Abstract
In The HIV Prevention Trials Network 061 study, 155 human immunodeficiency virus (HIV)–infected men reported no prior HIV diagnosis; 83 of those men had HIV RNA levels of <1000 copies/mL at enrollment. Antiretroviral drug testing revealed that 65 of the 83 (78.3%) men were on antiretroviral treatment. Antiretroviral drug testing can help distinguish between newly diagnosed and previously diagnosed HIV infection.
Keywords: HIV, antiretroviral, self-report, MSM, new diagnosis
Many human immunodeficiency virus (HIV) prevention and surveillance studies focus on identifying individuals with newly diagnosed HIV infection, and many prevention strategies include increasing access to and uptake of HIV testing [1]. Identification of individuals with newly diagnosed HIV infection is usually based on information obtained directly from study participants about prior HIV testing (self-report). However, self-report may be unreliable. Because many HIV-infected individuals are on antiretroviral (ARV) therapy, ARV drug testing may be useful in addition to self-report for distinguishing between newly diagnosed and previously diagnosed HIV infection.
In a recent study, retrospective ARV drug testing revealed that some clinic patients who reported no knowledge of their HIV status were on ARV treatment (ART) [2]. ARV drug testing also revealed that some participants in HIV prevention trials who reported that they were ARV drug-naive were on ART [3, 4]. ARV drug testing also provides an objective measure of adherence to ARV drug regimens in clinical trials [5]. In recent studies, ARV drug testing revealed that many participants who reported high levels of adherence to ARV regimens for preexposure prophylaxis (PrEP) were not taking study drugs [5].
We recently developed a low-cost ARV drug assay based on high-resolution accurate mass spectroscopy that can be multiplexed to detect multiple classes of ARV drugs in a single assay [6]. We used this assay to test for the presence of ARV drugs in samples from HIV-infected participants in a clinical trial who reported no prior HIV diagnosis or ARV drug use.
METHODS
Study Cohort
Samples were obtained from the HIV Prevention Trials Network (HPTN) 061 study, which evaluated the feasibility of a multicomponent intervention to reduce HIV incidence among black men who have sex with men (MSM) in the United States (NCT 0095129, 2009–2011) [7]. Men were recruited from the community or as sexual partners of index participants. Index participants were HIV-uninfected men, HIV-infected men who were aware of their infection but not in care (previously diagnosed), or HIV-infected men who reported no prior HIV diagnosis (characterized as newly diagnosed prior to this analysis) [7]. Enrollment of community-recruited participants was capped at 10 per site for HIV-infected men already in care or having unprotected anal intercourse with only HIV-infected partners. Follow-up visits were conducted 6 and 12 months after enrollment. Behavioral data, including HIV status, HIV testing history, and use of ARV drugs, was collected using audio computer-assisted self-interviews. HIV testing was performed at study sites; results were confirmed at a central laboratory [7].
ARV Drug Testing
ARV drug testing was performed using stored plasma samples from HIV-infected men who reported no prior HIV diagnosis and had viral loads <1000 copies/mL. Testing was performed using a qualitative assay that detects 15 ARV drugs, including nonnucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs; Supplementary Table 1) [6]. ARV testing was performed retrospectively; test results were not reported to study participants.
Statistical Analysis
The χ2 and Fisher exact tests were used to compare the difference in proportions of ARV drug detection for categorical variables in SAS software, version 9.2.
Ethical Considerations
Written informed consent was obtained for study participation. The study was approved by institutional review boards at the participating institutions.
RESULTS
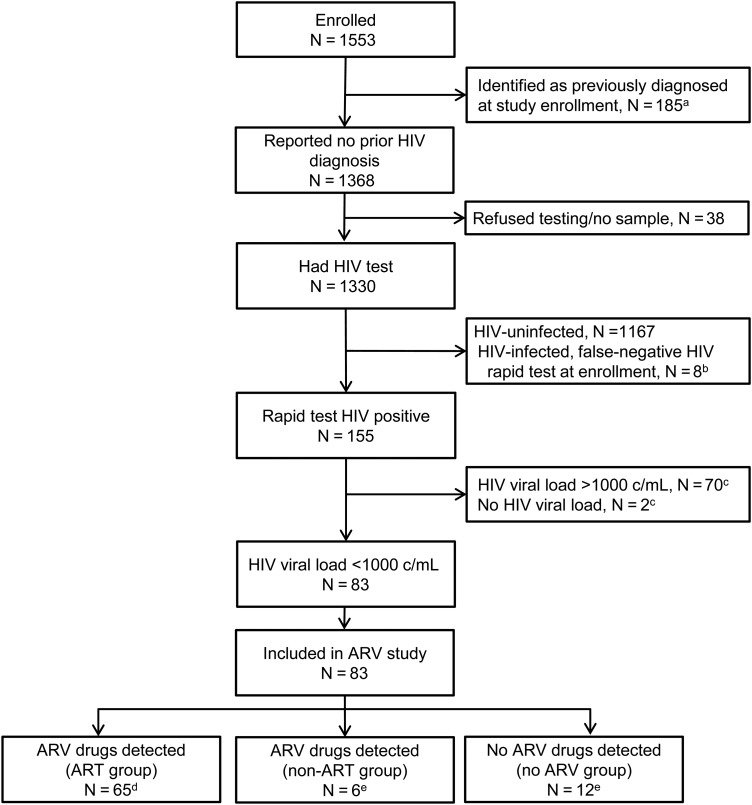
HPTN 061 enrolled 1553 men; 185 (11.9%) were identified as previously diagnosed at enrollment and 1368 (88.1%) reported no prior HIV diagnosis (Figure 1). HIV testing was completed for 1330 of the 1368 (97.2%) men who reported unknown HIV status; 155 (11.7%) of those men were confirmed to be HIV infected and were provisionally characterized as newly diagnosed. None of the 155 men reported prior or current use of ARV drugs. ARV drug testing was performed for 83 men who had viral loads <1000 copies/mL (68 with <400 copies/mL; 15 with 400–1000 copies/mL, Figure 1).
Figure 1.
Selection of study participants for antiretroviral (ARV) drug screening in HPTN 061. aIncludes 150 men who reported a prior diagnosis of human immunodeficiency virus (HIV) and 24 men who were identified as HIV infected in the HPTN 061 study (from review of public records or HIV testing). Eleven of these men reported ARV drug use. bHIV infection was identified retrospectively for these 8 men (based on retrospective testing performed at the HPTN Network Laboratory); they were not included in the ARV study. cMen were not included in the ARV study if they had a viral load >1000 copies/mL at enrollment or if viral load data were not available from the enrollment visit. dThese men had a pattern of ARV drugs detected that was consistent with ARV treatment. The classification for these men was changed from newly diagnosed (provisional) to previously diagnosed based on ARV drug test results. eThe classification for these men was not changed; their final classification was newly diagnosed. Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; c/mL, copies per milliliter; HIV, human immunodeficiency virus.
Seventy-one of the 83 (85.5%) men who had ARV testing (45.8% of 155 men initially characterized as newly diagnosed) had at least 1 ARV drug detected at enrollment (Figure 1, Supplementary Table 1). Detection of ARV drugs was more frequent among men with viral loads <400 copies/mL than among men with viral loads of 400–1000 copies/mL (63 of 68 [92.6%] vs 8 of 15 [53.3%], P =.0007). Among the 68 men with viral loads <400 copies/mL, detection of ARV drugs was associated with older age (>30 years), the absence of a sexually transmitted infection at enrollment, and lower household income (Supplementary Table 2). However, when the analysis included all 83 men with viral loads <1000 copies/mL, the only factor associated with ARV detection was older age.
In this study, detection of a PI and/or an NNRTI was considered to be indicative of ART. That pattern of drugs was detected in samples from 65 men (41.9% of 155 men initially characterized as newly diagnosed; Supplementary Table 1); those men were classified as being on ART at enrollment, and their enrollment classification was changed from newly diagnosed to previously diagnosed. In some cases, unusual combinations of drugs were detected (eg, 2 PIs other than ritonavir; 2 NNRTIs; PI with an NNRTI; Supplementary Table 1). Samples from 6 other men had 1 or 2 NRTIs detected in the absence of a PI or NNRTI. This suggested either incomplete adherence to an ART regimen or non-ART drug use (eg, PrEP). Twelve men did not have any ARV drugs detected.
Follow-up samples were available from 74 (89.2%) of the 83 men included in this substudy (10 from the 6-month visit; 64 from the 12-month visit); at least 1 ARV drug was detected in 45 (60.8%) of the 74 men with follow-up samples (Supplementary Table 1).
DISCUSSION
We used a qualitative, multidrug assay to identify black MSM in HPTN 061 who were on ART, but reported that they were unaware of their HIV status. ARV drugs were detected in 85.5% of samples with viral loads <1000 copies/mL and 92.6% of samples with viral loads <400 copies/mL; 65 of the 83 (78.3%) samples tested contained ARV drugs consistent with ART. Based on these data, 65 of the 155 (41.9%) men provisionally classified as newly diagnosed were reclassified as previously diagnosed. Most of those individuals continued to use ARV drugs after study enrollment. These results demonstrate that self-report may be unreliable for identifying newly diagnosed individuals. A variety of factors may influence the decision not to disclose prior HIV diagnosis when one is being considered for participation in a research study. In HPTN 061, study enrollment was capped for men with a prior HIV diagnosis who were already in care or reported only having unprotected anal intercourse with HIV-infected partners [7]. Therefore, some men may have chosen not to disclose their HIV status in order to be eligible for the study. Motivations to enroll in a research study are likely to vary considerably, based on the type of study, study population, incentives provided for participation, study interventions, improved access to clinical care, and other factors. An individual may also chose not to disclose his or her HIV infection status out of concern that this information will be shared with people other than the study staff (eg, sexual or network partners) and/or due to fear of stigma and discrimination.
ARV drugs are also used for PrEP or postexposure prophylaxis (PEP) [8, 9]; some individuals may continue to take a PrEP or PEP regimen after they become HIV infected, if they are not aware of their infection. ARV drugs are also used recreationally in some settings [10]. Therefore, some individuals who are taking ARV drugs may not be aware that they are infected. For this reason, it is important to consider the type of ARV drugs detected, to distinguish ART from other types of ARV drug use.
It is also important to note that many individuals who know they are infected may not be on ART. Therefore, ARV drug testing will only detect a subset of individuals with previously diagnosed HIV infection. Also, viral suppression may not be a reliable surrogate marker for ART. In HPTN 061, 12 of the 83 (14.5%) men with viral loads <1000 copies/mL who reported no prior diagnosis did not have ARV drugs detected (likely elite controllers); those men may not have known that they were HIV infected. Unusual drug combinations were observed in samples from some men in HPTN 061 (eg, multiple PIs, a PI with an NNRTI). This does not appear to be an artifact of the test method, as these combinations of drugs were not detected in >400 samples from other cohorts when the same method was used for ARV testing [6, 11].
Our findings demonstrate the importance of using an objective, biomedical measure in addition to self-report to distinguish between previously diagnosed and newly diagnosed HIV infections. Further studies are needed to understand why some participants may choose not to disclose their HIV status when enrolling in clinical trials. ARV drug testing may also be useful in surveillance studies and other studies assessing the frequency of undiagnosed HIV infection. The availability of a low-cost multidrug assay may facilitate ARV drug testing for these applications and for monitoring adherence to ARV drug regimens in research studies and HIV care settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the HPTN 061 study team and participants for providing the samples and data used in this study. We also thank the laboratory staff for assistance with sample management.
Financial support. This work was supported by the HIV Prevention Trials Network, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health, and the National Institute of Drug Abuse, Office of AIDS Research, of the National Institutes of Health (NIH; grants U01-AI068613/UM1-AI068613; U01-AI068617/UM1-AI068617; and U01-AI068619/UM1-AI068619). Additional support was provided by the NIAID, NIH (grant R01-AI095068).
Author contributions. M. A. M: developed methods used for antiretroviral drug testing; contributed to study design and data analysis; coordinated sample and data management for drug testing; reviewed results from antiretroviral drug testing. W. C.: developed methods used for antiretroviral drug testing; contributed to study design and data analysis; reviewed results from antiretroviral drug testing. L. W.: statistician for HPTN 061; responsible for statistical analyses. V. C.: HPTN Network Laboratory Quality Assurance/Quality Control Representative for HPTN 061; assisted with sample and data, management, and data analysis related to HIV infection status. T.-Y. L.: data analyst for HPTN 061; assisted with statistical analyses. E. P.-M.: responsible for overseeing laboratory testing in HPTN 061; assisted with data analysis related to HIV infection status. A. B.: performed antiretroviral drug testing; reviewed test results. S. G.: project coordinator for HPTN 061. S. B.: Principal Investigator for the HPTN 061 site in San Francisco. S. S.: Principal Investigator for the HPTN 061 site in Los Angeles. C. d. R.: Principal Investigator for the HPTN 061 site in Atlanta. M. M.: Principal Investigator for the HPTN 061 site in Washington, DC. S. M.: Principal Investigator for one HPTN 061 site in New York City. S. D. F.: chair of the HPTN 061 Black Caucus; assisted with cultural data interpretations. K. H. M.: protocol co-chair for HPTN 061; assisted with clinical interpretation of ARV test results; provided general information about the HPTN 061 study; Principal Investigator for the HPTN 061 site in Boston. D. P. W.: protocol co-chair for HPTN 061; assisted with clinical interpretation of ARV test results; provided general information about the HPTN 061 study. B. A. K.: protocol chair for HPTN 061; assisted with clinical interpretation of ARV test results; provided general information about the HPTN 061 study; Principal Investigator for one HPTN 061 site in New York City. S. H. E.: designed the study; coordinated the study; drafted and finalized the manuscript. J. M. F.: analyzed data related to HIV infection status and antiretroviral drug detection; drafted the manuscript. All authors contributed to the study and to the preparation of the manuscript. All authors reviewed and approved the final version of the manuscript prior to submission.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McNairy ML, Cohen M, El-Sadr WM. Antiretroviral therapy for prevention is a combination strategy. Curr HIV/AIDS Rep. 2013;10:152–8. doi: 10.1007/s11904-013-0152-1. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan AK, Savage EJ, Lowndes CM, et al. Non-disclosure of HIV status in UK sexual health clinics—a pilot study to identify non-disclosure within a national unlinked anonymous seroprevalence survey. Sex Transm Infect. 2013;89:120–1. doi: 10.1136/sextrans-2012-050801. [DOI] [PubMed] [Google Scholar]
- 3.Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multi-national clinical trial (HPTN 052) [epub ahead of print] J Infect Dis. 2013 doi: 10.1093/infdis/jit390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahle EM, Kashuba A, Baeten JM, et al. Unreported antiretroviral use by HIV-1 infected participants enrolling in a prospective research study. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e3182a2db02. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minnis AM, Gandham S, Richardson BA, et al. Adherence and acceptability in MTN 001: a randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2013;17:737–47. doi: 10.1007/s10461-012-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzinke MA, Breaud A, Clarke W. Development of a high resolution accurate mass method for the multiplexed monitoring of antiretroviral agents in human serum. In: 47th Meeting of the Academy of Clinical Laboratory Physicians and Scientists, Milwaukee, WI, 31 May–2 June, 2012. [Google Scholar]
- 7.Koblin BA, Mayer KH, Eshleman SH, et al. Correlates of HIV acquisition in a cohort of black men who have sex with men in the United States: HIV Prevention Trials Network (HPTN) 061. PLoS One. 2013;8:e70413. doi: 10.1371/journal.pone.0070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber J, Tatoud R, Fidler S. Postexposure prophylaxis, preexposure prophylaxis or universal test and treat: the strategic use of antiretroviral drugs to prevent HIV acquisition and transmission. AIDS. 2010;24(suppl 4):S27–39. doi: 10.1097/01.aids.0000390705.73759.2c. [DOI] [PubMed] [Google Scholar]
- 9.Zablotska IB, Prestage G, de Wit J, Grulich AE, Mao L, Holt M. The informal use of antiretrovirals for preexposure prophylaxis of HIV infection among gay men in Australia. J Acquir Immune Defic Syndr. 2013;62:334–8. doi: 10.1097/QAI.0b013e31827e854a. [DOI] [PubMed] [Google Scholar]
- 10.Grelotti DJ, Closson EF, Mimiaga MJ. Pretreatment antiretroviral exposure from recreational use. Lancet Infect Dis. 2013;13:10–12. doi: 10.1016/S1473-3099(12)70294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laeyendecker O, Piwowar-Manning E, Fiamma A, et al. Estimation of HIV incidence in a large, community-based, randomized clinical trial: NIMH Project Accept (HIV Prevention Trials Network 043) PLoS One. 2013;8:e68349. doi: 10.1371/journal.pone.0068349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.