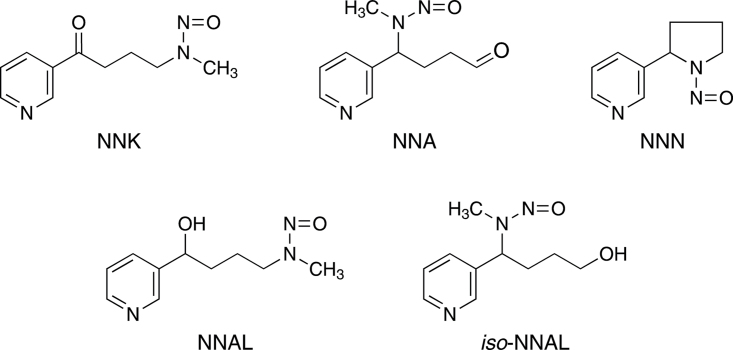
Figure 1.
Structures of the compounds discussed in the text. NNK, NNA, and NNN are tobacco-specific nitrosamines (Hecht & Hoffmann, 1988). NNK and NNN are tobacco-specific compounds found in all tobacco products, and both are potent carcinogens (International Agency for Research on Cancer, 2007). NNA has not been detected in tobacco or tobacco smoke, but is formed upon reaction of nicotine with nitrous acid (Hecht, Chen, Ornaf et al., 1978b; Sleiman et al., 2010). NNAL is found in the urine and blood of people exposed to NNK (International Agency for Research on Cancer, 2007). iso-NNAL has been suggested as a biomarker for NNA exposure but has yet to be detected in human urine or blood (Sleiman et al., 2010; Thomas et al., 2011).
In this study, we used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to analyze surfaces in homes of smokers and nonsmokers for the powerful tobacco-specific lung carcinogen NNK.
METHODS
Study Procedures
This study was approved by the University of Minnesota Research Subjects’ Protection Program Institutional Review Board: Human Subjects Committee. The participating homes were part of the “Project STARS: Start Taking Action to Restrict Smoking” study, a randomized clinical trial designed to test ultimately the efficacy of “biomarker feedback” (i.e., laboratory report of tobacco toxicants in child’s urine) on increasing parental implementation of complete home smoking restrictions and smoking cessation.
Participants were recruited through radio and news paper advertisements, flyers posted at retail stores, community centers, and medical clinics serving lower income persons, WIC (Women, Infants, and Children USDA Food and Nutrition Service) clinics, and word of mouth. Eligible participants lived in homes where smoking took place. They included adult females who were the parent or legal guardian of a child aged 10 years or younger living in their home. Eligible participants smoked at least 100 cigarettes in their lifetime and had smoked at least 1 cigarette/day on at least 20 of the past 30 days. Participants were excluded if they were currently pregnant, planning to become pregnant, planning to move in the next 3 months, and/or were receiving treatment for smoking cessation. Participant’s positive smoking status was verified via urine cotinine test strips (NicAlert; Cooke et al., 2008). To be considered eligible, the participants’ home environmental exposure to secondhand smoke was confirmed using a passive nicotine dosimeter (Hammond & Leaderer, 1987).
Study staff conducted the entire baseline visit in the participant’s home. During this visit, they obtained verbal and written consent (and assent for children aged 7–10), administered the baseline questionnaire, and collected samples, including the thirdhand smoke swab samples. All baseline visits were completed between June 2011 and July 2012. A random selection of 37 home thirdhand smoke swab samples, and 5 car samples were chosen from a total of 60 homes enrolled in the study during this time period.
During the baseline visit, enrolled parents were asked a series of questions about their smoking and other smoking-related behaviors, as well as questions to document demographic variables, socioeconomic status, residential characteristics, general health, and child exposure to smoke. Passive air nicotine dosimeters were used to verify and measure home exposure to secondhand smoke. At the baseline visit, the dosimeter was hung out of reach in an unobtrusive location, away from sources of air circulation in a common area of the home (e.g., kitchen, living room, dining room), a room in which the family reportedly spends the most waking hours. The dosimeter was retrieved approximately 1 week after placement and analyzed by gas chromatography in the laboratory of S. Katharine Hammond (University of California, Berkeley, CA) using a standardized procedure (Eisner, Katz, Yelin, Hammond, & Blanc, 2001).
Thirdhand Smoke Collection Protocol
Prior to the visit, 1.5ml of 0.1% aqueous ascorbic acid was added to a 4 oz. polypropylene sterile specimen cup. Ascorbic acid is an established inhibitor of artefactual nitrosamine formation under conditions of analysis for tobacco-specific nitrosamines (Hecht, Ornaf, & Hoffmann, 1974). The solution was stored at 4 °C for no longer than 1 week prior to use.
While in the home, staff members soaked a 100% cotton swab in the ascorbic acid solution from the prepared specimen cup. The swab was then wiped vertically and horizontally across a 100-cm2 sampling area using a 10×10 wire “frame” created for this study. Two samples were collected from two different locations in the room of the home in which the dosimeter was placed, and one of these from each home was randomly selected for analysis. Samples were ideally taken from a location infrequently cleaned where dust had visibly accumulated. Preferred locations were elevated, horizontal, porous surfaces such as wooden cabinets or shelves. If a porous surface was not available, the top of the refrigerator was used. When available, one additional sample was taken from the horizontal surface of the car dashboard, deep in a corner. Once a sample was obtained, the cotton swab was placed back into the cup, capped and transported in a cooler from the participant’s home to the laboratory and stored at −20 °C until analysis. Control swabs were prepared in the same manner except without swabbing any surface.
Nonsmoking volunteer staff and faculty associated with the study were interviewed regarding smoking in their homes. Those who denied any tobacco smoke exposure in their homes were invited to submit surface samples from their homes. The identical procedure was followed.
Sample Analysis
To the cup containing the swab and ascorbic acid solution was added 5.0ml H2O and internal standard [pyridine-D4]NNK (130 pg, Toronto Research Chemicals). The mixture was sonicated for 5min, then transferred to a 10-ml glass centrifuge tube. The sample was applied to a ChemElute 5ml cartridge (Agilent Technologies). After equilibration of the sample on the column for 5min, CH2Cl2 (5ml) was added to the column, and this was repeated 6 times. The eluants were combined and the sample was concentrated to dryness on a Speedvac. The residue was dissolved in 1ml of methanol, sonicated for 1min, and transferred to a glass “total recovery” autosampler vial (National Scientific). This was repeated with 0.5ml of methanol. The combined washings were concentrated to dryness and redissolved in 20 µl of 5mM ammonium acetate for LC-MS/MS analysis. The analysis was performed on a Thermo Scientific Ultra LC-Triple Quadrupole System equipped with a 50×0.5mm Luna C18(2) 3µ column (Phenomenex). Eight microliter was injected using a column temperature of 40 °C with elution by 30% aqueous methanol at a flow rate of 15 µl/min. The following MS parameters were used: Q1, 0.2; Q3, 0.7; ion transfer tube, 250 °C; scan width, 0.2 m/z; scan time, 0.2 s; spray voltage, 3,000V. Selected reaction monitoring was carried out for NNK (m/z 208 → m/z 122) and [pyridine-D4]NNK (m/z 212 → m/z 126). The retention time of NNK was 2.4min.
Some samples were treated with NaBH3CN at pH 7 for 4hr at room temperature, then analyzed for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, the metabolite of NNK) essentially as described above for NNK except that an additional sample purification was carried out by solid-phase extraction on Oasis MCX mixed-mode cation exchange cartridges (Waters Corporation), mainly as described previously (Church et al., 2010).
Statistical Analysis
Descriptive statistics, such as frequency, median, and range were calculated for smoking-related variables. Mean and standard deviation (SD) were calculated for detected NNK samples, and median and range were calculated for all NNK samples. Samples with a designation of “not determined due to chromatographic interferences” were treated as random missing. Fisher’s exact test was used to compare the number of samples with detected NNK in smokers’ versus nonsmokers’ homes. The rank test (Peto & Peto, 1972) was used to compare the NNK levels between the homes of smokers versus those of controls; values lower than the detection limit (30 pg/100cm) were treated as left-censored data. All data analyses were performed in SAS 9.3 (SAS Institute, Cary, NC) and R 2.15.2 (R Core Team, 2012) using the NADA package (Lee, 2012).
RESULTS
Some characteristics of the smokers’ homes are summarized in Table 1. The median number of cigarettes per day smoked in the homes was 6 (range, 0–20). The median number of smokers living in each home was 1 (range 0–3), and the number of visitors to the home who also smoked was 2 (range 0–5). Smoking was most common in the parent’s bedroom. Only one residence had a total restriction against smoking. The median level of cotinine in the urine of the children (n = 37) was 24ng/ml.
Table 1.
Characteristics of Smokers’ Homes
| Variable | Frequency (%) or median [range] (n = 37) |
|---|---|
| CPD smoked in home, median [range] | 6 [0–20] |
| No. of smokers who live in the home, median [range] | 1 [0–3] |
| No. of smokers who visit the home, median [range] | 2 [0–5] |
| Where is most smoking done, frequency | |
| Parent bedroom | 18 (49%) |
| Bathroom | 4 (11%) |
| Kitchen | 4 (11%) |
| Dining/eating area | 2 (5%) |
| Living room | 5 (14%) |
| Porch/patio | 3 (8%) |
| Yard | 1 (3%) |
| Home smoking restrictions | |
| No one is allowed to smoke anywhere, ever | 1 (3%) |
| Allowed in some places or at some times | 25 (68%) |
| Permitted anywhere | 11 (30%) |
| Enforcement of restrictions | |
| Smoking rules are always followed | 13 (35%) |
| Smoking rules are sometimes followed | 12 (32%) |
| Unsure or not answered | 12 (32%) |
| How often are visitors asked to smoke outside? | |
| Never | 11 (30%) |
| Sometimes | 17 (46%) |
| Often | 2 (5%) |
| Always | 1 (3%) |
| Not answered | 6 (16%) |
| Child cotinine (ng/ml), median [range] | 24 [2–3,590] |
Note. CPD = cigarettes per day
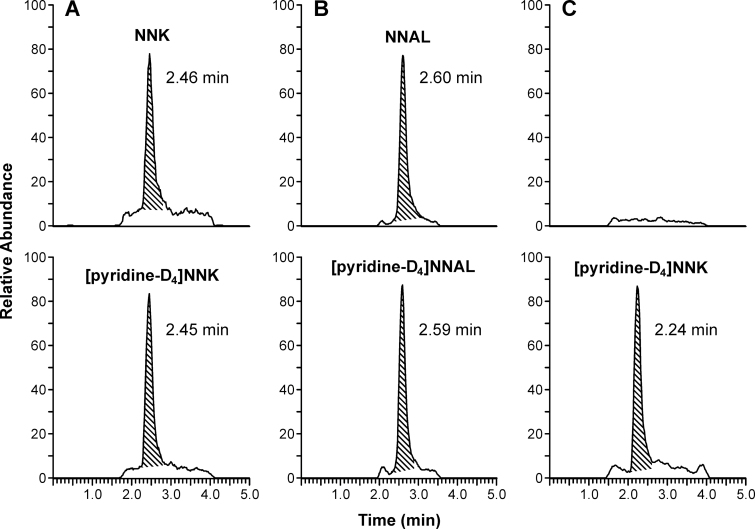
A typical LC-MS/MS chromatogram obtained upon analysis of a swab for NNK is illustrated in Figure 2A. The top panel is the chromatogram of the analyte and the bottom panel is the chromatogram of the internal standard, [pyridine-D4]NNK. A clear peak corresponding to NNK was observed in the sample and this eluted at the correct retention time. Further evidence for the identity of NNK was obtained by treatment of samples with NaBH3CN, which converts NNK to NNAL. As shown in Figure 2B, the peak corresponding to NNK disappeared and a new peak, resulting from conversion of NNK to NNAL, was observed. In 10 samples treated with NaBH3CN, the measured amounts of NNK and NNAL were highly correlated (R 2 = .995), with a slope of 1.08. The results of analysis of a sample from a typical nonsmokers’ home is shown in Figure 2C. NNK was not detected in this sample.
Figure 2.
Chromatograms obtained upon LC-MS/MS analysis of extracts of swabs for NNK and NNAL. (A) From a smokers’ home, monitored at m/z 208 → m/z 122 for NNK and m/z 212 → m/z 126 for [pyridine-D4]NNK; (B) from a smokers’ home, after treatment with NaBH3CN, monitored for m/z 210 → m/z 180 for NNAL and m/z 214 → m/z 184 for [pyridine-D4]NNAL; (C) from a nonsmokers’ home showing no peak for NNK.
The results of the analysis of NNK in samples obtained by swabbing surfaces in 37 smokers’ homes and 19 nonsmokers’ homes are summarized in Table 2. NNK was detected and quantified in 33 of 37 samples from smokers’ homes but only 3 of 19 from homes of nonsmokers, a significant difference (p < .001). NNK levels in smokers’ homes were as follows: (mean ± SD) 700±788 pg/100cm2; (median) 379 pg/100cm2; (range) not detected–3,500 pg/100cm2. Levels in nonsmokers’ homes were as follows: (mean ± SD) 235±176 pg/100cm2 for the samples in which NNK was detected; (median) below limit of detection; (range) below limit of detection–435 pg/100cm2. These differences were highly significant (p < .0001). NNK was not detected in any of the blank samples analyzed at the same time as the samples obtained from homes. NNK was also detected on the dashboards of 4 of the 5 smokers’ cars analyzed, in amounts of 306, 515, 550, and 4,860 pg/100cm2.
Table 2.
NNK (pg/100cm2) on Surfaces in Smokers’ and Nonsmokers’ Homes
| Smokers’ homes | NNK amount | Nonsmokers’ homes | NNK amount |
|---|---|---|---|
| 1 | 379 | 1 | LOD |
| 2 | 164 | 2 | LOD |
| 3 | 3,500 | 3 | LOD |
| 4 | 174 | 4 | LOD |
| 5 | 189 | 5 | LOD |
| 6 | 1,150 | 6 | LOD |
| 7 | 2,690 | 7 | LOD |
| 8 | 448 | 8 | LOD |
| 9 | 469 | 9 | LOD |
| 10 | LODa | 10 | LOD |
| 11 | 692 | 11 | LOD |
| 12 | 419 | 12 | 105 |
| 13 | 757 | 13 | LOD |
| 14 | 115 | 14 | LOD |
| 15 | 124 | 15 | LOD |
| 16 | 1,160 | 16 | LOD |
| 17 | 714 | 17 | 166 |
| 18 | 281 | 18 | 435 |
| 19 | 408 | 19 | LOD |
| 20 | 148 | ||
| 21 | 1,270 | ||
| 22 | 2,270 | ||
| 23 | 1,360 | ||
| 24 | 121 | ||
| 25 | 329 | ||
| 26 | 371 | ||
| 27 | 41 | ||
| 28 | 936 | ||
| 29 | 770 | ||
| 30 | NDb | ||
| 31 | 747 | ||
| 32 | 192 | ||
| 33 | 37 | ||
| 34 | ND | ||
| 35 | 233 | ||
| 36 | ND | ||
| 37 | 451 | ||
| Mean ± SD | 700±788 |
Note. aLOD = below limit of detection (30 pg/100cm2); NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
bND = Not determined due to chromatographic interference.
DISCUSSION
This is the first study to report the presence of NNK, a tobacco-specific lung carcinogen, on surfaces in homes. Our results clearly demonstrate the presence of NNK on surfaces in almost all the smokers’ homes tested whereas it was found only occasionally in nonsmokers’ homes. The difference between NNK detectability and levels in homes of smokers versus nonsmokers was significant. Since NNK is a tobacco-specific compound, chemically related to and derived from tobacco alkaloids, its presence on surfaces can be due only to contamination by tobacco smoke or, less likely, unburned tobacco. One previous study reported detection of NNK on the glove compartment door of a truck driven by a smoker (Sleiman et al., 2010). All other studies on tobacco-specific nitrosamines in thirdhand smoke have been carried out in model systems (Matt et al., 2011; Sleiman et al., 2010).
A recent study demonstrated the presence of PAH in settled household dust in homes (Hoh et al., 2012). A range of PAHs were analyzed and were detected in virtually all homes studied, independent of smoking status of the residents. Carcinogenic PAH levels were, however, significantly higher in smoker homes than nonsmoker homes (701 vs. 331ng/m2 dust, p = .014; compared with 70.0ng/m2 NNK on surfaces in smokers’ homes in this study). PAHs are combustion products with multiple environmental sources in addition to tobacco smoke, consistent with their ubiquitous presence in dust from the homes of nonsmokers.
Our findings are significant because NNK is a potent lung carcinogen and is absolutely tobacco specific. The results indicate the existence of a potential hazard in homes that have been occupied by smokers. This hazard would be most pertinent to children, who may come into contact with surfaces in the home during their activities. Children in homes occupied by smokers are exposed to NNK through secondhand smoke (Thomas et al., 2011) and our findings may signal additional exposures. Perhaps potentially more important are the residues of thirdhand smoke that may be left in a rental unit or home after smokers move out. If the new occupants are nonsmokers, they could be unknowingly exposed to this tobacco-specific lung carcinogen. We did detect NNK residues in the homes of three nonsmokers, which could have come from previous owners. It seems certain that nonsmoking potential tenants or buyers would not desire to move into a unit or home contaminated with NNK. It would be important to develop policies that would prevent unknowing exposure of potential tenants or buyers to tobacco carcinogens and toxicants.
Although the amounts of NNK detected here were relatively small when compared with that delivered in the smoke of a cigarette, they undeniably signal a potential hazard, particularly to nonsmokers. We made no attempt to investigate a statistically meaningful sample of surfaces in each home. The samples that we analyzed were taken randomly. We do not know if the results are representative of other surfaces in the same homes.
NNK is a member of the nitrosamine class of carcinogens. A common feature of most nitrosamine carcinogens is organo-selectivity (Druckrey, Preussmann, Ivankovic, & Schmähl, 1967; Preussmann & Stewart, 1984). Depending on their structure, most nitrosamines tend to affect a particular tissue or organ, independent of the route of administration. For NNK, that organ is the lung. The lung is the major target tissue of NNK in mice, rats, and hamsters independent of the route of administration (Hecht, 1998). Lung tumors have been observed in rats after administration of NNK by subcutaneous injection, in the drinking water, by oral swabbing, or intravesicularly. Similar results have been obtained in mice and Syrian golden hamsters (Hecht, 1998). In one study, Sencar mice were treated with NNK by topical application to the skin (LaVoie et al., 1987). In addition to skin tumors, a significant incidence of lung tumors was also observed. The skin and oral administration results, producing mainly lung tumors, are potentially relevant to the present study.
Sleiman and co-workers have studied surface-mediated reactions of nicotine with nitrous acid, observing the formation of NNK and two other nicotine-derived nitrosamines, N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-4-(3-pyridyl)butanal (NNA) (Sleiman et al., 2010). The predominant product in those model studies was NNA. We did not analyze for NNA in this study because it is not known to have carcinogenic activity (Hecht, Chen, Hirota et al., 1978). Furthermore, it is quite reactive and its analysis may require specially developed technology. Sleiman and co-workers suggested that urinary 1-(methylnitrosamino)-1-(3-pyridyl)butan-4-ol (iso-NNAL), a likely metabolic reduction product of NNA, could be a specific urinary biomarker for thirdhand smoke exposure because NNA is not present in cigarette mainstream or sidestream smoke but could be present on surfaces, having been formed in the reaction of surface nicotine with gaseous nitrous acid. In a previous study, we were unable to detect iso-NNAL in the urine of children who lived in homes where the parents smoked, indicating limited or no uptake of NNA from surfaces in these children (Thomas et al., 2011). We could not analyze for exposure of children to NNK on surfaces in that study because all of the children were exposed to NNK from secondhand smoke and therefore excreted its metabolite NNAL in their urine. It was not possible to determine whether the NNAL detected in children’s urine in that study originated from exposure of the children to NNK from secondhand smoke, from thirdhand smoke, or from both. It is likely, however, that thirdhand smoke exposure is a less important source of NNK uptake in children than secondhand smoke exposure.
A limitation of this is that we do not have adequate information on the overall frequency of NNK contamination because to date we have not carried out a comprehensive examination of household surfaces nor have we surveyed a large and statistically representative sampling of homes. Nevertheless, we did detect NNK in 33 of the 37 smokers’ households sampled, indicating that its presence on surfaces of smokers’ homes is common.
In summary, this study presents the first evidence for contamination of surfaces in smokers’ homes with the potent tobacco-specific lung carcinogen NNK. Potential buyers or renters of homes or apartments should be notified whether smokers lived there previously. Given a choice, presumably anyone looking for housing would not choose an apartment or house with NNK or other thirdhand tobacco smoke constituents on its surfaces.
FUNDING
This work was supported by the National Cancer Institute (CA-81301 to SSH) and the National Center on Minority Health and Health Disparities (P60MD003422 to JSA).
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Belinsky S. A., Foley J. F., White C. M., Anderson M. W., Maronpot R. R. (1990). Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Research, 50, 3772–3780 [PubMed] [Google Scholar]
- Burton A. (2011). Does the smoke ever really clear? Thirdhand smoke exposure raises new concerns. Environmental Health Perspectives, 119, A70–A74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church T. R., Anderson K. E., Le C., Zhang Y., Kampa D. M., Benoit A. R, … Hecht S. S. (2010). Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers, 15, 345–352.10.3109/13547501003753881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke F., Bullen C., Whittaker R., McRobbie H., Chen M. H., Walker N. (2008). Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine & Tobacco Research, 10, 607–612.10.1080/14622200801978680 [DOI] [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. (1967). Organotrope carcinogen Wirkungen bei 65 verschiedenen N-Nitrosoverbindungen an BD-ratten. Zeitschrift fur Krebsforschung und klinische Onkologie, 69, 103–201 [PubMed] [Google Scholar]
- Eisner M. D., Katz P. P., Yelin E. H., Hammond S. K., Blanc P. D. (2001). Measurement of environmental tobacco smoke exposure among adults with asthma. Environmental Health Perspectives, 109, 809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S. K., Leaderer B. P. (1987). A diffusion monitor to measure exposure to passive smoking. Environmental Science & Technology, 21, 494–497 [DOI] [PubMed] [Google Scholar]
- Hecht S. S. (1998). Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology, 11, 559–603.10.1021/tx980005y [DOI] [PubMed] [Google Scholar]
- Hecht S. S. (2012). Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine & Tobacco Research, 14, 18–28.10.1093/ntr/ntq216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. S., Chen C. B., Hirota N., Ornaf R. M., Tso T. C., Hoffmann D. (1978). Tobacco specific nitrosamines: Formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. Journal of the National Cancer Institute (1988), 60, 819–824.10.1093/jnci/60.4.819 [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Chen C. B., Ornaf R. M., Jacobs E., Adams J. D., Hoffmann D. (1978). Reaction of nicotine and sodium nitrite: Formation of nitrosamines and fragmentation of the pyrrolidine ring. Journal of Organic Chemistry, 43, 72–76.10.1021/jo00395a017 [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Hoffmann D. (1988). Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis, 9, 875–884.10.1093/carcin/9.6.875 [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Ornaf R. M., Hoffmann D. (1974). N′-Nitrosonornicotine in tobacco: Analysis of possible contributing factors and biologic implications. Journal of the National Cancer Institute (1988), 54, 1237–1244 [DOI] [PubMed] [Google Scholar]
- Hoh E., Hunt R. N., Quintana P. J., Zakarian J. M., Chatfield D. A., Wittry B. C, … Matt G. E. (2012). Environmental tobacco smoke as a source of polycyclic aromatic hydrocarbons in settled household dust. Environmental Science and Technology, 46, 4174–4183.10.1021/es300267g [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2004). Tobacco smoke and involuntary smoking. In IARC monographs on the evaluation of carcinogenic risks to humans (pp. 33–1187). [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2007). Smokeless tobacco and tobacco-specific nitrosamines.In IARC monographs on the evaluation of carcinogenic risks to humans, v. 89 (pp. 41–583). Lyon, FR: Author [PMC free article] [PubMed] [Google Scholar]
- LaVoie E. J., Prokopczyk G., Rigotty J., Czech A., Rivenson A., Adams J. D. (1987). Tumorigenic activity of the tobacco- specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK), 4-(methylnitrosamino)-4-(3-pyridyl)-1- butanol (iso-NNAL) and N’-nitrosonornicotine (NNN) on topical application to Sencar mice. Cancer Letters, 37, 277–283 [DOI] [PubMed] [Google Scholar]
- Lee L. (2012). NADA: Nondetects and data analysis for environmental data.Retrieved from http://cran.r-project.org/package=NADA
- Matt G. E., Quintana P. J., Destaillats H., Gundel L. A., Sleiman M., Singer B. C, … Hovell M. F. (2011). Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environmental Health Perspectives, 119, 1218–1226.10.1289/ehp.1103500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Peto J. (1972). Asymptotically efficient rank invariant test procedures. Journal of the Royal Statistical Society, Series A, 135, 185–207 [Google Scholar]
- Preussmann R., Stewart B. W. (1984). N-nitroso carcinogens. In Searle C. E. (Ed.), Chemical carcinogens, second edition, ACS monograph 182 (pp. 643–828). Washington, DC: American Chemical Society; [Google Scholar]
- R Core Team (2012). R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- Rivenson A., Hoffmann D., Prokopczyk B., Amin S., Hecht S. S. (1988). Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Research, 48, 6912–6917 [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. (2012). Cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 62, 10–29.10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- Sleiman M., Gundel L. A., Pankow J. F., Jacob P., III, Singer B. C., Destaillats H. (2010). Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of the National Academy of Sciences of the United States of America, 107, 6576–6581.10.1073/pnas.0912820107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. L., Guo H., Carmella S. G., Balbo S., Han S., Davis A, … Hecht S. S. (2011). Metabolites of a tobacco-specific lung carcinogen in children exposed to secondhand or thirdhand tobacco smoke in their homes. Cancer Epidemiology, Biomarkers & Prevention, 20, 1213–1221.10.1158/1055–9965.EPI-10–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2006). The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. Washington, DC: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; [Google Scholar]
- Winickoff J. P., Friebely J., Tanski S. E., Sherrod C., Matt G. E., Hovell M. F., McMillen R. C. (2009). Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics, 123, e74–e79.10.1542/peds.2008–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2012). Cancer Fact Sheet No. 297. Retrieved February 2012, from www.who.int/mediacentre/factsheets/fs297/en/index.html