Abstract
Purpose The aim of this study was to assess the impact of 3-D navigation for pedicle screw placement accuracy in minimally invasive transverse lumbar interbody fusion (MIS-TLIF).
Methods A retrospective review of 52 patients who had MIS-TLIF assisted with 3D navigation is presented. Clinical outcomes were assessed with the Oswestry Disability Index (ODI), Visual Analog Scales (VAS), and MacNab scores. Radiographic outcomes were assessed using X-rays and thin-slice computed tomography.
Result The mean age was 56.5 years, and 172 screws were implanted with 16 pedicle breaches (91.0% accuracy rate). Radiographic fusion rate at a mean follow-up of 15.6 months was 87.23%. No revision surgeries were required. The mean improvement in the VAS back pain, VAS leg pain, and ODI at 11.3 months follow-up was 4.3, 4.5, and 26.8 points, respectively. At last follow-up the mean postoperative disc height gain was 4.92 mm and the mean postoperative disc angle gain was 2.79 degrees. At L5–S1 level, there was a significant correlation between a greater disc space height gain and a lower VAS leg score.
Conclusion Our data support that application of 3-D navigation in MIS-TLIF is associated with a high level of accuracy in the pedicle screw placement.
Keywords: minimally invasive spine surgery, transforaminal lumbar interbody fusion, 3D-NAV, neuronavigation, pedicle screw
Advances in minimally invasive spinal surgery (MIS) are changing the way we approach the operative treatment of some spinal disorders. Since the development of tubular retractors facilitating a microsurgical decompression of spinal degenerative pathology,1 MIS has evolved to permit placement of instrumentation percutaneously or through mini-open approaches.2 3 4 These techniques may show improved outcomes and reduced complications, such as a reduction of persistent back problems caused by muscular atrophy and decreased trunk extensor strength from iatrogenic muscle denervation.5 6 7 8 9 10
Transforaminal lumbar interbody fusion (TLIF) is one of the most commonly performed lumbar fusion procedures. Evolved from a posterior lumbar interbody fusion approach, it allows an anterior fusion through a unilateral posterior approach while avoiding the risks associated with bilateral posterior lumbar dissection and graft placement. The traditional open TLIF procedure has been adapted for minimally invasive approaches (MIS-TLIF) (Fig. 4).
Figure 4.
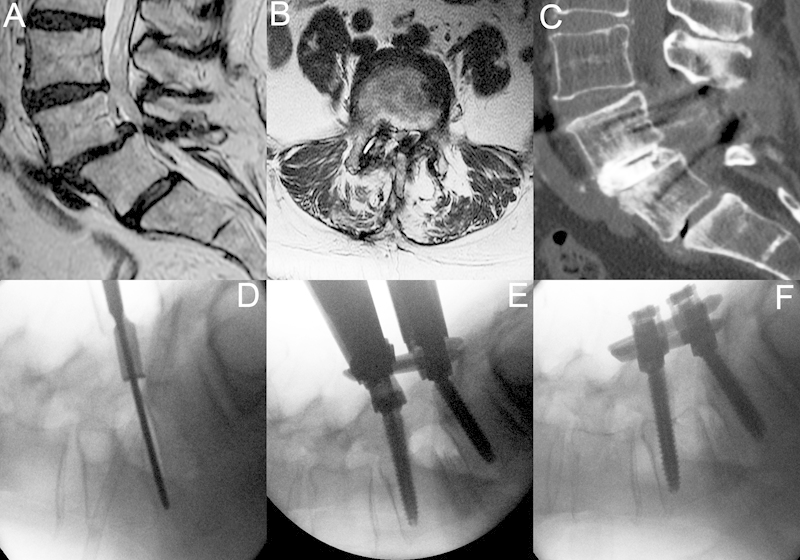
A 55 year old man with history of back pain and neurogenic claudication. This patient had failed a previous laminectomy and further nonoperative treatment and underwent a minimally invasive lumbar redo laminectomy, discectomy, interbody fusion, and instrumentation through a 22-mm tubular retractor. (A, B) Lumbar magnetic resonance imaging shows grade I/II spondylolisthesis with severe stenosis. (C) Postoperative computed tomography 18 months after surgery. (D) Lateral X-ray on the operating room table reveals a grade II spondylolisthesis. A 22-mm tubular retractor is in place, and the disc space is entered and discectomy is performed. (E, F) An expandable cage has been inserted and bone graft has been placed. Instrumentation has been placed and the spondylolisthesis is reduced by locking down the L5 cap and reducing L4.
Accurate pedicle screw placement using MIS techniques requires significant alterations in the operative technique and a reliance on radiographic guidance. Either intraoperative fluoroscopy with sequential biplanar imaging, or intraoperative guidance with stereotactic real-time neuronavigation based on a computed tomography (CT) scan (preoperatively or intraoperative acquisition), can be undertaken.
Conventional 2-D fluoroscopic-guided techniques have higher screw misplacement rates in both cadaveric and clinical studies when compared with 3-D navigation and require greater X-ray exposure.11 12 13 14 15 16 17
The use of frameless navigation systems combined with 3-D fluoroscopy (3D-NAV) may be an important contribution to MIS, providing the surgeon an intraoperative visual 3-D approximation of the anatomy. The purpose of our study was to review our MIS-TLIF with 3D-NAV-assisted pedicle screw instrumentation surgical series to assess radiographic and clinical outcome parameters.
Materials and Methods
Fifty-two consecutive patients who had undergone MIS-TLIF surgery for degenerative disease with 3D-NAV were included; surgeries were performed by a single surgeon between July 2005 and January 2010. Mini-open or percutaneously placed pedicle screws were used in all cases, either unilaterally or bilaterally. Degenerative spondylolisthesis was present in 59.6% of the patients; 46.1% (24 patients) had grade I spondylolisthesis, and 13.4% (7 patients) had grade II. Patient demographics are shown in as Table 1. Single-level MIS-TLIF was performed in all patients.
Table 1. Summary of Patient Demographic and Clinical Characteristics in 52 Cases.
| Characteristic | Value |
|---|---|
| Male:female | 30:22 |
| Mean age, y (range) | 56.5 (31–84) |
| Preoperative diagnosis | |
| Degenerative grade I spondylolisthesis | 24 (46.2%) |
| Degenerative grade II spondylolisthesis | 7 (13.4%) |
| Degenerative disc disease with back or leg pain | 17 (32.7%) |
| Previous surgery/recurrent disc herniation | 4 (7.7%) |
| Levels involved | |
| L2–L3 | 2 (3.8%) |
| L3–L4 | 2 (3.8%) |
| L4–L5 | 35 (67.3%) |
| L5–S1 | 13 (25.0%) |
Surgical Technique
Endotracheal general anesthesia and a radiolucent Jackson table were utilized for all patients. Fluoroscopic imaging guided the incision placement; in an anterior-posterior plane this generally aligned with the outer margins of the facet joint of interest. A Wiltse transmuscular approach18 was utilized, and serial dilators (Insight Access® system, Synthes Spine, Westchester, PA; or METRx® retractors, Medtronic Sofamor Danek, Memphis TN) were introduced on the side of decompression and positioned toward the facet joint to be removed. A 22-mm tubular retractor was fixed into position. The surgical microscope was introduced (ZEISS Pentero, Carl Zeiss AG, Jena, Germany) and a complete facetectomy was undertaken with a high-speed drill (Anspach, Palm Beach Gardens FL). In most cases, a laminectomy was performed by angling the tube medially and undercutting the spinous process and contralateral lamina. A discectomy was then performed and the vertebral endplates carefully prepared. All interbody fusions were performed with a polyetheretherketone (PEEK) implant, recently with an expandable PEEK cage (Spine Wave Inc., Shelton, CT). Synthetic bone graft materials (Actifuse®, Apatech, Hertfordshire, UK; DBX®, Synthes Spine, Westchester, PA; bone morphogenic protein [BMP], Medtronic Sofamor Danek, Memphis, TN) were utilized in addition to morselized bone. Fusion was confined only to the disc space, and neither the contralateral facets nor the intertransverse processes were fused.
The navigation reference array (VectorVision®, BrainLAB AG, Feldkirchen, Germany), was attached with two percutaneous Steinman pins to the posterior iliac crest or on a spinous process using the SP clamp for cases including L3 and above. A 3-D image set was obtained using the Siremobil ISO-C 3D (Siemens AG, Munich, Germany) and the data imported into the navigation system. The ideal transpedicular trajectory was determined, and the diameter and length of the planned screws simulated. A navigated drill guide was then used to create a 3.2-cm starting hole into the pedicle. Kirschner wires were introduced, and a second spin of the ISO-C 3-D arm was performed to verify position. The pedicles were undertapped and the appropriate MIS screw inserted. Every attempt was made to use the longest and largest diameter pedicle screw (usually 7 mm). Connecting rods were then inserted. The fascia was closed with an absorbable suture, and the subcutaneous tissue and skin were closed conventionally.
Clinical Evaluation
Prospectively collected clinical outcome measures were reviewed. The 100-point Oswestry Disability Index (ODI)19 and 10-point Visual Analog Scale (VAS) score for back and leg pain were collected pre- and postoperatively. The MacNab rating20 was recorded postoperatively. Hospital records and surgical notes were reviewed to assess surgical times, blood loss, complications, and length of stay. A specialist-trained nurse who collected the follow-up data assessed all patients.
Radiological Evaluation
A thin-slice CT scan was performed within 24 hours postoperatively and repeated at 1 year; plain film radiography was used for intermediate follow-up at 3 and 6 months. A standardized technique21 22 was used to evaluate the TLIF implant, with disc space height and angle measurements, as shown in Fig. 1.
Figure 1.
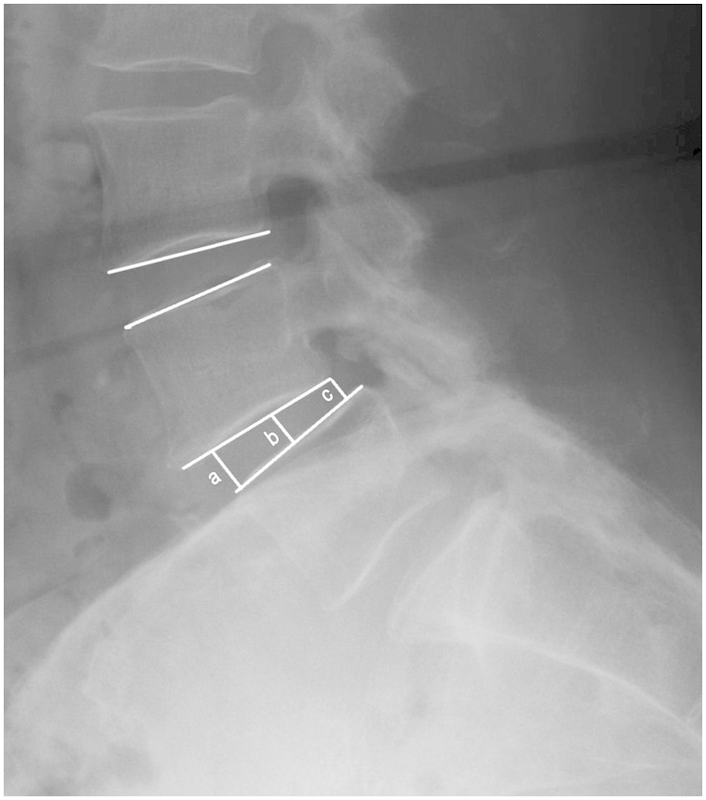
Disc space height ([a + b + c]/3) and angle measurement techniques.
The accuracy of pedicle screw placement using the 3D-NAV system was determined on the postoperative CT scan, using the grading system described by Rajasekaran et al12:
Grade 0: no pedicle perforation;
Grade 1: <2-mm threads outside the pedicle;
Grade 2: 2 to 4 mm of core screw diameter outside pedicle; and
Grade 3: entire screw outside the pedicle.
Fusion was defined as osseous bridging between the end plates in coronal and sagittal reconstruction images of CT scans or absence of mobility on flexion/extension lateral radiographs23 (<5 degrees of angular motion, <3 mm of translational motion). Fusion was determined by two neuroradiologists (A.J.T., C.G.) and a Neurosurgical Fellow (J.T.).
Statistical Analysis
Descriptive statistics (including frequency, percent, mean, median, standard deviation, and range) for demographic and clinical variables were calculated to characterize the study sample. The paired t test was used to assess pre- versus postoperative changes in the VAS back, VAS leg, and ODI scores.
The Pearson correlation coefficient was used to assess the correlation between postoperative VAS/ODI and average postoperative disc height, postoperative interspace angle, postoperative average disc height gain, and postoperative average angle gain.
Correlations for height/angle gain were also stratified by level of the interbody fusion (L5–S1 versus all other levels).
The two-sample t test was used to compare the above four parameters between MacNab function score categories of good/excellent and fair/poor. The two-sample t test was also used to evaluate differences in mean postoperative VAS/ODI between age groups (≤60 versus >60), gender, and body mass index (BMI; ≤27 versus >27). The chi-square test was used to assess the association between age/gender/BMI categories and MacNab functional categories. Similarly, the chi-square test was used to evaluate the association between fusion status (yes/no) and (1) age category, (2) gender, (3) BMI category, (4) smoking status, (5) diabetes status, and (6) steroid use. All p values are two-sided with statistical significance evaluated at the 0.05 α level. All analyses were performed in SPSS Version 18.0 (SPSS Inc., Chicago, IL) by an independent statistician.
Results
Clinical Parameters
At a mean clinical follow-up of 11.3 (±9.2) months, the mean improvement in the VAS back score was 4.3 points and in the VAS leg score was 4.5 (both p < 0.0001 based on 50 patients with pre- and postoperative scores). Mean ODI improvement was 26.8 points (p < 0.0001 based on 28 patients with pre- and postoperative scores. Excellent/good McNab outcomes were recorded for 84.6%. There was one dural tear, which was repaired intraoperatively. Two patients developed superficial surgical site infections and were successfully treated with oral antibiotics. One patient was admitted 10 days after surgery with a diagnosis of deep vein thrombosis and bilateral pulmonary embolism and appropriate anticoagulation was initiated. Clinical and operative parameters are depicted in Figs. 2 and 3 and Tables 2 and 3.
Figure 2.
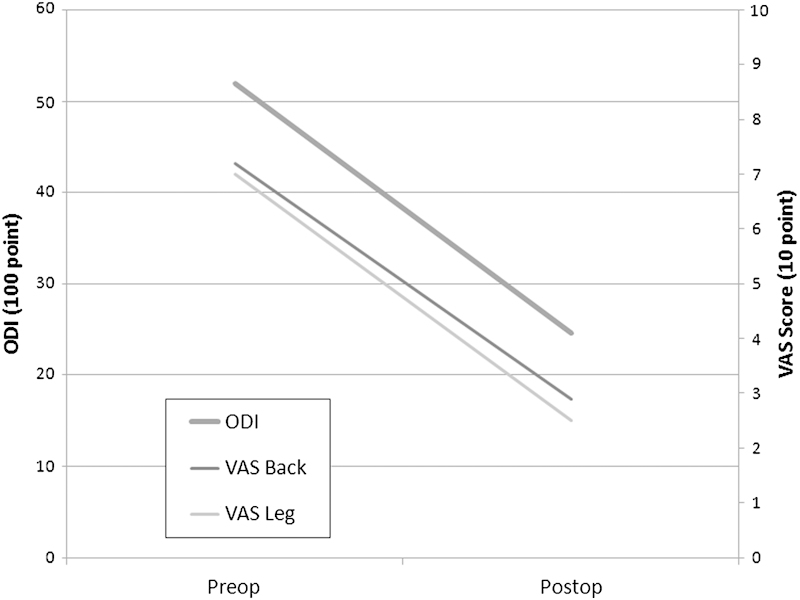
Preoperative versus postoperative functional outcomes. ODI, Oswestry Disability Index; VAS, Visual Analog Scale.
Figure 3.
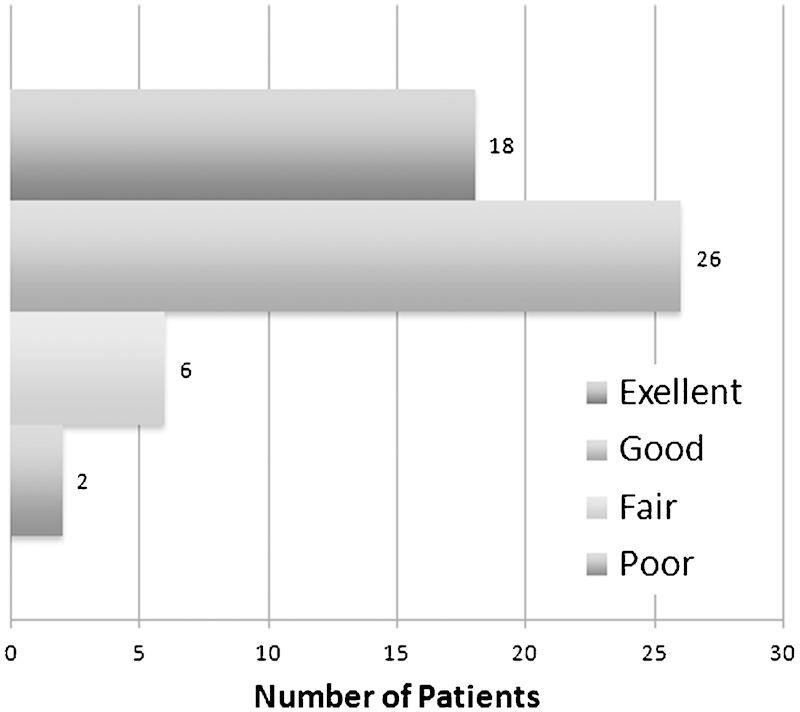
MacNab outcome scores.
Table 2. Operative Outcomes.
| Value (SD) | |
|---|---|
| Operative time (min) | 247 (±59) |
| Length of stay (d) | 4.0 (±2.2) |
| Blood loss (mL) | 166 (±158) |
| Complications | |
| Dural tear | 1 |
| Surgical site infection | 2 |
| DVT/PE | 1 |
DVT, deep venous thrombosis; PE, pulmonary embolism; SD, standard deviation.
Table 3. Clinical and Radiological Outcomes.
| Preoperative (Range) | Postoperative (Range) | Change | |
|---|---|---|---|
| Clinical outcome | |||
| VAS back | 7.2 (0–10) | 2.9 (0–10) | −4.3 |
| VAS leg | 7.0 (0–10) | 2.5 (0–9) | −4.5 |
| ODI | 51.9 (8–76) | 24.6 (0–70) | −26.8 |
| McNab | |||
| Excellent | 18 (34.6%) | ||
| Good | 26 (50%) | ||
| Fair | 6 (11.5%) | ||
| Poor | 2 (3.8%) | ||
| Radiological outcome | |||
| Disc space height (mm) | |||
| L2–L5 | 4.5 (0–12.04) | 9.2 (6.65–12.15) | +4.7 (−4.28–11.04) |
| L5–S1 | 2.6 (0–7.96) | 8.9 (6.19–12.21) | +6.3 (1.36–12.21) |
| Disc space angle (degrees) | |||
| L2-L5 | 6.6 (−2.8–17.9) | 7.0 (0.9–16.9) | +2.2 (−5.8–10.4) |
| L5–S1 | 7.6 (1.1–15.3) | 8.7 (0.8–17.2) | +4.3 (−6.9–17.2) |
| Fusion rate | |||
| Unilateral screws | 75.0% | ||
| Bilateral screws | 93.3% | ||
ODI, Oswestry Disability Index; VAS, Visual Analog Scale.
Radiological Outcomes
Disc Space Height and Angle Measurements
At a mean of 91 days postoperatively the overall mean disc height gain was 5.1 mm; L2–L5 interspaces gained 4.7 mm (range 4.2 to 11.0) and L5–S1 interspaces gained 6.3 mm (range 1.3 to 12.2). When disc height gain was compared with outcome for the L5–S1 level, there was a significant correlation between larger disc height gain and improved leg pain VAS (r = −0.56, p = 0.049) and lower ODI score (r = −0.62, p = 0.057) but not VAS back pain or McNab criteria; no correlation was present at other levels.
The mean postoperative disc angle gain at L2–L5 was 2.2 degrees (range 5.8 to 10.4 degrees) and at L5–S1 was 4.3 degrees (range 6.9 to 17.2 degrees), and the overall mean postoperative disc space angle gain was 2.7 degrees. No significant correlation with any outcome was found.
Screw Precision Measurements
One hundred seventy-two pedicle screws in 50 patients were visualized adequately on CT scan to enable accuracy assessment. Eighteen patients underwent unilateral screw placement. There were 16 pedicle breaches (9% misplacement); 6 (37.5%) were classified as grade 1 and 10 (62.5%) as grade 2. All medial breaches were grade 1. There were no neurological or clinical sequelae associated any breach, and no revision surgery was required. There were 12 (75%) lateral breaches, of which 8 (75%) were in the upper MIS-TLIF screw and 4 (25%) were medial breaches (Table 4). Misplacement rates were assessed by spinal levels, and a positive correlation was found between the distance from the iliac crest reference array and the misplacement rate. Nonetheless, due to limited numbers, this was not statistically significant.
Table 4. Type and Direction of Pedicle Breaches.
| Breach | Medial Breach | Lateral Breach | Total | |
|---|---|---|---|---|
| Cranial Screw | Caudal Screw | |||
| Grade 1 | 4 | 1 | 1 | 6 (37.5%) |
| Grade 2 | 0 | 7 | 3 | 10 (62.5%) |
| Grade 3 | 0 | 0 | 0 | 0 (0%) |
| Total | 4 (25%) | 8 (50%) | 4 (25%) | 16 (100%) |
Fusion Rates
At a mean follow-up of 15.6 (±13.8) months, the overall fusion rate was 87.2% in the 49 patients who had a study adequate to evaluate fusion. Fusion was statistically greater with the use of bilateral screws (93.3%) than with unilateral screws (75%; p = 0.036), although the results are limited due to the low numbers. No significant differences in fusion rates were demonstrated for gender, BMI, or smoking. Overall fusion rates for smokers was 80% (12/15), for diabetic patients was 75% (3/4), and for chronic steroid users was 100% (4/4).
The fusion rate with Actifuse® alone was 22/25 (88.0%), for BMP alone was 11/13 (84.6%), for DBX alone was 1/3 (33.3%), and for a combination of autogenous bone, Actifuse, and BMP was 7/7 (100%). Due to limited numbers, no conclusions could be made with regards to the difference between the graft materials. Autologous bone marrow with Healos® bone graft was used in one case without obtaining fusion (0%).
Discussion
Clinical Results
Several studies have shown that clinical results after MIS-TLIF are comparable to open surgery while being associated with less blood loss and frequently shorter hospital stay.24 25 26 Nonetheless, relative higher costs are among the factors that also need to be taken into consideration. Our study showed similar perioperative results with the previous studies. In Shin et al's recent meta-analysis, no significant differences were found in the operative time between navigated and nonnavigated pedicle screw insertion techniques.27 For clinical outcome, although in our study no conclusions could have been made due to the limited numbers of patients with preoperative ODI scores, our values were found to be similar to the values previously published in the literature.
Radiographic Parameters
Our results corroborate a recent meta-analysis that found a fusion rate of 94.8% with MIS-TLIF versus 90.9% with open TLIF. Our fusion results are within the range reported in the literature. We found that fusion rates were superior with the use of bilateral pedicle screws (93.3%) versus unilateral pedicle screws (75%). For this reason, we have discontinued the use of unilateral screw constructs in our practice.
We observed a postoperative mean gain in disc height of 4.5 mm at L2–L5 and 5.8 mm at L5–S1, with corresponding gains in disc space angle of 2.2 and 4.3 degrees. MIS-TLIF is therefore capable of disc height improvement and lordosis correction.
Pedicle Screw Accuracy
Pedicle screws were introduced by Boucher28 in 1959 and were improved and standardized by the efforts of Roy-Camille et al in 1976.29 Pedicle screws have become the most common type of instrumentation used in spinal surgery. The Kosmopoulos and Schizas30 meta-analysis from 2007 of in vivo lumbar pedicle screws reported a weighted mean accuracy rate of 87.3% (of 1674) for nonnavigated screws and 92.1% (of 864) for navigated screw insertion.
Navigation for Pedicle Screw Placement
The first report of the combination of CT and stereotaxy for the placement of transpedicular screws was by Nolte et al31 in 1995. Subsequent studies reporting the accuracy of screw positioning using various intraoperative navigation aids32 33 34 35 36 have reported misplacement rates ranging from 6 to 25%.
Most recently, Tian and Lang37 in a meta-analysis reported a significantly reduced incidence of pedicle violation with CT-based navigation. The superiority of navigation systems was especially clear when applied to abnormal spinal anatomy, and no significant difference between navigation systems was revealed. This is consistent with Nottmeier et al's38 series, which used a similar grading system as the present study, that showed a 7.5% overall misplacement rate. Additionally when evaluating the diameter of lumbar pedicle screws, they found that 71% of the 765 lumbar screws were ≥7.5 mm in diameter. This is in accordance with our series where we found that the use of 3D-NAV generally facilitated safe and accurate placement of larger (≥7 mm) diameter screws.
In their retrospective cohort study, Larson et al reported their results on the application of intraoperative CT and image-guided navigation system for the placement of pedicle screws. They assessed the accuracy rate of screw placement in their group of 50 pediatric scoliotic patients (984 screws placed) and compared it with the results on adults (1151 screws) who were operated on by the same imaging-guided technique. Although the accuracy rate of pedicle screw placement in their pediatrics group was statistically lower compared with the adult group (98.2% versus 96.4%), their accuracy rate for navigation in children was significantly higher than the findings from a meta-analysis of predominantly nonnavigated screws in children, which was reported to be 94.9%.39
In a recent meta-analysis on the perforation risk with computer-navigated pedicle screw insertion versus freehand insertion, the relative risk for pedicle screw perforation was determined to be 0.39, favoring navigation. The perforation risk was 6% with navigated insertion (4814 screws total), whereas it was 15% with the nonnavigated conventional insertion (3725 screws total).27
MIS and Navigation
Few studies specifically evaluate the accuracy of 3D-NAV for pedicle screw insertion with MIS techniques (Table 5). In 2003 Holly and Foley40 conducted a cadaveric study utilizing 3D-NAV to introduce percutaneous pedicle screws in the thoracic and lumbar spine, with a 94.7% accuracy (89/94 screws). Villavicencio et al41 reported their clinical experience with percutaneously placed pedicle screws showing a malposition rate of 1.5% in 220 lumbosacral screws. In a subsequent study considering breaches greater than 2 mm, Villavicencio et al42 reported 2/43 screws malpositioned in MIS-TLIF. Nakashima et al43 published a series of 67 patients with degenerative spondylolisthesis comparing conventional fluoroscopically guided pedicle screw placement on one side to subsequent contralateral placement using ISO-C 3D-NAV screws. The median pedicle screw placement accuracy was 79.0% and 96.1%, respectively. Fraser et al,44 reporting their initial experience comparing 29 MIS patients using Iso-C 3D-NAV with 13 patients with conventional fluoroscopy, demonstrated an accuracy (no breaches) of 90.9% and 73.7%, respectively.
Table 5. Studies in MIS 3D-NAV.
| Author | Definition of Accuracy (mm) | Type of Study | Screws | Breaches (Grade) | Accuracy (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thoracic | Lumbar | Overall | 1 | 2 | 3 | Thoracic | Lumbar | Overall | |||
| Holly et al (2003)40 | a | Cadaver | 64 | 30 | 94 | Not available | 92 | 100 | 94.7 | ||
| Villavicencio et al (2005)41 | <2 | Patient | — | 220 | — | 2 | 0 | 2 (1 M,1 L) | — | 98.2 | — |
| Nakashima et al (2009)43 | <2 | Patient | — | 150 | — | 11c (2 M,1 L) | 0 | — | 93 | — | |
| Torres et al (2010) | b | Patient | — | 178 | — | 6 (4 M, 2 L) | 10 (0 M,10 L) | 0 | — | 91 | — |
| Fraser et al (2010)44 | a | Patient | — | 66 | — | 6 | 0 | 0 | — | 91 | — |
MIS-3D-NAV, minimally invasive frameless navigation systems combined with 3-D fluoroscopy; M, medial; L, lateral.
Breach/no breach.
None, threads <2 mm, 2–4 mm, full screw.
Combined grade 1 and 2.
Analysis of pedicle screw accuracy is complicated by many factors, the most important of which is that the evaluation of pedicle integrity is not standardized. The Kosmopoulos and Schizas meta-analysis30 of 130 papers demonstrated that only 50% stated how pedicle screw placement was assessed, and 35 different assessment methods were reported. Different approaches (mini-open, percutaneous) and incision size can offer various degrees of digital feedback for the surgeon. The navigation systems themselves offer different accuracy.45 Surgeon experience improves placement.34 Cadaveric studies generally seem to report higher accuracy rates perhaps due to “softer” bone quality.
We documented a pedicle screw misplacement rate of 9%, which is comparable to findings in the literature. Most of the breaches were lateral and all breaches were clinically insignificant and no revision was necessary. This finding is in accordance with reports of low clinical sequelae associated with minor pedicle breaches with computer-assisted navigation.33 34 35 Of the 16 pedicle breaches, four were medial and all were grade 1 (<2 mm). We believe that the majority of our lateral breaches were related to the surgeon's preference for an insertion point of the cranial screws in the lateral aspect of the inferior facet, to avoid injury and involvement of the adjacent facet. This led to “purposeful” lateral breaches that were detected intraoperatively and accepted.
When misplacement rates were assessed by spinal level, it was evident that as the distance from the iliac crest reference array grew, the misplacement rate increased. This relation was also evident in the studies by Quiñones-Hinojosa et al46 and Fraser et al44 emphasizing the importance of appropriate placement of the reference array. Therefore, for instrumentation at L3 and above we now use the L2 or L3 spinous process for placement.
Limitations
There are limitations of this study, common to retrospective series. Some of the preoperative imaging studies and clinical outcomes were not available for all patients, data on radiation exposure were not collected, and some patients were unavailable for final assessment. The use of various types of instrumentation and fusion technologies over the study period may have affected the learning curve and thus the results—for example, different types of cages and graft materials were used and these may have affected radiographic outcome and fusion rates. The extent of radiation exposure to the patient and to the surgical team was not evaluated in this study. However, Nottmeier et al recently reported that there was no radiation exposure to the surgeon in a series of spine fusion cases, when navigation was used with a technique and workflow very similar to ours.47
Conclusions
In comparison to fluoroscopy, application of 3D-NAV in MIS-TLIF has the potential to facilitate surgery, leading to radiographic and clinical results that are comparable to open surgery. As improved software and newer imaging technologies such as intraoperative CT scanners become more widely available, we expect 3-D navigation to gain wider acceptance.
Acknowledgments
We thank AO Spine for fellowship support of Dr. Andrew R. James. We thank Dr. Paul Christos from Weill Cornell Biostatistics and Research Methodology Core, who helped with the statistical analysis, and also Michael Macielak, B.S., who helped with the preparation of the manuscript.
Footnotes
Disclosures Jorge Torres, None Andrew R. James, None Marjan Alimi, None Apostolos John Tsiouris, None Christian Geannette, None Roger Härtl, Consulting: Synthes, SpineWave, Brainlab, Lanx
References
- 1.Perez-Cruet M J, Foley K T, Isaacs R E. et al. Microendoscopic lumbar discectomy: technical note. Neurosurgery. 2002;51(5, Suppl):S129–S136. [PubMed] [Google Scholar]
- 2.Tredway T L Santiago P Hrubes M R Song J K Christie S D Fessler R G Minimally invasive resection of intradural-extramedullary spinal neoplasms Neurosurgery 2006581, SupplONS52–ONS58.; discussion ONS52–ONS58 [DOI] [PubMed] [Google Scholar]
- 3.O'Toole J E, Eichholz K M, Fessler R G. Minimally invasive far lateral microendoscopic discectomy for extraforaminal disc herniation at the lumbosacral junction: cadaveric dissection and technical case report. Spine J. 2007;7:414–421. doi: 10.1016/j.spinee.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh P C, Koski T R, Sciubba D M. et al. Maximizing the potential of minimally invasive spine surgery in complex spinal disorders. Neurosurg Focus. 2008;25:E19. doi: 10.3171/FOC/2008/25/8/E19. [DOI] [PubMed] [Google Scholar]
- 5.Weber B R, Grob D, Dvorák J, Müntener M. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine. 1997;22:1765–1772. doi: 10.1097/00007632-199708010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi Y, Yabuki S, Styf J. et al. Back muscle injury after posterior lumbar spine surgery. Topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine. 1996;21:2683–2688. doi: 10.1097/00007632-199611150-00019. [DOI] [PubMed] [Google Scholar]
- 8.Hyun S J, Kim Y B, Kim Y S. et al. Postoperative changes in paraspinal muscle volume: comparison between paramedian interfascial and midline approaches for lumbar fusion. J Korean Med Sci. 2007;22:646–651. doi: 10.3346/jkms.2007.22.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D Y, Lee S H, Chung S K, Lee H Y. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine. 2005;30:123–129. [PubMed] [Google Scholar]
- 10.Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19:316–324. doi: 10.1007/s00586-009-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berlemann U, Monin D, Arm E, Nolte L P, Ozdoba C. Planning and insertion of pedicle screws with computer assistance. J Spinal Disord. 1997;10:117–124. [PubMed] [Google Scholar]
- 12.Rajasekaran S, Vidyadhara S, Ramesh P, Shetty A P. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine. 2007;32:E56–E64. doi: 10.1097/01.brs.0000252094.64857.ab. [DOI] [PubMed] [Google Scholar]
- 13.Laine T, Schlenzka D, Mäkitalo K, Tallroth K, Nolte L P, Visarius H. Improved accuracy of pedicle screw insertion with computer-assisted surgery. A prospective clinical trial of 30 patients. Spine. 1997;22:1254–1258. doi: 10.1097/00007632-199706010-00018. [DOI] [PubMed] [Google Scholar]
- 14.Schwarzenbach O, Berlemann U, Jost B. et al. Accuracy of computer-assisted pedicle screw placement. An in vivo computed tomography analysis. Spine. 1997;22:452–458. doi: 10.1097/00007632-199702150-00020. [DOI] [PubMed] [Google Scholar]
- 15.Nolte L, Zamorano L, Arm E. et al. Image-guided computer-assisted spine surgery: a pilot study on pedicle screw fixation. Stereotact Funct Neurosurg. 1996;66:108–117. doi: 10.1159/000099677. [DOI] [PubMed] [Google Scholar]
- 16.Nolte L P, Zamorano L, Visarius H. et al. Clinical evaluation of a system for precision enhancement in spine surgery. Clin Biomech (Bristol, Avon) 1995;10:293–303. doi: 10.1016/0268-0033(95)00004-5. [DOI] [PubMed] [Google Scholar]
- 17.Merloz P, Tonetti J, Pittet L, Coulomb M, Lavalleé S, Sautot P. Pedicle screw placement using image guided techniques. Clin Orthop Relat Res. 1998;(354):39–48. doi: 10.1097/00003086-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Wiltse L L, Bateman J G, Hutchinson R H, Nelson W E. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg Am. 1968;50:919–926. [PubMed] [Google Scholar]
- 19.Fairbank J C, Couper J, Davies J B, O'Brien J P. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 20.Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891–903. [PubMed] [Google Scholar]
- 21.Inoue H, Ohmori K, Miyasaka K, Hosoe H. Radiographic evaluation of the lumbosacral disc height. Skeletal Radiol. 1999;28:638–643. doi: 10.1007/s002560050566. [DOI] [PubMed] [Google Scholar]
- 22.Quint D J, Tuite G F, Stern J D. et al. Computer-assisted measurement of lumbar spine radiographs. Acad Radiol. 1997;4:742–752. doi: 10.1016/s1076-6332(97)80078-5. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration. Guidance for Industry and/or FDA Staff: Guidance Document for the Preparation of IDEs for Spinal Systems. 2010 Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm073771.htm#_Toc472296067. Accessed January 13, 2000
- 24.Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Orthop. 2009;33:1683–1688. doi: 10.1007/s00264-008-0687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacs R E, Podichetty V K, Santiago P. et al. Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine. 2005;3:98–105. doi: 10.3171/spi.2005.3.2.0098. [DOI] [PubMed] [Google Scholar]
- 26.Scheufler K M Dohmen H Vougioukas V I Percutaneous transforaminal lumbar interbody fusion for the treatment of degenerative lumbar instability Neurosurgery 2007604, Suppl 2203–212.; discussion 212–213 [DOI] [PubMed] [Google Scholar]
- 27.Shin B J, James A R, Njoku I U, Härtl R. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine. 2012;17(2):113–122. doi: 10.3171/2012.5.SPINE11399. [DOI] [PubMed] [Google Scholar]
- 28.Boucher H H. A method of spinal fusion. J Bone Joint Surg Br. 1959;41-B:248–259. doi: 10.1302/0301-620X.41B2.248. [DOI] [PubMed] [Google Scholar]
- 29.Roy-Camille R, Saillant G, Berteaux D, Salgado V. Osteosynthesis of thoraco-lumbar spine fractures with metal plates screwed through the vertebral pedicles. Reconstr Surg Traumatol. 1976;15:2–16. [PubMed] [Google Scholar]
- 30.Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine. 2007;32:E111–E120. doi: 10.1097/01.brs.0000254048.79024.8b. [DOI] [PubMed] [Google Scholar]
- 31.Nolte L P, Zamorano L J, Jiang Z, Wang Q, Langlotz F, Berlemann U. Image-guided insertion of transpedicular screws. A laboratory set-up. Spine. 1995;20:497–500. doi: 10.1097/00007632-199502001-00016. [DOI] [PubMed] [Google Scholar]
- 32.Merloz P, Huberson C, Tonetti J. Computer-assisted pedicle screw insertion. Tech Orthop. 2003;18:149–159. [Google Scholar]
- 33.Rampersaud Y R, Pik J H, Salonen D, Farooq S. Clinical accuracy of fluoroscopic computer-assisted pedicle screw fixation: a CT analysis. Spine. 2005;30:E183–E190. doi: 10.1097/01.brs.0000157490.65706.38. [DOI] [PubMed] [Google Scholar]
- 34.Rampersaud Y R, Simon D A, Foley K T. Accuracy requirements for image-guided spinal pedicle screw placement. Spine. 2001;26:352–359. doi: 10.1097/00007632-200102150-00010. [DOI] [PubMed] [Google Scholar]
- 35.Schizas C, Michel J, Kosmopoulos V, Theumann N. Computer tomography assessment of pedicle screw insertion in percutaneous posterior transpedicular stabilization. Eur Spine J. 2007;16:613–617. doi: 10.1007/s00586-006-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjardes T, Shafizadeh S, Rixen D. et al. Image-guided spine surgery: state of the art and future directions. Eur Spine J. 2010;19:25–45. doi: 10.1007/s00586-009-1091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian W, Lang Z. Placement of pedicle screws using three-dimensional fluoroscopy-based navigation in lumbar vertebrae with axial rotation. Eur Spine J. 2010;19:1928–1935. doi: 10.1007/s00586-010-1564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nottmeier E W, Seemer W, Young P M. Placement of thoracolumbar pedicle screws using three-dimensional image guidance: experience in a large patient cohort. J Neurosurg Spine. 2009;10:33–39. doi: 10.3171/2008.10.SPI08383. [DOI] [PubMed] [Google Scholar]
- 39.Larson A N, Santos E R, Polly D W Jr. et al. Pediatric pedicle screw placement using intraoperative computed tomography and 3-dimensional image-guided navigation. Spine. 2012;37:E188–E194. doi: 10.1097/BRS.0b013e31822a2e0a. [DOI] [PubMed] [Google Scholar]
- 40.Holly L T, Foley K T. Three-dimensional fluoroscopy-guided percutaneous thoracolumbar pedicle screw placement. Technical note. J Neurosurg. 2003;99(3, Suppl):324–329. doi: 10.3171/spi.2003.99.3.0324. [DOI] [PubMed] [Google Scholar]
- 41.Villavicencio A T, Burneikiene S, Bulsara K R, Thramann J J. Utility of computerized isocentric fluoroscopy for minimally invasive spinal surgical techniques. J Spinal Disord Tech. 2005;18:369–375. doi: 10.1097/01.bsd.0000168511.67189.64. [DOI] [PubMed] [Google Scholar]
- 42.Villavicencio A T, Burneikiene S, Nelson E L, Bulsara K R, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3:436–443. doi: 10.3171/spi.2005.3.6.0436. [DOI] [PubMed] [Google Scholar]
- 43.Nakashima H, Sato K, Ando T, Inoh H, Nakamura H. Comparison of the percutaneous screw placement precision of isocentric C-arm 3-dimensional fluoroscopy-navigated pedicle screw implantation and conventional fluoroscopy method with minimally invasive surgery. J Spinal Disord Tech. 2009;22:468–472. doi: 10.1097/BSD.0b013e31819877c8. [DOI] [PubMed] [Google Scholar]
- 44.Fraser J, Gebhard H, Irie D, Parikh K, Härtl R. Iso-C/3-dimensional neuronavigation versus conventional fluoroscopy for minimally invasive pedicle screw placement in lumbar fusion. Minim Invasive Neurosurg. 2010;53:184–190. doi: 10.1055/s-0030-1267926. [DOI] [PubMed] [Google Scholar]
- 45.Amiot L P, Labelle H, DeGuise J A, Sati M, Brodeur P, Rivard C H. Computer-assisted pedicle screw fixation. A feasibility study. Spine. 1995;20:1208–1212. doi: 10.1097/00007632-199505150-00019. [DOI] [PubMed] [Google Scholar]
- 46.Quiñones-Hinojosa A, Robert Kolen E, Jun P, Rosenberg W S, Weinstein P R. Accuracy over space and time of computer-assisted fluoroscopic navigation in the lumbar spine in vivo. J Spinal Disord Tech. 2006;19:109–113. doi: 10.1097/01.bsd.0000168513.68975.8a. [DOI] [PubMed] [Google Scholar]
- 47.Nottmeier E W, Bowman C, Nelson K L. Surgeon radiation exposure in cone beam computed tomography-based, image-guided spinal surgery. Int J Med Robot. 2012;8(2):196–200. doi: 10.1002/rcs.450. [DOI] [PubMed] [Google Scholar]


