Abstract
Introduction:
The rate of smokeless tobacco use in India is 20%; its use causes serious health problems, and no trial has assessed behavioral or pharmacological treatments for this public health concern. This trial evaluated varenicline for treating smokeless tobacco dependence in India.
Methods:
This was a double-blind placebo-controlled randomized trial of varenicline (12 weeks, 1mg, twice per day) with 237 smokeless tobacco users in India. All participants received behavioral counseling. Outcomes included self-reported and biochemically verified abstinence at the end of treatment (EOT), lapse and recovery events, safety, and medication adherence.
Results:
Self-reported EOT abstinence was significantly greater for varenicline (43%) versus placebo (31%; adjusted odds ratio [AOR] = 2.6, 95% CI = 1.2–4.2, p = .009). Biochemically confirmed EOT abstinence was greater for varenicline versus placebo (25.2% vs. 19.5%), but this was not statistically different (AOR = 1.6, 95% CI = 0.84–3.1, p = .15). Compared with placebo, varenicline did not reduce the risk for a lapse (hazard ratio [HR] = 0.86, 95% CI = 0.69–1.1, p = .14), but it did increase the likelihood of recovery to abstinence (HR = 1.2, 95% CI = 1.02–1.4, p = .02). Greater adherence increased EOT cessation rates for varenicline (39% vs. 18%, p = .003) but not for placebo (28% vs. 14%, p = .06). There were no significant differences between varenicline and placebo in rate of side effects, serious adverse events, hypertension, or stopping or reducing medication.
Conclusions:
Varenicline is safe for treating smokeless tobacco dependence in India, and further examination of this medication for this important public health problem is warranted.
INTRODUCTION
In contrast to the United States, where the rate of smokeless tobacco use is less than 5% (Substance Abuse and Mental Health Services Administration, 2011), the rate of smokeless tobacco use in India is 20% and exceeds 50% in certain provinces (Global Adult Tobacco Survey, 2009). Smokeless tobacco contains known cancer-causing agents, such as nitrosamines and polycyclic aromatic hydrocarbons, and its use may account for at least 10,000 deaths each year from oral cancer (Critchley & Unal, 2003). Although public health campaigns designed to educate users across the community about the health risks of smokeless tobacco have helped to reduce the incidence of smokeless tobacco use and leukoplakia in certain geographic regions of India, to date, there have been no clinical trials of individual-level treatments for smokeless tobacco use in India (Gupta & Ray, 2003).
In the United States, behavioral interventions, including telephone counseling, web-based counseling, and oral examinations with risk feedback, have been found to be efficacious for reducing smokeless tobacco dependence when tested in clinical trials (Ebbert, Montori, Erwin, & Stead, 2011a). However, quit rates in such trials rarely exceed 15%. Clinical trials of nicotine replacement therapies (NRTs) have shown efficacy for the lozenge on withdrawal and short-term cessation but not for long-term cessation (Ebbert, Severson, Croghan, Danaher, & Schroeder, 2009). Further, clinical trials testing other NRTs and bupropion have not yielded positive results (Ebbert et al., 2009, 2011a). In contrast, a large placebo-controlled trial in Scandinavia found that varenicline was efficacious for smokeless tobacco dependence (Fagerström et al., 2010), and a recent pilot trial in the United States reported preliminary support for an additional large clinical trial evaluation of varenicline for smokeless tobacco users in the United States (Ebbert, Croghan, Severson, Schroeder, & Hays, 2011b). Given that varenicline is relatively more effective for treating tobacco smoking than NRT (Aubin et al., 2008) and bupropion (Gonzales et al. 2006) and that varenicline significantly reduces craving to use smokeless tobacco (Ebbert et al., 2011b), varenicline may be efficacious for treating smokeless tobacco dependence in India. In light of the paucity of any pharmacotherapy trials for smokeless tobacco dependence in India, the relatively few clinical trial evaluations of varenicline for smokeless tobacco dependence, worldwide, and the potential increase in the prevalence of smokeless tobacco use in the United States as a harm reduction approach or as new marketing campaigns are initiated; the results of this trial could have broad implications. We hypothesized, therefore, that varenicline would increase rates of smokeless tobacco cessation. Given conflicting studies about the potential adverse risks associated with varenicline (e.g., Prochaska & Hilton, 2012; Singh, Loke, Spangler, & Furberg, 2011;), this study also sought to assess varenicline safety for use in this group of tobacco users.
METHODS
Participants
The study was conducted at the National Drug Dependence Treatment Centre, All India Institute of Medical Sciences (AIIMS), New Delhi, India. Participants (N = 237) were recruited from the Centre for Dental Education and Research (CDER) at AIIMS. Potential participants were screened in person for eligibility, including a physical examination and a psychiatric evaluation using the MINI International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Eligibility criteria included the following: use of smokeless tobacco each day for the past year (confirmed with urinary cotinine assessment; ≥50ng/ml), age over 18, and residing within 60 miles of New Delhi. Exclusions were as follows: current cigarette use (confirmed with breath carbon monoxide [CO] >10 ppm); current or planned use of tobacco cessation treatment; current use of cocaine, marijuana, or opioids or current consumption of ≥25 alcoholic drinks/week; current or recent use of psychiatric, pain, or asthma medications; current pregnancy or lactation; history or current diagnosis of psychosis, schizophrenia, bipolar disorder, or suicidality; current diagnosis of depression; diagnosis of cancer, heart disease, or HIV/AIDS in past 6 months; history of epilepsy/seizures; history or diagnosis within the last 6 months of abnormal heart rhythms and/or tachycardia (>100 beats/min); history or current diagnosis of chronic obstructive pulmonary disease, cardiovascular disease, heart attack in the last 6 months, and uncontrolled hypertension (systolic blood pressure > 150 or diastolic blood pressure > 90); and history of kidney or liver failure. Illiterate participants were not excluded from the trial.
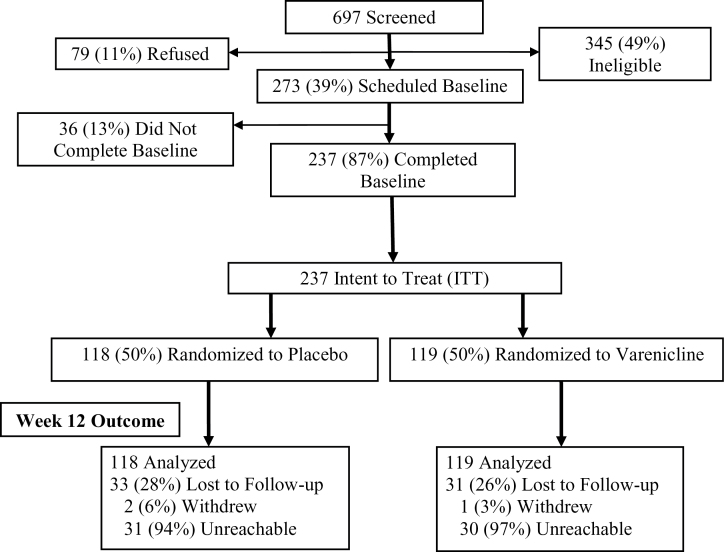
Accrual and retention data are shown in Figure 1. A total of 697 individuals were assessed for study eligibility; 345 individuals were considered ineligible (including 64 for a CO level above 10 ppm and 17 for current use of a contraindicated medication), 79 individuals refused to complete the assessment, and 273 individuals were considered eligible for the trial and were scheduled for the initial treatment session. Of the 273 participants scheduled for treatment, 36 participants failed to attend the session, leaving 237 individuals who were considered the intent-to-treat (ITT) sample. There was no significant difference in the rate of missed outcome data due to participant withdrawal or being unreachable across the treatment arms (χ 2[1] = 0.11, p = .77).
Figure 1.
CONSORT diagram. There were no significant differences between treatment arms with respect to the number of participants who were lost to follow-up, withdrew from the trial, or were not reachable for biochemical confirmation of tobacco use.
Procedures
The procedures were approved by the University of Pennsylvania Institutional Review Board, the AIIMS Ethics Committee, Drug Controller General India, and the Indian Council for Medical Research. During routine visits to the AIIMS CDER, a trained research assistant approached patients to assess their interest in participating in the clinical trial. Those who interested were scheduled for an in-person visit to assess their eligibility (Week 1). At this visit, the study physician and psychiatrist evaluated inclusion and exclusion criteria, including confirmation of smokeless tobacco use with urinary cotinine (cutoff for inclusion ≥50ng/ml) and assessment of tobacco smoking (participants were excluded if they had a breath CO > 10 ppm). Quantitative urinary cotinine was done using ELISA kits (Calbiotech), which uses solid-phase competitive ELISA. Assays were carried out as directed by the manufacturers, and the detection limit of the cotinine assay was 1ng/ml. A urine drug screen was conducted as was a pregnancy test for female participants. Eligible participants were randomized to 12 weeks of placebo or varenicline 1mg twice per day. Consistent with drug labeling and past trials (Gray, Carpenter, Lewis, Klintworth, & Upadhyaya, 2012), participants whose weight was <55kg were given varenicline 1mg once per day. All participants received behavioral counseling, a six-session, manual-based intervention modeled on past studies (Dale et al., 2007; Severson, Andrews, Lichtenstein, Gordon, & Barckley, 1998) and revised for cultural relevance based on studies conducted in India (e.g., Stigler et al., 2007). The intervention was designed to enhance awareness of the harmful effects of smokeless tobacco, assist the person in developing skills to quit and avoid relapse, and instruct the participant on medication use (Ebbert, Carr, & Dale, 2004).
Treatment was initiated at baseline (Week 0). Medication (varenicline or matching placebo) was taken for 12 weeks following labeling and previous varenicline trials (Gonzales et al., 2006): 0.5mg taken once per day for 1–3 days, 0.5mg taken twice per day for 4–7 days, and 1.0mg taken twice per day for 11 weeks. Counseling was provided at 0, 1, 3, 5, 7, and 9 weeks in person at AIIMS. A target quit date was established for Week 1. Assessments were conducted at baseline (Week 0) and at 1, 3, 5, 7, and 9 weeks. Participants reporting abstinence at the EOT (Week 12) were asked to attend AIIMS to provide a urine sample for cotinine assessment.
Assessments
Covariates
At baseline, demographic (e.g., age, education), and smokeless tobacco use (e.g., rate, years of use) data were collected. The smokeless tobacco version of the Fagerström Test for Nicotine Dependence (FTND-ST; Ebbert et al., 2006) was completed.
Medication Adherence
Medication adherence was measured by self-report pill count. At each assessment from Week 0–12, participants indicated if they used the appropriate number of pills each day. Participants were classified as adherent if they reported taking ≥80% of the prescribed medication across the 12 weeks (Catz et al., 2011; Ebbert et al., 2011b), a method previously validated (Buchanan et al., 2012).
Side Effects
Side effects were assessed at 0, 1, 3, 5, 7, 9, and 12 weeks using a symptom checklist from past trials (Patterson et al., 2009; Schnoll et al., 2011). Each symptom (e.g., nausea, depressed mood, suicidal ideation) was rated from 1 (none) to 4 (severe). Participants were instructed to contact study personnel if they experienced any serious medical problems between assessments. Adverse events were considered serious if the participant considered them debilitating or if they required hospitalization. Blood pressure was assessed at each visit.
Tobacco Cessation
At the EOT (Week 12), tobacco quit rates were assessed. Self-reported 7-day point prevalence abstinence was assessed at this time, and those who indicated abstinence were asked to attend clinic for biochemical confirmation of smokeless tobacco cessation with urinary cotinine (cutoff for abstinence ≤50ng/ml; Benowitz et al., 2002) and absence of tobacco smoking using breath CO (>10 ppm). For lapse and recovery events, a smokeless-tobacco-use lapse was defined as any day between the quit date and EOT on which participants used smokeless tobacco; recovery was defined as any 24-hr period of self-reported abstinence postlapse (Schnoll et al., 2010; Wileyto et al., 2005). The outcome of interest was time to transition between runs of smokeless-tobacco-use days and runs of abstinent days.
Statistical Analysis
Sample characteristics were examined using descriptive statistics and differences across treatment arms were assessed using chi-square test or analysis of variance. The rates of completion of the EOT assessment across treatment arms were compared using chi-square test. Next, we examined differences in abstinence rates across the treatment arms using logistic regression, computing odds ratios (ORs), and 95% CIs with alpha of .05. We present the unadjusted models and the models adjusted for covariates, controlling for variables related to rates of smokeless tobacco use and abstinence (FTND-ST, baseline tobacco use, duration of use, duration of past abstinence, and medication adherence). Separate models were conducted using self-reported versus biochemically verified abstinence rates and separate models were conducted for ITT, with missing outcome data considered continued use of smokeless tobacco, and completers only (including only participants who completed the Week 12 assessment; n = 173). Multivariate time-to-event (cox regression) models determined whether treatment arm predicted transitions from abstinence to lapse and from lapse to recovery using alpha of .05. This type of alternating-state multivariate data consists of times to transition between runs of tobacco-use days (≥1 day) and runs of tobacco abstinent days (≥1 day; Wileyto et al., 2005). Up to 16 cycles of lapse and recovery events were evaluated, and participants could cycle through multiple events. Standard errors were adjusted for repeated measures using the cluster-correlated robust variance estimate (Williams, 2000). Participants lost to follow-up on the timeline follow-back were removed from the risk set without registering an event. The clock is reset to zero every time there is a move (e.g., from smoking to abstinence). The analysis is stratified by the sequence of events, so the baseline is adjusted for each lapse (in the lapse model) and for each recovery (in the recovery model). Finally, the frequency of moderate-to-severe side effects, serious adverse events, and hypertension (systolic > 160 or diastolic > 90) at 1, 3, 5, 7, 9, and 12 weeks were compared across treatment arms using chi-square test (α = .05). Chi-square test was also used to evaluate rates of medication adherence (>80% of medication taken) and counseling adherence across the treatment arms (α = .05).
RESULTS
Sample Characteristics
On average, participants were 34.2 years old (SD = 9.2 years). Ninety-seven percent of the sample was male, 32% of the sample had a primary school education or less, 76% of the sample reported an annual family income of ≤1 lakh (≤$1,782U.S. dollars), 78% of the sample was married, and the average weight of participants was 62.9kg (SD = 11.2). In addition, most (81%) of the sample were Hindu. The average FTND score was 7.0 (SD = 1.9), the average number of times smokeless tobacco was used per day at baseline was 12.7 (SD = 7.8), the number of years smokeless tobacco was used before entering the trial was 11.2 years (SD = 7.6), the longest previous duration of abstinence from smokeless tobacco use prior to study entry was 22.0 days (SD = 55.0), and the average baseline cotinine value was 7360.5ng/ml (SD = 7612.7; range = 84–49065ng/ml). There were no significant differences in these characteristics across the treatment arms (ps > .05; Table 1).
Table 1.
Baseline Participant Characteristics by Treatment Arm Assignment
| Characteristic | Placebo (n = 118) | Varenicline (n = 119) | Overall (n = 237) |
|---|---|---|---|
| Sex (% male) | 96.6 | 97.5 | 97.0 |
| Age (years; M, SD) | 34.7 (9.9) | 33.8 (8.4) | 34.2 (9.2) |
| Marital status (% married) | 78.0 | 77.3 | 77.6 |
| Education (% ≤ primary school) | 29.7 | 34.5 | 32.1 |
| Income (% < 1 lakh or ≤ $1,782U.S. dollars, annually) | 77.1 | 75.6 | 76.4 |
| Religion (% Hindu) | 83.1 | 79.8 | 81.4 |
| Weight (kg; M, SD) | 63.1 (10.8) | 62.8 (11.7) | 62.9 (11.2) |
| FTND-ST (M, SD) | 7.1 (1.7) | 7.0 (2.0) | 7.0 (1.9) |
| Smokeless tobacco use per day (M, SD) | 12.3 (6.7) | 13.0 (8.8) | 12.7 (7.8) |
| Years of smokeless tobacco use (M, SD) | 11.4 (7.6) | 10.9 (7.6) | 11.2 (7.6) |
| Longest duration of previous abstinence from smokeless tobacco (days; M, SD) | 22.1 (48.7) | 21.9 (60.8) | 22.0 (55.0) |
| Cotinine (ng/ml; M, SD) | 7871.0 (8811.9) | 6854.3 (6196.6) | 7360.5 (7612.7) |
Note. FTND-ST = Fagerström Test for Nicotine Dependence-ST; n = number of participants.
Treatment Arm Effect on Tobacco Abstinence
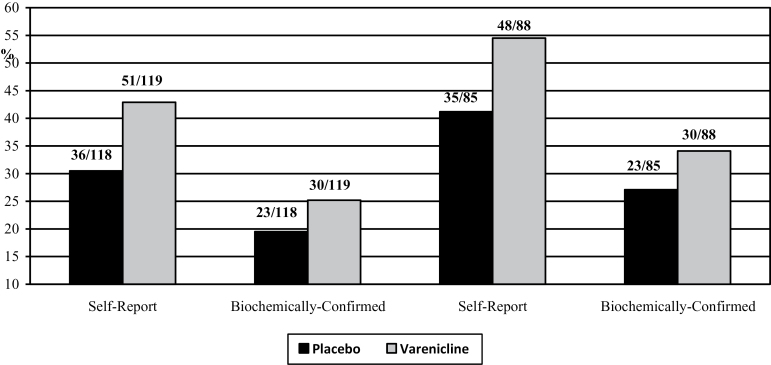
The left part of Figure 2 presents the quit rates across treatment arm for the ITT analyses. At Week 12, a significantly greater proportion of participants were treated with varenicline self-reported abstinence from smokeless tobacco us compared with participants treated with placebo (42.9% vs. 30.5%; unadjusted OR = 1.7, 95% CI = 1.001–2.92, p = .05; adjusted OR [AOR]= 2.6, 95% CI = 1.2–4.2, p = .009). A greater proportion of varenicline-treated participants showed biochemically confirmed abstinence from smokeless tobacco compared with placebo-treated participants (25.2% vs. 19.5%), but this comparison was not statistically significant (unadjusted OR = 1.4, 95% CI = 0.75–2.58, p = .29; AOR = 1.6, 95% CI = 0.84–3.1, p = .15).
Figure 2.
End-of-treatment 7-day point prevalence abstinence rates across treatment arms, ITT (left), and completers only (right). ITT indicates intent-to-treat analysis; bars show proportion quit, and numbers atop columns show number of participants. Logistic regression models controlled for nicotine dependence (Fagerström Test for Nicotine Dependence), baseline rate of smokeless tobacco use, duration of smokeless tobacco use, longest previous quit duration, gender, and adherence.
The right part of Figure 2 presents the quit rates across treatment arm for the completers-only analyses. At Week 12, a greater proportion of participants treated with varenicline self-reported smokeless tobacco abstinence versus placebo-treated participants (54.5% vs. 41.2%). This comparison approached significance in the unadjusted model (OR = 1.7, 95% CI = 0.94–3.13, p = .08) and was significant in the adjusted model (AOR = 2.4, 95% CI = 1.2–4.8, p = .01). A greater proportion of varenicline-treated participants showed biochemically confirmed abstinence from smokeless tobacco compared with placebo-treated participants (34.1% vs. 27.1%), but this comparison was not statistically significant (unadjusted OR = 1.4, 95% CI = 0.73–2.67, p = .32; adjusted OR = 1.7, 95% CI = 0.71–2.8, p = .15).
The analysis of transitions from alternating periods of abstinence and tobacco use showed that varenicline did not affect the likelihood of a lapse event (hazard ratio [HR] = 0.85, 95% CI = 0.69–1.05, p = .14), but it did increase the likelihood of recovery from a lapse to recovery (i.e., abstinence; HR = 1.2, 95% CI = 1.2–1.38, p = .02; Table 2). Participants in the varenicline arm were 20% more likely to recover from a lapse than participants in the placebo arm.
Table 2.
Models of Lapse and Recovery Events
| Transition | HR (95% CI) | p Value |
|---|---|---|
| To lapse | 0.85 (0.69–1.05) | 0.14 |
| To recovery | 1.20 (1.02–1.38) | 0.02 |
Note. HR = hazard ratio; CI = confidence interval.
Participants were allowed up to 16 transition events; models controlled for gender, nicotine dependence, baseline smokeless-tobacco-use rate, years of smokeless tobacco use, and longest duration of previous cessation; separate models with adherence added as interaction with treatment showed no interaction effect for lapse (HR = 1.19, 95% CI = 0.85–1.90, p = .24) but a significant interaction for recovery (HR = 1.34, 95% CI = 1.01–1.77, p = .04).
Side Effects and Adherence
There were no significant differences in the frequency of self-reported moderate-to-severe side effects from the checklist administered at 1, 3, 5, 7, 9, and 12 weeks between the treatment arms (Table 3; all ps > .05). The most frequently reported moderate-to-severe side effects were sleep problems (including general sleep problems, insomnia, and abnormal dreams): 11 reports for placebo participants (9.3%) versus 8 reports for varenicline participants (6.7%); and gastrointestinal problems (nausea, pain, flatulence, indigestion, vomiting, constipation, diarrhea): 9 reports for placebo participants (7.6%) versus 8 reports for varenicline participants (6.7%). Notably, there were no reports of suicidal ideation or behavior or depressed mood, and there was no significant difference across treatment arms in the rate of high blood pressure at any assessment timepoint. There were two side effects reported by participants in addition to the checklist: one reported erectile dysfunction at Week 9 and another reported loss of libido at 1 and 3 weeks; both received placebo. There were no reported serious adverse events.
Table 3.
Frequency of Moderate-Severe Side Effects by Treatment Arm
| Variable | Week 1 (N = 213) | Week 3 (N = 191) | Week 5 (N = 178) | Week 7 (N = 171) | Week 9 (N = 166) | Week 12 (N = 173) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA | VAR | PLA | VAR | PLA | VAR | PLA | VAR | PLA | VAR | PLA | VAR | |
| Nausea | 3 (2.9) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 1 (1.0) | 2 (1.9) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 0 (0.0) | 0 (0.0) | 1 (1.1) | 2 (2.1) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Irritability | 1 (1.0) | 0 (0.0) | 1 (1.1) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dry mouth | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Insomnia | 1 (1.0) | 1 (0.9) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abnormal dreams | 1 (1.0) | 1 (0.9) | 1 (1.1) | 3 (3.1) | 0 (0.0) | 1 (1.1) | 3 (3.7) | 2 (2.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sleep problems | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Irregular heartbeat | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Increased heart rate | 0 (0.0) | 1 (0.9) | 1 (1.1) | 2 (2.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anxiety | 1 (1.0) | 0 (0.0) | 1 (1.1) | 1 (1.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin redness | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Swelling | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dizziness | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hostility | 1 (1.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Agitation | 1 (1.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chest pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weakness (one side) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Depressed mood | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Suicidal ideation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| High blood pressure | 5 (4.8) | 5 (4.6) | 5 (5.3) | 6 (6.2) | 5 (5.7) | 4 (4.4) | 3 (3.7) | 3 (3.4) | 2 (2.5) | 2 (2.5) | 2 (2.5) | 3 (3.6) |
Note. PLA = placebo, VAR = varenicline.
Number listed indicates number of participants at timepoint reporting moderate or severe side effect; number in parentheses represents proportion of treatment group at that timepoint; there were two reports provided by participants in addition to the checklist: one participant reported erectile dysfunction at Week 9, and one participant reported loss of libido at 1 and 3 weeks. Both participants received placebo.
Finally, there were no significant differences across treatment arms in the rate of medication adherence. Among varenicline-treated participants, 34.5% of participants indicated taking at least 80% of the doses versus 39% of placebo-treated participants (χ 2[1] = .52, p = .50). Rate of adherence, which was controlled for in the logistic regression and lapse-recovery event models, was correlated with abstinence. Specifically, in the varenicline treatment arm, abstinence rates among participants who adhered to treatment were significantly greater than abstinence rates among those who did not (39% vs. 18%; χ 2[1] = 9.26, p = .003). In contrast, in the placebo treatment arm, abstinence rates among participants who adhered to treatment were not significantly different from abstinence rates among those who did not (28% vs. 14%; χ 2[1] = 3.60, p = .06). There was no significant difference in the rate of reducing or stopping medication between placebo (4.2%) and varenicline groups (8.4%; χ 2[1] = 1.77, p = .29). Rates of completion of the counseling sessions were equivalent across treatment arms (all ps > .05). For varenicline-treated participants, counseling session adherence was 100%, 91%, 82%, 77%, 74%, and 71%, whereas for placebo-treated participants, it was 100%, 89%, 80%, 75%, 70%, and 69%.
DISCUSSION
This study was designed to evaluate the efficacy and safety of varenicline for treating smokeless tobacco dependence in India where there are exceptionally high rates of smokeless tobacco use and very little existing data on which to formulate initiatives to address this significant public health problem. Several notable results were yielded from this trial.
First, in terms of the efficacy of varenicline for treating smokeless tobacco dependence in India, the results were mixed. On the one hand, compared with placebo, varenicline yielded significantly higher self-reported EOT smokeless tobacco abstinence rates in unadjusted and adjusted models and significantly increased the likelihood of a recovery to abstinence after a lapse. Further, cessation rates—biochemically confirmed—were significantly higher for those who adhered to varenicline, versus those who did not. On the other hand, biochemically verified abstinence was not significantly different for varenicline versus placebo, and varenicline was not associated with a reduced likelihood of a lapse to tobacco use following a period of abstinence. Thus, overall, the results concerning efficacy suggest that larger clinical trials, which incorporate interventions to promote medication adherence (e.g., Anton et al., 2006), are needed to discern the potential efficacy of varenicline for treating smokeless tobacco use in India.
Second, the results of this trial were supportive of the safety of varenicline for clinical use in this population. In addition to overall low rates of varenicline-related side effects, such as headache, nausea, and insomnia, this study found no evidence that varenicline was associated with adverse cardiovascular or psychiatric effects, including hypertension and suicidality. There was also no significant difference in the rate of reducing or stopping medication between the treatment arms. These results converge with findings from previous varenicline trials for treating smokeless tobacco (Ebbert et al., 2011b; Fagerström et al., 2010) and tobacco smokers (Garza, Murphy, Tseng, Riordan, & Chatterjee, 2011; Gunnell, Irvine, Wise, Davies, & Martin, 2009; Prochaska & Hilton, 2012; Svanström, Pasternak, & Hviid, 2012), and challenge recent studies indicating psychiatric and cardiovascular risks of varenicline (Moore, Furberg, Glenmullen, Maltsberger, & Singh, 2011: Singh et al., 2011). Although the present study did not conduct an inferiority test to more rigorously evaluate the safety of varenicline for treating smokeless tobacco dependence in India, the present sample size does compare with several previous studies that report safety of varenicline. As such, these safety data can make a contribution to future meta-analytic analyses of varenicline safety.
Two additional findings are worth highlighting. First, relative to other clinical trials of varenicline for smokeless tobacco dependence, the present study showed that adherence to medication use is low in this population. For instance, the current adherence rate among varenicline users was about one-half that reported by Ebbert et al. (2011b). There are data to suggest that, like the present trial, adherence to varenicline may require particular attention among subgroups of users such as light smokers (de Dios, Anderson, Stanton, Audet, & Stein, 2012) and among those receiving care through a general medical practice (Blak, Wilson, Metcalfe, Maguire, & Hards, 2010). Further, there are fairly convincing data from numerous studies showing that varenicline efficacy is strongly affected by level of adherence (Catz et al., 2011; Hays, Leischow, Lawrence, & Lee, 2010; Nollen et al., 2011). Thus, what can be done to boost varenicline adherence? First, a systematic assessment of reasons for nonadherence may be needed to understand unique barriers to adherence in this population of tobacco users. Once completed, a medication adherence component could be integrated into the behavioral counseling program as has been done in previous trials (Gariti, Lynch, Alterman, Kampman, Xie, & Varillo, 2009; Schnoll et al., 2008). This counseling module could be modeled on the medical management framework that was used in Project COMBINE (Anton et al., 2006), which formally assesses participant reasons for nonadherence using scenarios and uses specific strategies to enhance compliance. At each counseling session, adherence would be assessed, and for those who are classified as nonadherent, counselors would attempt to identify the reasons for nonadherence (e.g., unintentional forgetting, convinced the medication is placebo, concern about side effects, convinced that they do not need medication, lack of trust). Counselors could then use established strategies to directly address the participant’s reasons for nonadherence (e.g., place medication in prominent place or use reminders; discuss difficulties discerning medication effects and that everyone receives counseling; emphasize that side effects typically diminish and problem solve about ways to decrease side effects; and indicate that stopping medication prematurely can lead to relapse). Future studies to assess the efficacy of varenicline for smokeless tobacco dependence in India should integrate such adherence counseling elements into standard care.
Finally, it is worth noting the high rate of false self-reported abstinence. It was 36% in this trial, although not statistically different between varenicline and placebo participants. Typically, clinical trials for tobacco dependence see a false reporting rate of about 10% (Gariti, Alterman, Ehrman, Mulvaney, & O’Brien, 2002), although false reporting rates as high as 25% have been found for subgroups who may face unique stigma from continuing to smoke, such as pregnant women (Shipton et al., 2009). Consequently, this population may represent a subgroup of tobacco users for whom biochemical verification procedures is required in a clinical trial such as this.
This trial is not without limitations. First, although the sample size is relatively large for a tobacco cessation clinical trial, our a priori power analysis indicated that a 17% difference between treatment arms was needed to detect a statistically significant treatment effect with the current sample size and 80% power. Thus, the present sample was not adequately powered to detect small, yet clinically meaningful, differences. Second, the present study does not report long-term treatment effects to assess if any benefits are maintained. Third, the adjusted models controlled for covariates associated with smokeless tobacco use and cessation, and although this was planned a priori and done in other clinical trials, this should be considered when interpreting the present results. Finally, as described above, adherence to medication was considerably lower than typically found and false reporting of abstinence was considerably higher than is typically found. As such, if varenicline is to be examined further as a treatment for smokeless tobacco dependence in India, researchers should target a large sample, include long-term follow-up, utilize biochemical verification procedures for all reports of abstinence, and integrate a medication adherence component into cessation counseling regimens.
Nevertheless, this trial provides the first systematic evaluation of the efficacy and safety of varenicline for treating smokeless tobacco dependence in India. Although the efficacy results were mixed, the safety data were consistent. Overall, the results of this study suggest that a future trial to evaluate the efficacy of varenicline for smokeless tobacco use in India is warranted, but such a trial should be designed to address the potential lower effect size and lower medication adherence rates in this population. Such a trial may provide more conclusive findings to support the use of varenicline to treat smokeless tobacco use in a region of the world with exceptionally high rates of use and a limited evidence base on which to base treatment decisions.
FUNDING
This research was supported by grants from the National Institute on Drug Abuse (R21 DA026404, R01 DA025078), the National Institute on Drug Abuse and the National Cancer Institute (P50 CA143187), and the National Institute on Drug Abuse, the National Cancer Institute, the National Institute of General Medical Sciences, and the National Human Genome Research Institute (U01 DA020830).
DECLARATION OF INTERESTS
Dr. Schnoll has served as a consultant to GlaxoSmithKline and Pfizer, and Pfizer provided the study medication and placebo for this study free of charge.
ACKNOWLEDGMENTS
The authors would like to thank the following individuals who participated or assisted in the implementation of this research project: Caryn Lerman, Elisa Martinez, Angela Pinto, Ainsley Backman, Herb Severson, and Melissa Stigler.
REFERENCES
- Anton R. F., O’Malley S. S., Ciraulo D. A., Cisler R. A., Couper D., Donavan D. M, … COMBINE Study Research Group (2006). Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. Journal of the American Medical Association, 295, 2003–2017 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16670409 [DOI] [PubMed] [Google Scholar]
- Aubin H. J., Bobak A., Britton J. R., Oncken C., Billing C. B., Jr, Gong J, … Reeves K. R. (2008). Varenicline versus transdermal nicotine patch for smoking cessation: Results from a randomised open-label trial. Thorax, 63, 717–724.10.1136/thx.2007.090647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blak B. T., Wilson K., Metcalfe M., Maguire A., Hards M. (2010). Evaluation of varenicline as an aid to smoking cessation in UK general practice - A THIN database study. Current Medical Research and Opinion, 26, 861–870.10.1185/03007990903526461 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P., Ahijevych K., Jarvis M. J., Hall S., LeHouezec J. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12028847 12028847 [Google Scholar]
- Buchanan T. S., Berg C. J., Cox L. S., Nazir N., Benowitz N. L., Yu L, … Nollen N. A. (2012). Adherence to varenicline among African American smokers: An exploratory analysis comparing plasma concentration, pill count, and self-report. Nicotine & Tobacco Research, 14, 1083–1091.10.1093/ntr/ntr333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz S. L., Jack L. M., McClure J. B., Javitz H. S., Deprey M., Zbikowski S. M, … Swan G. E. (2011). Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research, 13, 361–368.10.1093/ntr/ntr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley J. A., Unal B. (2003). Health effects associated with smokeless tobacco: A systematic review. Thorax, 58, 435–443 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12728167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L. C., Ebbert J. O., Glover E. D., Croghan I. T., Schroeder D. R., Severson H. H., Hurt R. D. (2007). Bupropion SR for the treatment of smokeless tobacco use. Drug and Alcohol Dependence, 90, 56–63 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17353101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios M. A., Anderson B. J., Stanton C., Audet D. A., Stein M. (2012). Project impact: A pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. Journal of Substance Abuse Treatment, 43, 322–330.10.1016/j.jsat.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert J., Montori V. M., Erwin P. J., Stead L. F. (2011a). Interventions for smokeless tobacco use cessation. Cochrane Database Systematic Reviews, CD004306.10.1002/14651858.CD004306.pub4 [DOI] [PubMed] [Google Scholar]
- Ebbert J. O., Carr A. B., Dale L. (2004). Smokeless tobacco: An emerging addiction. The Medical Clinics of North America, 88, 1593–1605 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15464115 [DOI] [PubMed] [Google Scholar]
- Ebbert J. O., Croghan I. T., Severson H. H., Schroeder D. R., Hays J. T. (2011b). A pilot study of the efficacy of varenicline for the treatment of smokeless tobacco users in Midwestern United States. Nicotine & Tobacco Research, 13, 820–826.10.1093/ntr/ntr078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert J. O., Patten C. A., Schroeder D. R. (2006). The Fagerström Test for Nicotine Dependence-Smokeless Tobacco (FTND-ST). Addictive Behaviors, 31, 716–721 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16448783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert J. O., Severson H. H., Croghan I. T., Danaher B. G., Schroeder D. R. (2009). A randomized clinical trial of nicotine lozenge for smokeless tobacco use. Nicotine & Tobacco Research, 11, 1415–1423.10.1093/ntr/ntp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K., Gilljam H., Metcalfe M., Tonstad S., Messig M. (2010). Stopping smokeless tobacco with varenicline: Randomised double blind placebo controlled trial. British Medical Journal, 341, c6549.10.1136/bmj.c6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariti P., Alterman A. I., Ehrman R., Mulvaney F. D., O’Brien C. P. (2002). Detecting smoking following smoking cessation treatment. Drug and Alcohol Dependence, 65, 191–196 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11772480 [DOI] [PubMed] [Google Scholar]
- Gariti P., Lynch K., Alterman A., Kampman K., Xie H., Varillo K. (2009). Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment, 37, 247–255.10.1016/j.jsat.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza D., Murphy M., Tseng L. J., Riordan H. J., Chatterjee A. (2011). A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biological Psychiatry, 69, 1075–1082.10.1016/ j.biopsych.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Global Adult Tobacco Survey, India (2009). Retrieved from http://www.who.int/tobacco/surveillance/gats_india/en/index.html
- Gonzales D., Rennard S. I., Nides M., Oncken C., Azoulay S., Billing C. B, … Varenicline Phase 3 Study Group (2006). Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association, 296, 47–55 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16820546 [DOI] [PubMed] [Google Scholar]
- Gray K. M., Carpenter M. J., Lewis A. L., Klintworth E. M., Upadhyaya H. P. (2012). Varenicline versus bupropion XL for smoking cessation in older adolescents: A randomized, double-blind pilot trial. Nicotine & Tobacco Research, 14, 234–239.10.1093/ntr/ntr130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D., Irvine D., Wise L., Davies C., Martin R. M. (2009). Varenicline and suicidal behaviour: A cohort study based on data from the General Practice Research Database. British Medical Journal, 339, b3805.10.1136/bmj.b3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. C., Ray C. S. (2003). Smokeless tobacco and health in India and South Asia. Respirology, 8, 419–431 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14708551 [DOI] [PubMed] [Google Scholar]
- Hays J. T., Leischow S. J., Lawrence D., Lee T. C. (2010). Adherence to treatment for tobacco dependence: Association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research, 12, 574–581.10.1093/ntr/ntq047 [DOI] [PubMed] [Google Scholar]
- Moore T. J., Furberg C. D., Glenmullen J., Maltsberger J. T., Singh S. (2011). Suicidal behavior and depression in smoking cessation treatments. PLoS One, 6, e27016.10.1371/journal.pone.0027016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen N. L., Cox L. S., Nazir N., Ellerbeck E. F., Owen A., Pankey S, … Ahluwalia J. S. (2011). A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine & Tobacco Research, 13, 868–873.10.1093/ntr/ntr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F., Jepson C., Strasser A. A., Loughead J., Perkins K. A., Gur R. C, … Lerman C. (2009). Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry, 65, 144–149.10.1016/ j.biopsych.2008.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J. J., Hilton J. F. (2012). Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: Systematic review and meta-analysis. British Medical Journal, 344, e2856.10.1136/bmj.e2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll R. A., Cappella J., Lerman C., Pinto A., Patterson F., Wileyto P, … Leone F. (2011). A novel recruitment message to increase enrollment into a smoking cessation treatment program: Preliminary results from a randomized trial. Health Communication, 10, 1–8.10.1080/10410236.2011.566829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll R. A., Patterson F., Wileyto E. P., Heitjan D. F., Shields A. E., Asch D. A., Lerman C. (2010). Effectiveness of extended-duration transdermal nicotine therapy: A randomized trial. Annals of Internal Medicine, 152, 144–151.10.1059/0003-4819-152-3-201002020-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll R. A., Wileyto E. P., Pinto A., Leone F., Gariti P., Siegel S, … Lerman C. (2008). A placebo-controlled trial of modafinil for nicotine dependence. Drug and Alcohol Dependence, 98, 86–93.10.1016/ j.drugalcdep.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson H. H., Andrews J. A., Lichtenstein E., Gordon J. S., Barckley M. F. (1998). Using the hygiene visit to deliver a tobacco cessation program: Results of a randomized clinical trial. Journal of the American Dental Association, 129, 993–999 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9685764 [DOI] [PubMed] [Google Scholar]
- Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E, … Dunbar G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59 (Suppl. 20)22–33 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9881538 [PubMed] [Google Scholar]
- Shipton D., Tappin D. M., Vadiveloo T., Crossley J. A., Aitken D. A., Chalmers J. (2009). Reliability of self-reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. British Medical Journal, 339, b4347.10.1136/bmj.b4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Loke Y. K., Spangler J. G., Furberg C. D. (2011). Risk of serious adverse cardiovascular events associated with varenicline: A systematic review and meta-analysis. Canadian Medical Association Journal, 183, 1359–1366.10.1503/cmaj.110218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigler M. H., Perry C. L., Arora M., Shrivastav R., Mathur C., Reddy K. S. (2007). Intermediate outcomes from Project MYTRI: Mobilizing youth for tobacco-related initiatives in India. Cancer Epidemiology Biomarkers & Prevention, 16, 1050–1056 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17548662 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2011). Results from the 2010 national survey on drug use and health: Summary of national findings (Vol. 2012). Rockville, MD: Substance Abuse and Mental Health Services Administration; [Google Scholar]
- Svanström H., Pasternak B., Hviid A. (2012). Use of varenicline for smoking cessation and risk of serious cardiovascular events: Nationwide cohort study. British Medical Journal, 345, e7176.10.1136/bmj.e7176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto E. P., Patterson F., Niaura R., Epstein L. H., Brown R. A., Audrain-McGovern J, … Lerman C. (2005). Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research, 7, 257–268 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16036283 [DOI] [PubMed] [Google Scholar]
- Williams R. L. (2000). A note on robust variance estimation for cluster-correlated data. Biometrics, 56, 645–646 [DOI] [PubMed] [Google Scholar]