Abstract
The cryptococcal antigen (CRAG) lateral flow assay (LFA) had 100% sensitivity and specificity on cerebrospinal fluid samples. Pretreatment LFA titers correlated with quantitative cultures (R2 = 0.7) and predicted 2- and 10-week mortality. The CRAG LFA is an accurate diagnostic assay for CSF and should be considered for point-of-care diagnosis of cryptococcal meningitis.
Keywords: HIV, cryptococcal meningitis, diagnosis, point-of-care, sub-Saharan Africa
A lateral flow immunochromatographic assay (LFA; Immy Inc, Norman, Oklahoma) for detection of cryptococcal antigen (CRAG) has been recently developed. The test can be performed without electricity in ≤10 minutes, is stable at room temperature, and costs $2 per strip in low-income countries. Those features make it an attractive option for diagnosis of cryptococcal disease in resource-limited settings.
Reports of the CRAG LFA diagnostic accuracy are limited to studies with few cerebrospinal fluid (CSF) samples, and those performed in clinical laboratories [1, 2]. Use of the CRAG LFA as a clinical point-of-care test in CSF specimens would enable rapid meningitis diagnosis, and differentiation between uncomplicated infection and meningitis among those patients whose samples are positive by serum CRAG testing [3]. Furthermore, the LFA's prognostic significance or ability to monitor treatment response is unknown. We estimated the accuracy and prognostic value of the CRAG LFA as a point-of-care test on CSF specimens by comparing LFA to standard diagnostic tests and correlating LFA CRAG titers with mortality among human immunodeficiency virus (HIV)–infected persons with suspected meningitis at a regional hospital in southwestern Uganda.
MATERIALS AND METHODS
Participants and Ethics Statement
Samples were collected as part of 2 prospective cohorts: (1) a randomized trial from 2011–2012 including 112 patients with suspected meningitis (clinicaltrials.gov: NCT01075152), in which patients with confirmed cryptococcal meningitis received induction therapy with 2 weeks of amphotericin B and fluconazole, and were randomized to early or deferred antiretroviral therapy (ART) initiation [4]; and (2) a prospective cohort study from 2008–2009 of 30 patients with confirmed cryptococcal meningitis (by India ink testing), who received short-course amphotericin B (5 days) and fluconazole for 2 weeks [5]. All participants were recruited after written informed consent at Mbarara Regional Referral Hospital. Study procedures were approved by all relevant institutional review boards.
Laboratory Procedures
We collected CSF for CRAG latex agglutination assay (Meridian Bioscience, Cincinnati, Ohio) and CRAG LFA (IMMY, Inc, Norman, Oklohoma); both were performed according to the manufacturer's instructions, as well as India ink staining and quantitative CSF culture with quantitative dilutions, as described previously [6, 7]. In the cohort study (n = 30), we tested additional CSF samples on days 3, 7, and 14 for LFA and fungal culture, which were performed on cryopreserved specimens. Semiquantitative LFA titers were performed with 2-fold CSF dilutions from 1:10 up to the greatest dilution with a positive reaction.
Statistical Analyses
We first assessed accuracy of the CSF CRAG LFA compared to a composite reference standard, defined as ≥2 positive tests, to avoid incorporation bias [8]. Repeated lumbar punctures within 14 days could further inform the reference standard for initially discordant specimens. We restricted this analysis to specimens from the randomized clinical trial (n = 112) because the cohort study had positive India ink as an inclusion criterion.
Next, we used repeated measurements from the cohort study (n = 30) to correlate CSF CRAG titers and quantitative fungal cultures at baseline, and at days 3, 7, and 14 of treatment. Specifically, we compared fungal culture log10 colony-forming units (CFU)/mL and log2 LFA titers by calculating R2 and fitting linear regression models. We also compared the change in log10 CFU/mL per day (also known as early fungicidal activity) to change in log2 LFA titer per day [9].
Finally, we used data from both studies to measure the association between baseline CSF CRAG LFA titer and quantitative fungal culture and both 2- and 10-week mortality, adjusted for Glasgow Coma Scale (GCS; dichotomized by <15 or 15). All statistical analyses were performed with Stata software, version 11.2 (StataCorp, College Station, Texas).
RESULTS
Forty-seven of 112 subjects (42%) with suspected meningitis had cryptococcal meningitis by the reference standard: 41 aseptic meningitis, 12 suspected tuberculous meningitis, 6 normal CSF parameters, 3 Streptococcus pneumoniae meningitis, 2 suspected bacterial meningitis, and 1 cerebral malaria. Subjects with cryptococcal meningitis had a median GCS of 15 (range, 8–15) and median CD4 count of 27 cells/µL (interquartile range, 12–66). The diagnostic performance of each assay is presented in Table 1. Both the sensitivity and specificity of LFA CRAG were 100%, and there was high concordance between diagnostic modalities (107/112).
Table 1.
Diagnostic Performance of Tests for Cryptococcal Meningitis Using Cerebrospinal Fluid Specimens Collected in Southwestern Uganda (n = 112)
| Test | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|
| CRAG LFA | 100% (47/47) | 92.5%–100% | 100% (65/65) | 94.5%–100% |
| CRAG latex | 100% (47/47) | 92.5%–100% | 98.5% (64/65) | 91.7%–100% |
| CSF culture | 95.7% (45/47)a | 85.5%–99.5% | 100% (65/65) | 94.5%–100% |
| India ink | 93.6% (44/47) | 82.5%–98.7% | 100% (65/65) | 94.5%–100% |
Abbreviations: CI, confidence interval; CRAG, cryptococcal antigen; CSF, cerebrospinal fluid; LFA, lateral flow assay.
a Of 2 culture-negative patients, 1 was positive by the 3 other tests; the second was CRAG positive by LFA and latex, but was diagnosed with culture positive cryptococcal meningitis 2 weeks later.
We found a high correlation between log10 CFU and log2 LFA titers on day 1 (n = 142, R2 = 0.70, P < .001). However, in the subgroup with repeated titers on treatment (n = 30), the correlation between log10 CFU and log2 LFA CRAG titers decreased from day 3 (R2 = 0.69, P < .001) to day 7 (R2 = 0.30, P = .005) to day 14 (R2 = 0.20, P = .03) (Supplementary Figure 1). On day 14, 9 of 11 culture-negative samples had positive LFA titers. There was no correlation between change in log10 CFU (median, −0.31) and change in log2 LFA titers during time on treatment (median, −0.06) (R2 = 0.10, P = .11; Supplementary Figure 2).
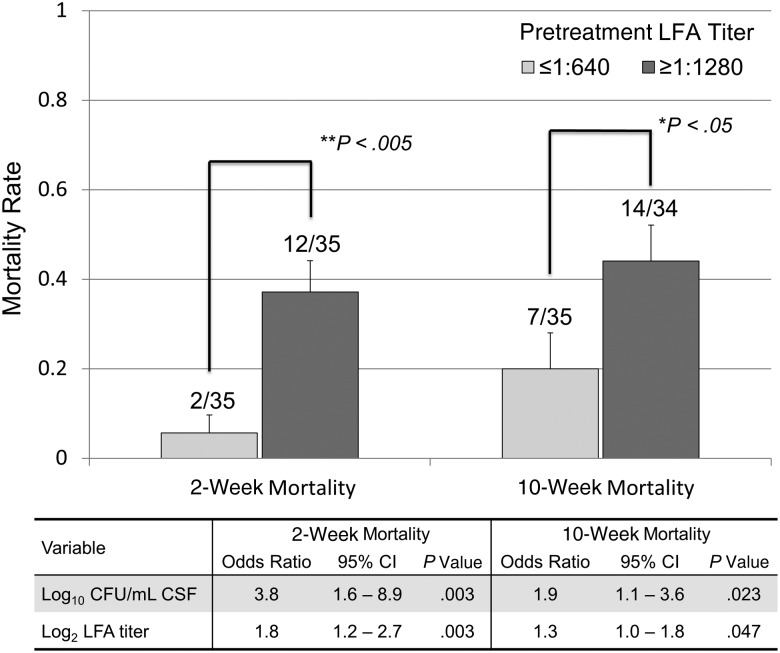
Among 70 participants followed prospectively, the crude mortality rate was 21% (15/70) at 2 weeks and 32% (22/69) at 10 weeks. Mortality rates were significantly higher for those with an LFA titer ≥1:1280 at both 2 weeks (12/35 vs 2/35) and 10 weeks (14/34 vs 7/35, P < .05 for both comparisons Figure 1). In multivariable models adjusted for GCS score, both pretreatment CSF quantitative culture CFU/mL and LFA titer were associated with mortality. Each doubling of the LFA titer was associated with an estimated 1.8-fold increased odds of 2-week mortality and a 30% increased odds of 10-week mortality.
Figure 1.
Two- and 10-week mortality by pretreatment cryptococcal antigen lateral flow assay titers. Error bars signify 1 standard deviation. P values denote results of χ2 testing between groups. Multivariable analyses are adjusted for pretreatment Glasgow Coma Scale score at diagnosis. Odds ratios are per each increase in pretreatment log10 colony-forming units per milliliter of cerebrospinal fluid culture at diagnosis, and per each 2-fold dilution >1:10 in pretreatment cryptococcal antigen lateral flow assay titer at diagnosis. Abbreviations: CFU, colony-forming unit; CI, confidence interval; CSF, cerebrospinal fluid; LFA, lateral flow assay.
DISCUSSION
We report that the CRAG LFA point-of-care assay in CSF specimens is a highly sensitive and specific test for cryptococcal meningitis. Additionally, CSF CRAG LFA titers at time of diagnosis correlate with fungal burden and are associated with mortality at 2 and 10 weeks. Because the CRAG LFA is a rapid, low-cost assay, and can be performed without electricity by healthcare workers with minimal training, the CRAG LFA fills the need for a point-of-care test for cryptococcal meningitis diagnosis in resource-limited settings. This need is increasingly relevant as Cryptococcus is the most common cause of adult meningitis in Africa [10], causing 20%–25% of AIDS-related mortality in Africa [11]. World Health Organization guidelines recommend systematic implementation of pre-ART CRAG screening from serum or whole blood specimens for HIV-infected persons with CD4 counts ≤100 cells/μL [3]. The CRAG LFA on CSF is a feasible option for confirming or excluding meningitis in those with positive blood tests.
Our findings add to the evidence that LFA is a highly accurate assay for the diagnosis of cryptococcal meningitis. A study that included 9 patients with positive CSF fungal cultures found that all were positive by CSF LFA [1]. A second study compared CSF LFA to enzyme immunoassay CRAG testing and found 100% sensitivity (15/15) and 99.7% specificity (395/396) [2]. Notably, we found some evidence that the LFA is possibly more sensitive than fungal culture at early stages of disease. Two subjects had low LFA titers (1:10) with negative fungal culture; both were CRAG-latex positive and 1 was India ink positive. Both subjects underwent repeat testing within 14 days and were found to have culture-confirmed cryptococcal meningitis.
We also found evidence for a prognostic value of pretreatment CSF LFA titers. Both quantitative culture and higher latex-CRAG titers were predictive of adverse outcomes [12–15]. We found a higher correlation between pretreatment quantitative fungal cultures and pretreatment LFA titers (R2 = 0.7, P < .001) than a prior study correlating latex agglutination CRAG titer and culture CFU (R2 = 0.5) [16]. Moreover, we found increasing 2- and 10-week mortality with each 2-fold titer increase in CSF LFA. This finding is in keeping with a prior study that found a relationship between latex agglutination CRAG titers and mortality risk [16]. Because quantitative cultures are unavailable in much of the developing world, the LFA titers, which can be simply performed with serial dilutions, might serve an important additional prognostic role to advise clinicians on mortality risk. Although the cost of additional titers ($2 per titer) might present a challenge, a titer threshold of >1:1000 could be considered prognostic to minimize need for additional dilutions, based on our finding that a titer ≥1:1280 vs ≤640 was associated with 6-fold greater mortality at 2 weeks (Figure 1).
The LFA, however, should not be used to monitor treatment response. Because clearance of the cryptococcal capsule polysaccharide (ie, CRAG) by macrophages is an independent and slower process than killing of the yeast by antifungal therapy [16, 17], LFA titers remain elevated during effective treatment [16, 18, 19]. Thus, CRAG titers are not a suitable substitute for quantitative culture for determination of treatment response.
Limitations included the moderate sample size at a single center and use of archived specimens in the prospective cohort subgroup. However, given the confidence intervals around our accuracy estimates, a larger sample size would likely not have altered our conclusions greatly. Testing of samples in real time outside of a research setting will also be important to confirm the feasibility of LFA as a point-of-care test.
In summary, the LFA in CSF is an accurate and prognostic point-of-care test for cryptococcal meningitis. As cryptococcal meningitis remains the most common cause of adult meningitis in sub-Saharan Africa, and standard diagnostic techniques pose challenges in terms of cost and human resource needs, adoption of LFA as a point-of-care test on CSF should be urgently considered.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Nicky Longley and Tom Harrison for collaboration and support with the short-course amphotericin B cohort. We thank Neal Wetherall, Mandana Godard, and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS team for support of the laboratory's external quality assurance testing in 2010–2012.
Financial support. Research and salary support was provided by the NIAID (U01AI089244, K23AI073192, T32AI007433), the National Institute of Mental Health (K23099916), and the Medical Research Council (UK).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McMullan BJ, Halliday C, Sorrell TC, et al. Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS One. 2012;7:e49541. doi: 10.1371/journal.pone.0049541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen J, Slechta ES, Gates-Hollingsworth MA, et al. Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin Vaccine Immunol. 2013;20:52–5. doi: 10.1128/CVI.00536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescent and children. Available at: www.who.int/hiv/pub/cryptococcal_disease2011/en/ . Accessed 22 March 2013. [PubMed]
- 4.Boulware DR, Meya D, Muzoora C, et al. ART initiation within the first 2 weeks of cryptococcal meningitis is associated with higher mortality: a multisite randomized trial. 20th Conference on Retroviruses and Opportunistic Infections,; Atlanta, Georgia,. 3–6 March 2013. [Google Scholar]
- 5.Muzoora CK, Kabanda T, Ortu G, et al. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect. 2012;64:76–81. doi: 10.1016/j.jinf.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–7. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 8.Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess. 2007;11 doi: 10.3310/hta11500. iii, ix-51. [DOI] [PubMed] [Google Scholar]
- 9.Bicanic T, Muzoora C, Brouwer AE, et al. Independent association between rate of clearance of infection and clinical outcome of HIV associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49:702–9. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource limited settings. J Acquir Immune Defic Syndr. 2013;63:e101–8. doi: 10.1097/QAI.0b013e31828e1e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 12.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176–81. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 13.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–91. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 14.Sungkanuparph S, Filler SG, Chetchotisakd P, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49:931–4. doi: 10.1086/605497. [DOI] [PubMed] [Google Scholar]
- 15.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer AE, Teparrukkul P, Pinpraphaporn S, et al. Baseline correlation and comparative kinetics of cerebrospinal fluid colony-forming unit counts and antigen titers in cryptococcal meningitis. J Infect Dis. 2005;192:681–4. doi: 10.1086/432073. [DOI] [PubMed] [Google Scholar]
- 17.Grinsell M, Weinhold LC, Cutler JE, Han Y, Kozel TR. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J Infect Dis. 2001;184:479–87. doi: 10.1086/322787. [DOI] [PubMed] [Google Scholar]
- 18.Aberg JA, Watson J, Segal M, Chang LW. Clinical utility of monitoring serum cryptococcal antigen (sCRAG) titers in patients with AIDS-related cryptococcal disease. HIV Clin Trials. 2000;1:1–6. doi: 10.1310/NQXR-ULMG-MM1B-3T2B. [DOI] [PubMed] [Google Scholar]
- 19.Antinori S, Radice A, Galimberti L, Magni C, Fasan M, Parravicini C. The role of cryptococcal antigen assay in diagnosis and monitoring of cryptococcal meningitis. J Clin Microbiol. 2005;43:5828–9. doi: 10.1128/JCM.43.11.5828-5829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.