We found increased acute myocardial infarction risk among hypertensive and prehypertensive HIV-infected veterans compared to normotensive uninfected veterans, independent of confounding comorbidities.
Keywords: blood pressure, prehypertension, HIV, myocardial infarction
Abstract
Background. Compared to uninfected people, human immunodeficiency virus (HIV)–infected individuals may have an increased risk of acute myocardial infarction (AMI). Currently, HIV-infected people are treated to the same blood pressure (BP) goals (<140/90 or <130/80 mm Hg) as their uninfected counterparts. Whether HIV-infected people with elevated BP have excess AMI risk compared to uninfected people is not known. This study examines whether the association between elevated BP and AMI risk differs by HIV status.
Methods. The Veterans Aging Cohort Study Virtual Cohort (VACS VC) consists of HIV-infected and -uninfected veterans matched 1:2 on age, sex, race/ethnicity, and clinical site. For this analysis, we analyzed 81 026 people with available BP data from VACS VC, who were free of cardiovascular disease at baseline. BP was the average of the 3 routine outpatient clinical measurements performed closest to baseline (first clinical visit after April 2003). BP categories used in the analyses were based on criteria of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Analyses were performed using Cox proportional hazards regression.
Results. Over 5.9 years (median), 860 incident AMIs occurred. Low/high prehypertensive and untreated/treated hypertensive HIV-infected individuals had increased AMI risk compared to uninfected, untreated normotensive individuals (hazard ratio [HR], 1.60 [95% confidence interval {CI}, 1.07–2.39]; HR, 1.81 [95% CI, 1.22–2.68]; HR, 2.57 [95% CI, 1.76–3.76]; and HR, 2.76 [95% CI, 1.90–4.02], respectively).
Conclusions. HIV, prehypertensive BP, and hypertensive BP were associated with an increased risk of AMI in a cohort of HIV-infected and -uninfected veterans. Future studies should prospectively investigate whether HIV interacts with BP to further increase AMI risk.
Compared to uninfected people, individuals infected with human immunodeficiency virus (HIV) may have an increased risk of acute myocardial infarction (AMI) [1–3]. Hypertension and prehypertension are recognized risk factors for cardiovascular disease (CVD) in the general population [4–7]. Currently, HIV-infected people are treated to the same blood pressure goals (<140/90 or <130/80 mm Hg) as their uninfected counterparts. Whether HIV-infected people with elevated blood pressure have excess AMI risk compared to uninfected persons is not known. The objective of this study was to examine the relationship among HIV status, blood pressure, and the risk of AMI and to determine if HIV-infected people with prehypertension had an increased risk of AMI compared to uninfected people.
METHODS
Subject Selection
The Veterans Aging Cohort Study virtual cohort (VACS VC) [8] is a prospective longitudinal cohort of HIV-infected veterans, each matched on age, race/ethnicity, and clinical site to 2 uninfected veterans in care. Subjects have been continuously enrolled in VACS VC each year since 1998 using a validated existing algorithm from the US Department of Veterans Affairs (VA) national electronic medical record system [8]. The institutional review boards at the University of Pittsburgh, Yale University, and the West Haven VA Medical Center approved this study.
All VACS VC participants who were alive and enrolled in VACS VC on or after 2003 were eligible for this study. Baseline was a participant's first clinical encounter on or after 1 April 2003. All participants were followed from their baseline date to an AMI event, death, or the date of last follow-up (1 January 2010).
These AMI data were merged with AMI data from Medicare and the Ischemic Heart Disease Quality Enhancement Research Initiative, an initiative designed to improve the quality of care and health outcomes of veterans with ischemic heart disease [9, 10]. We excluded any participant who had prevalent CVD based on International Classification of Diseases, Ninth Revision (ICD-9) codes for AMI, unstable angina, cardiovascular revascularization, stroke or transient ischemic attack, peripheral vascular disease, or heart failure up to 6 months after their baseline date (N = 17 229) [11, 12]. After this exclusion, 81 026 veterans (33% HIV infected) with available blood pressure data were included in this study.
Independent Variable
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and antihypertensive medication prescription were used to create the independent variables. Blood pressures were obtained using standard clinical protocols within the VA by trained clinical staff. SBP and DBP were averaged across the 3 routine outpatient clinical blood pressure measurements performed closest to the baseline date. Data on antihypertensive medication were obtained from the pharmacy management benefits package. Blood pressure status was based on the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) [4], as follows: normal (SBP 90–120 mm Hg and DBP 60–80 mm Hg and no antihypertensive medication prescription); prehypertension (SBP 120–139 mm Hg or DBP 80–89 mm Hg and no antihypertensives); hypertension (SBP ≥ 140 mm Hg or DBP ≥ 100 mm Hg and no antihypertensives); treated hypertension. We further stratified the prehypertension category into low (120–129 mm Hg or 80–84 mm Hg) and high (130–139 mm Hg or 85–89 mm Hg) prehypertension given reports of increased CVD risk in the upper range of this category [5, 7, 13]. In this cohort and in prior work, those with very low blood pressure (<90/60 mm Hg) had higher mortality rates compared to those with more optimal blood pressure (90–119/60–79 mm Hg). This indicates that very low blood pressure reflects poor health rather than optimal health. We separated out these 2 groups (defined as normal in JNC 7) to lessen potential bias in our results due to this competing risk of mortality.
Participants were classified as being in the higher category if SBP and DBP fell in different categories. HIV infection was present if a participant had ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for HIV infection and was included in the VA Immunology Case Registry [8].
Dependent Variable
The definition of fatal and nonfatal AMI incidence has previously been described [1]. In brief, AMI incidence was determined using VA, Medicare, and death certificate data. AMI events in the VA were collected by trained abstractors from the VA External Peer Review program [10, 14]. Clinical confirmation required documentation of AMI in the discharge summary followed by a review of the physician notes and medical chart. Medical information abstracted included evidence of elevated serum markers of myocardial damage and electrocardiographic findings. Non-VA AMI events were captured using ICD-9 code 410, which had strong agreement with adjudicated AMI outcomes in the Cardiovascular Health Study (CHS) [11]. Based on CHS criteria, we defined definite or possible fatal AMI as a death within 4 weeks of a clinically confirmed AMI or a death certificate documenting AMI as the underlying cause (ICD-10 code I21.0–I21.9), respectively. Deaths were identified using the VA vital status file, the Social Security Administration death master file, the Beneficiary Identification and Records Locator Subsystem, and the Veterans Health Administration medical Statistical Analysis Systems inpatient datasets. Cause of death was obtained from the National Death Index on 94.2% and 95.5% of HIV-infected and -uninfected decedents, respectively.
Covariates
Sociodemographic data included age, sex, and race/ethnicity. Framingham CVD risk factors including diabetes [15], hyperlipidemia (ie, low-density lipoprotein cholesterol [LDL], high-density lipoprotein cholesterol [HDL], and triglycerides) were measured using outpatient and clinical laboratory data collected closest to the baseline date. Smoking was measured from the VA Health Factors data repository [16] using a standardized form within the VA. For other CVD risk factors, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor use was based on pharmacy data, body mass index (BMI) was measured from Health Factors data, and renal disease and anemia were measured using outpatient and clinical laboratory data collected closest to the baseline date. Hepatitis C virus (HCV) infection was defined as a positive HCV antibody test or ≥1 inpatient and/or ≥2 outpatient ICD-9 codes for this diagnosis [17]. History of cocaine and alcohol abuse or dependence was defined using ICD-9 codes [18].
Specifically, diabetes was diagnosed using glucose measurements, use of insulin or oral hypoglycemic agents, and/or ≥1 inpatient and/or 2 outpatient ICD-9 codes [19]. Hyperlipidemia variables were categorized based on National Cholesterol Education Program Adult Treatment Panel III criteria [20]. Cholesterol measurements were obtained from the VA Decision Support System. Renal disease was defined as an estimated glomerular filtration rate of <60 mL/minute/1.73 m2, per National Kidney Foundation Kidney Disease Outcomes Quality Initiative thresholds for chronic kidney disease [21]. Smoking was categorized into current, past, and never smoking.
For HIV-specific covariates, we collected data on HIV type 1 (HIV-1) RNA, CD4+ T-lymphocyte counts (CD4+ cell counts), and current use of highly active antiretroviral therapy (HAART). We used CD4+ cell counts and HIV-1 RNA measurements obtained as part of clinical care from within 180 days of our baseline date. We included all antiviral medications that were on VA formulary during the study period. We have previously shown in a nested sample that 98% of HIV-infected veterans on antiretroviral therapy (ART) obtain their medications from the VA [8].
Statistical Analyses
Variables were compared by HIV and blood pressure category using Kruskal-Wallis, Wilcoxon rank-sum, or χ2 tests as appropriate. We calculated unadjusted and age- and race/ethnicity-adjusted AMI rates per 10 000 person-years for each blood pressure category overall and by HIV status using Poisson regression models. Using Cox proportional hazard models, we estimated hazard ratios (HRs) and 95% confidence intervals (CIs) to assess whether HIV combined with blood pressure category was an independent risk factor for incident AMI after adjusting for age, sex, and race/ethnicity. We additionally adjusted these analyses for traditional CVD risk factors (diabetes, lipids, smoking), comorbid diseases (HMG-CoA reductase inhibitor use, HCV infection, renal disease, anemia, obesity) and substance use or abuse (cocaine, alcohol). We accounted for ART, HIV viremia, and immune status in models restricted to HIV-infected participants.
In secondary analyses, we investigated the impact of widening pulse pressure (SBP minus DBP) and HIV on AMI risk.
Missing covariate data were included in the analyses using multiple imputation techniques that generated 5 data sets with complete covariate values to increase the robustness of the estimated HRs (see Supplementary Data for additional details on imputation). Analyses were performed using Stata software, version 11.0 (StataCorp).
RESULTS
Of 81 026 veterans with available blood pressure data, 16% were categorized as normal, 44% as prehypertensive (half of whom had high prehypertension), and 39% as hypertensive. Mean age ranged from 46 to 53 years. Compared to veterans with normal blood pressure, those with prehypertension or hypertension had greater prevalence of diabetes, triglycerides ≥150 mg/dL, HMG-CoA inhibitor use, and BMI ≥30 kg/m2, but less prevalent cocaine use. Hypertensive veterans were older and had more prevalent renal dysfunction than normotensive veterans (Table 1). HIV-infected veterans had a higher prevalence of low HDL, high triglycerides, low hemoglobin, and HCV infection, but a lower prevalence of diabetes and BMI ≥30 kg/m2 than uninfected veterans (Table 1). HAART use was similar across blood pressure categories. Median CD4+ counts were higher and HIV-1 RNA was lower among those with elevated blood pressure compared to those with normal blood pressure (Table 1).
Table 1.
Baseline Characteristics of Study Population
| Characteristic | Normotension (90–119/60–79 mm Hg) |
Prehypertension (120–139/80–89 mm Hg) |
Hypertension (≥140/90, No BP Meds) |
Hypertension (on BP Meds) |
Low BP (<90/60) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | |
| No. | 7634 | 5722 | 23 446 | 12 063 | 11 021 | 4613 | 11 425 | 4256 | 441 | 405 |
| Demographics | ||||||||||
| Age, y, mean (SD) | 47.0 (8.5) | 47.0 (8.7) | 47.0 (9.0) | 47.0 (9.3) | 49.0 (9.0) | 49.0 (9.6) | 53.0 (8.9) | 53.0 (8.9) | 46.3 (11.1) | 46.6 (10.6) |
| Female | 5 | 4 | 3 | 3 | 2 | 2 | 2 | 2 | 8 | 6 |
| Race | ||||||||||
| African American | 47 | 48 | 44 | 45 | 48 | 47 | 57 | 58 | 44 | 45 |
| White | 38 | 36 | 41 | 41 | 38 | 39 | 32 | 31 | 41 | 37 |
| Hispanic | 10 | 10 | 8 | 7 | 7 | 6 | 7 | 6 | 7 | 9 |
| Other | 6 | 7 | 7 | 7 | 7 | 8 | 4 | 5 | 7 | 8 |
| Framingham risk factors | ||||||||||
| Systolic blood pressure, mm Hg, mean (SD) | 112.9 (5.3) | 112.0 (5.8) | 129.1 (5.9) | 128.6 (6.0) | 147.6 (10.0) | 146.7 (9.4) | 141.4 (15.6) | 140.1 (16.9) | 106.5 (8.0) | 103.5 (9.6) |
| Diastolic blood pressure, mm Hg, mean (SD) | 70.3 (4.9) | 69.9 (4.9) | 77.8 (6.2) | 77.6 (6.2) | 87.2 (8.2) | 86.9 (8.1) | 83.7 (10.0) | 83.6 (10.8) | 57.3 (2.4) | 57.2 (2.8) |
| Diabetes | 11 | 8 | 16 | 11 | 20 | 16 | 39 | 30 | 8 | 7 |
| LDL cholesterol | ||||||||||
| Optimal (<100 mg/dL) | 32 | 49 | 30 | 44 | 29 | 44 | 37 | 50 | 35 | 51 |
| Near optimal (100–129 mg/dL) | 35 | 30 | 33 | 30 | 33 | 31 | 33 | 28 | 35 | 31 |
| Borderline high (130–159 mg/dL) | 22 | 14 | 24 | 17 | 24 | 16 | 20 | 14 | 21 | 12 |
| High/very high (≥160 mg/dL) | 11 | 7 | 13 | 9 | 14 | 9 | 10 | 7 | 9 | 6 |
| HDL cholesterol | ||||||||||
| High (≥60 mg/dL) | 18 | 11 | 14 | 11 | 16 | 12 | 13 | 11 | 15 | 12 |
| Medium (40–59 mg/dL) | 49 | 37 | 48 | 38 | 47 | 38 | 45 | 36 | 54 | 44 |
| Low (<40 mg/dL) | 34 | 53 | 38 | 51 | 37 | 50 | 42 | 52 | 31 | 44 |
| Triglycerides ≥150 mg/dL | 29 | 41 | 38 | 47 | 41 | 53 | 42 | 50 | 20 | 32 |
| HMG-CoA reductase inhibitor use | 6 | 4 | 8 | 6 | 8 | 7 | 18 | 11 | 2 | 3 |
| Smoking | ||||||||||
| Current | 59 | 66 | 54 | 59 | 56 | 58 | 48 | 56 | 53 | 66 |
| Past | 12 | 11 | 15 | 13 | 16 | 13 | 20 | 16 | 20 | 11 |
| Never | 28 | 23 | 30 | 27 | 28 | 29 | 33 | 28 | 27 | 22 |
| Other risk factors | ||||||||||
| BMI ≥30 kg/m2 | 20 | 6 | 36 | 13 | 44 | 19 | 53 | 23 | 15 | 5 |
| Renal disease | ||||||||||
| eGFR ≥60 mL/min/1.73 m2 | 98 | 96 | 97 | 96 | 96 | 94 | 89 | 82 | 96 | 96 |
| eGFR 30–59 mL/min/1.73 m2 | 2 | 4 | 3 | 4 | 4 | 5 | 9 | 12 | 3 | 3 |
| eGFR <30 mL/min/1.73 m2 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 6 | 1 | 1 |
| Anemia | ||||||||||
| Hemoglobin ≥14 mg/dL | 71 | 49 | 76 | 60 | 75 | 61 | 64 | 45 | 59 | 39 |
| Hemoglobin 12–13.9 mg/dL | 24 | 35 | 21 | 30 | 21 | 30 | 29 | 35 | 34 | 35 |
| Hemoglobin 10–11.9 mg/dL | 4 | 12 | 2 | 7 | 3 | 7 | 6 | 14 | 5 | 16 |
| Hemoglobin <10 mg/dL | 1 | 4 | 1 | 2 | 1 | 2 | 2 | 6 | 1 | 10 |
| HIV-related risk factors | ||||||||||
| HCV infection | 17 | 36 | 15 | 32 | 16 | 34 | 17 | 44 | 12 | 32 |
| Cocaine abuse/dependence | 10 | 13 | 7 | 11 | 6 | 9 | 6 | 13 | 8 | 11 |
| Alcohol abuse/dependence | 15 | 15 | 13 | 13 | 13 | 12 | 14 | 18 | 12 | 12 |
| On HAART at baseline | 43 | 45 | 44 | 45 | 34 | |||||
| HIV specific biomarkers | ||||||||||
| CD4+ T-lymphocyte count, cells/µL | ||||||||||
| ≥500 | 27 | 34 | 34 | 32 | 23 | |||||
| 200–499 | 39 | 41 | 40 | 41 | 37 | |||||
| <200 | 34 | 26 | 26 | 27 | 40 | |||||
| Median, mean ± SD | 314 (359 ± 295) | 369 (415 ± 303) | 374 (413 ± 298) | 365 (414 ± 304) | 289 (316 ± 272) | |||||
| HIV RNA (copies/mL) | ||||||||||
| ≥500 | 60 | 55 | 54 | 52 | 61 | |||||
| Median (mean ± SD) × 104 | 0.3 (7.9 ± 22.0) | 0.1 (5.6 ± 18.4) | 0.09 (4.6 ± 12.1) | 0.07 (4.9 ± 13.3) | 0.5 (11.1 ± 31.3) | |||||
Data are % nonmissing of column unless otherwise specified. All variables had complete data except for LDL cholesterol (22 952 missing), HDL cholesterol (20 170 missing), triglycerides (16 234 missing), smoking (5283 missing), eGFR (8009 missing), hemoglobin (10 175 missing), CD4+ T-lymphocyte count (5425 missing), and HIV-1 RNA (4616 missing). All variables differed significantly across HIV and blood pressure categories (P < .05).
Abbreviations: BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HIV−, HIV uninfected; HIV+, HIV infected; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; LDL, low-density lipoprotein; SD, standard deviation.
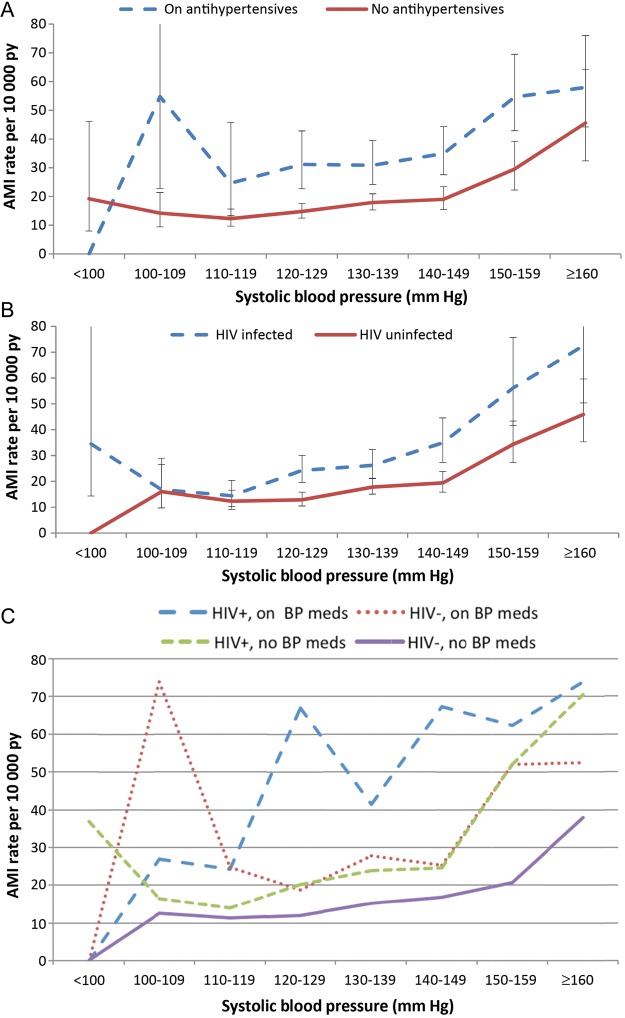
Over a total of 405 938.7 person-years (median follow-up time, 5.9 years), 860 incident AMIs occurred. AMI rates and risk increased with increasing blood pressure (Figure 1, Table 2) among HIV-infected and -uninfected veterans (Table 2). These associations persisted among HIV-infected veterans after adjustment for HIV-1 RNA, CD4+ count, and/or HAART use (Supplementary Table 1). AMI rates were significantly higher among hypertensive HIV-infected veterans compared to their hypertensive uninfected counterparts (Table 3).
Figure 1.
Unadjusted rate of incident acute myocardial infarction by systolic and diastolic blood pressure increments stratified by antihypertensive therapy (A), human immunodeficiency virus (HIV) infection status (B), and both HIV and antihypertensive therapy (C). Vertical bars represent 95% confidence intervals for rates. Abbreviations: AMI, acute myocardial infarction; BP, blood pressure; HIV, human immunodeficiency virus; py, person-years.
Table 2.
Rate and Risk of Incident Acute Myocardial Infarction by Blood Pressure Status and HIV Status (Separate Reference Groups)
| Blood Pressure Category, mm Hg, SBP/DBP | Definition | No. (All) | No. of AMIs (All) | Age-/Race- Adjusted AMI Rate/10 000 py (All) | Hazard Ratio (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Adjusted for Age, Sex, Race/Ethnicity | Adjusted for All Covariatesa |
|||||||
| (All) | All | HIV− | HIV+ | |||||
| 90–119/60–79 | Normal | 13 356 | 82 | 12.8 (9.9–16.4) | 1 | 1 | 1 | 1 |
| 120–129/80–84 | Low prehypertension | 17 812 | 123 | 13.8 (11.1–17.3) | 1.06 (.80–1.41) | 1.10 (.83–1.46) | 0.99 (.67–1.47) | 1.25 (.84–1.87) |
| 130–139/85–89 | High prehypertension | 17 697 | 156 | 17.5 (14.3–21.5) | 1.28 (.98–1.67) | 1.35 (1.03–1.76) | 1.29 (.89–1.87) | 1.41 (.95–2.09) |
| ≥140/90 | Hypertension | 15 634 | 190 | 24.5 (20.3–29.8) | 1.62 (1.25–2.11) | 1.65 (1.27–2.15) | 1.43 (1.00–2.06) | 2.06 (1.40–3.01) |
| Use of antihypertensive medication | Treated hypertension | 15 681 | 302 | 39.1 (33.1–46.1) | 2.16 (1.68–2.77) | 1.97 (1.52–2.56) | 1.81 (1.27–2.59) | 2.32 (1.58–3.39) |
| <90/60 | Low blood pressure | 846 | 7 | 18.6 (8.8–39.7) | 1.41 (.65–3.06) | 1.38 (.63–2.98) | 1.71 (.61–4.80) | 1.08 (.33–3.50) |
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; DBP, diastolic blood pressure; HIV, human immunodeficiency virus; py, person-years; SBP, systolic blood pressure.
a Includes age, sex, and race/ethnicity, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, diabetes, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor use, hepatitis C virus infection, renal disease, anemia, body mass index, cocaine, and alcohol use.
Table 3.
Rate and Risk of Incident Acute Myocardial Infarction by Blood Pressure and HIV Status (Common Reference Group)
| Blood Pressure Category, mm Hg, SBP/DBP | Definition | HIV Status | No. | No. of AMIs | Age-/Race-Adjusted AMI Rate/10 000 py | Hazard Ratio (95% CI) Age/Race/ Sex Adjusteda | Hazard Ratio (95% CI) Adjusted for All Covariatesa |
|---|---|---|---|---|---|---|---|
| 90–119/60–79 | Normal | HIV− | 7634 | 41 | 10.81 (7.75–15.1) | 1 | 1 |
| HIV+ | 5722 | 41 | 15.53 (11.13–21.7) | 1.43 (.93–2.20) | 1.28 (.82–1.98) | ||
| 120–129/80–84 | Low prehypertension | HIV− | 11 503 | 64 | 11.06 (8.37–14.62) | 0.99 (.67–1.47) | 0.99 (.67–1.47) |
| HIV+ | 6309 | 59 | 19.08 (14.31–25.47) | 1.76 (1.18–2.62) | 1.60 (1.07–2.39) | ||
| 130–139/85–89 | High prehypertension | HIV− | 11 943 | 92 | 15.28 (11.99–19.5) | 1.31 (.91–1.89) | 1.30 (.90–1.89) |
| HIV+ | 5754 | 64 | 22.26 (16.88–29.4) | 1.92 (1.30–2.85) | 1.81 (1.22–2.68) | ||
| ≥140/90, no antihypertensive medication | Hypertension | HIV− | 11 021 | 108 | 19.61 (15.6–24.69) | 1.54 (1.07–2.20) | 1.47 (1.02–2.11) |
| HIV+ | 4613 | 82 | 36.61 (28.4–47.29) | 2.84 (1.95–4.13) | 2.57 (1.76–3.76) | ||
| Use of antihypertensive medication | Treated hypertension | HIV− | 11 425 | 192 | 32.92 (27.31–39.77) | 2.14 (1.52–3.01) | 1.92 (1.35–2.72) |
| HIV+ | 4256 | 110 | 57.83 (46.27–72.41) | 3.80 (2.64–5.46) | 2.76 (1.90–4.02) | ||
| <90/60 | Low blood pressure | HIV− | 441 | 4 | 19.54 (7.25–52.66) | 1.74 (.62–4.86) | 1.73 (.62–4.84) |
| HIV+ | 405 | 3 | 17.58 (5.61–55.04) | 1.57 (.49–5.09) | 1.35 (.42–4.39) |
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; DBP, diastolic blood pressure; HIV, human immunodeficiency virus; py, person-years; SBP, systolic blood pressure.
a Includes age, sex, and race/ethnicity, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, diabetes, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor use, hepatitis C virus infection, renal disease, anemia, body mass index, cocaine, and alcohol use.
We did not detect significant interactions between elevated blood pressure and HIV status on incident AMI (P > .05) in regression models even when blood pressure was considered as a continuous systolic or diastolic measure.
Compared to uninfected veterans with normal blood pressure, HIV-infected veterans with low or high prehypertension or hypertension had significantly increased AMI risk (HR, 1.60 [95% CI, 1.07–2.39]; HR, 1.81 [95% CI, 1.22–2.68]; HR, 2.57 [95% CI, 1.76–3.76]; HR, 2.76 [95% CI, 1.90–4.02]) after adjustment for confounders (Table 3). Results were similar when SBP and DBP were analyzed separately (Supplementary Table 2).
A 10 mm Hg increase in pulse pressure was associated with a small but significantly increased risk of AMI (HR, 1.12 [95% CI, 1.06–1.19], P < .001) after adjusting for confounders. HIV infection did not modify the association between pulse pressure and AMI risk (HR for interaction, 0.92 [95% CI, .83–1.03]; data otherwise not shown).
DISCUSSION
In this cohort of veterans, high prehypertension and hypertension were associated with an increased risk of AMI. Compared to normotensive uninfected veterans, HIV-infected veterans with low/high prehypertension or hypertension had increased risk of AMI.
Prior research has reported an association between hypertension and AMI risk among HIV-infected people [22, 23]. This study extends those results by demonstrating that HIV status and blood pressure are associated with AMI risk independent of each other. By comparing all participants to a common referent group (HIV-uninfected normotensive), we are better able to assess the individual and combined effects of HIV status and elevated blood pressure.
Whether HIV infection and/or its treatment interact with blood pressure to increase the risk of AMI is not clear. Prior studies examining the association between HIV, ART, and blood pressure are inconsistent [24–26]. We found no statistical interaction between HIV, elevated blood pressure, and AMI risk. This suggests that HIV may not modify the association between blood pressure and AMI risk. This finding, however, should be interpreted carefully because we may have been underpowered to detect a significant interaction. Moreover, there are prior studies reporting significant associations between HIV and renal disease [27], endothelial dysfunction [28], reduced arterial elasticity [29], and progression of atherosclerosis [30–32], which in turn are associated with elevated blood pressure and cardiac events.
Our findings have important clinical implications for HIV-infected individuals. Prior work in the VACS and other studies demonstrate that HIV infection, ART use, and traditional CVD risk factors, including hypertension, are associated with an increased risk of AMI [1]. HIV-infected people have an increased risk of AMI (including those who achieve HIV-1 RNA levels <500 copies/mL over time), compared to uninfected people. Our data suggest that traditional CVD risk factors such as hypertension contribute additional AMI risk independent of and in addition to that contributed by HIV infection. Performing similar studies for other CVD risk factors such as diabetes and substance abuse may be warranted to better guide CVD risk stratification in HIV.
There are limitations that warrant discussion. First, as this is an observational study, our findings alone are insufficient to change current guidelines for blood pressure management for HIV-infected people. Second, as our population is overwhelmingly male, our results may not be generalizable to women. Third, there is the possibility of unmeasured confounding—eg, we were unable to adjust these analyses for family history of CVD or duration of antihypertensive therapy. Fourth, there is also the possibility of residual confounding present in this analysis. For example, alcohol is associated with blood pressure and coronary heart disease outcomes. In this study, alcohol abuse and alcohol dependence were assessed with ICD-9 codes, which can result in some misclassification. However, the fact that alcohol use is common in both HIV-infected and -uninfected veterans suggests that misclassification would not be differential by HIV status. Fifth, blood pressure, HIV-1 RNA, and CD4+ cell count were assessed only at baseline. Participants could have moved between blood pressure, HIV-1 RNA, and/or CD4+ cell count categories between baseline and censoring.
In summary, prehypertensive and hypertensive blood pressure were associated with an increased risk of AMI in a cohort of HIV-infected and -uninfected veterans. Future studies should prospectively investigate whether HIV interacts with blood pressure to further increase AMI risk.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The National Institutes of Health (NIH) did not participate in the design and conduct of the study or the collection, management, analysis, or interpretation of the data; nor did the NIH prepare, review, or approve of the article. The views expressed in this article are those of the authors and do not necessarily reflect the position or policies of the Department of Veterans Affairs.
Financial support. This work was supported by the National Heart, Lung, and Blood Institute and the National Institute on Alcohol Abuse and Alcoholism at the NIH (grant numbers HL095136–04 to M. S. F. and A. C. J., and AA013566–10, AA020790, and AA020794 to A. C. J.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Int Med. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 3.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–53. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 6.Liszka HA, Mainous AG, 3rd, King DE, Everett CJ, Egan BM. Prehypertension and cardiovascular morbidity. Ann Fam Med. 2005;3:294–9. doi: 10.1370/afm.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006;119:133–41. doi: 10.1016/j.amjmed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Veterans Affairs. Ischemic Heart Disease (IHD) Quality Enhancement Research Initiative. 2011. Available at: http://www.queri.research.va.gov/ihd/default.cfm . Accessed 4 October 2013.
- 10.Every NR, Fihn SD, Sales AE, Keane A, Ritchie JR. Quality Enhancement Research Initiative in ischemic heart disease: a quality initiative from the Department of Veterans Affairs. QUERI IHD Executive Committee. Med Care. 2000;38(6 suppl 1):I49–59. [PubMed] [Google Scholar]
- 11.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 12.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14:555–8. doi: 10.1046/j.1525-1497.1999.10198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon NS, Jeong MH, Ahn Y, et al. Impact of high-normal blood pressure measured in emergency room on adverse cardiac events in acute myocardial infarction. Korean Circ J. 2012;42:304–10. doi: 10.4070/kcj.2012.42.5.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maynard C, Lowy E, Rumsfeld J, et al. The prevalence and outcomes of in-hospital acute myocardial infarction in the Department of Veterans Affairs Health System. Arch Int Med. 2006;166:1410–6. doi: 10.1001/archinte.166.13.1410. [DOI] [PubMed] [Google Scholar]
- 15.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115–9. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 16.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–9. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(suppl 3):S99–105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer KL, McGinnis KA, Skanderson M, et al. Alcohol problems and health care services use in human immunodeficiency virus (HIV)-infected and HIV-uninfected veterans. Med Care. 2006;44(8 suppl 2):S44–51. doi: 10.1097/01.mlr.0000223703.91275.78. [DOI] [PubMed] [Google Scholar]
- 19.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–266. [PubMed] [Google Scholar]
- 22.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the Data Collection on Adverse Effects of Anti-HIV Drugs Study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 23.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis. 2011;53:84–91. doi: 10.1093/cid/cir269. [DOI] [PubMed] [Google Scholar]
- 24.Thiebaut R, El-Sadr WM, Friis-Moller N, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther. 2005;10:811–23. doi: 10.1177/135965350501000706. [DOI] [PubMed] [Google Scholar]
- 25.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–60. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 26.Khalsa A, Karim R, Mack WJ, et al. Correlates of prevalent hypertension in a large cohort of HIV-infected women: Women's Interagency HIV Study. AIDS. 2007;21:2539–41. doi: 10.1097/QAD.0b013e3282f15f7b. [DOI] [PubMed] [Google Scholar]
- 27.Phair J, Palella F. Renal disease in HIV-infected individuals. Curr Opin HIV AIDS. 2011;6:285–9. doi: 10.1097/COH.0b013e3283476bc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–76. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker JV, Duprez D, Rapkin J, et al. Untreated HIV infection and large and small artery elasticity. J Acquir Immune Defic Syndr. 2009;52:25–31. doi: 10.1097/qai.0b013e3181b02e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker JV, Henry WK, Patel P, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis. 2011;53:826–35. doi: 10.1093/cid/cir497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 32.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.