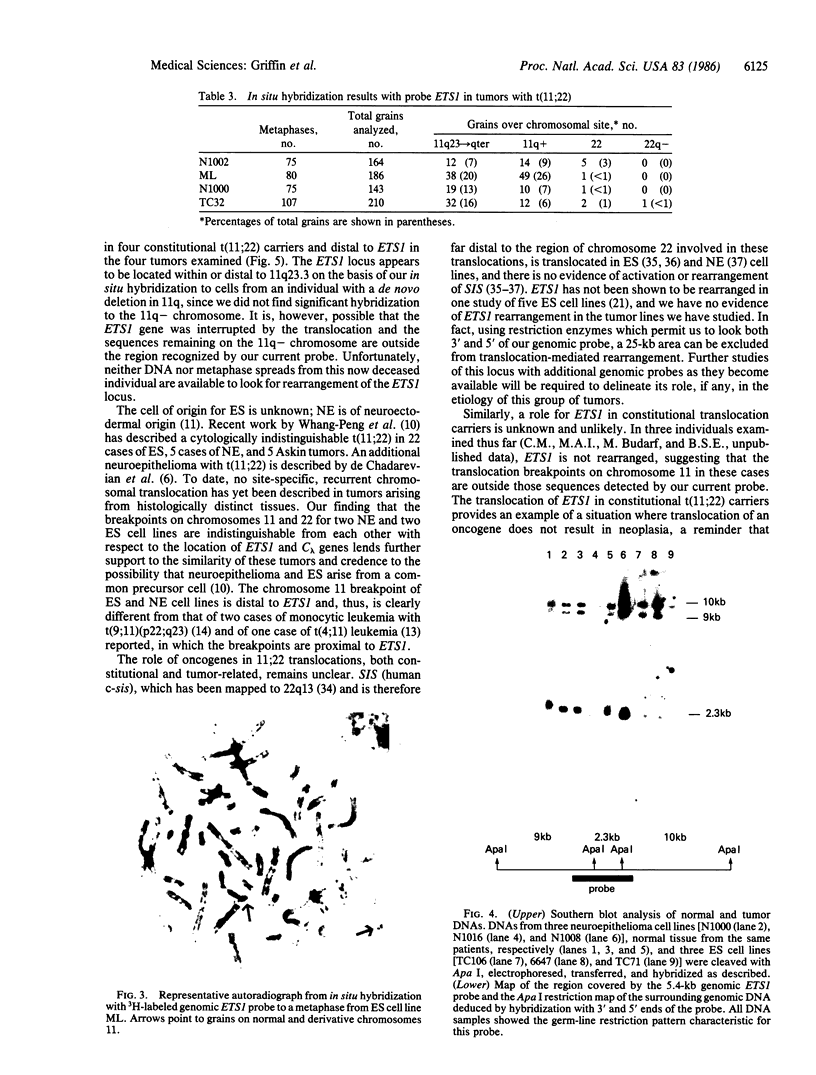
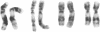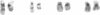Abstract
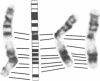
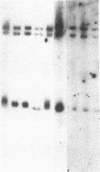
Recurring, site-specific chromosomal rearrangements are associated with several human syndromes and malignant disorders. Such nonrandom translocations involving chromosome 22 in band q11 are numerous and found to be associated with a diversity of neoplasms as well as constitutional disorders. Chromosome 11 in bands q23-q24 is similarly involved in several types of tumors as well as in a recurring constitutional reciprocal translocation with chromosome 22. Here we report the use of chromosomal in situ hybridization to compare the translocation breakpoints in the cytologically indistinguishable constitutional t(11;22) and the tumor-related t(11;22) associated with Ewing sarcoma and peripheral neuroepithelioma. We have shown that the breakpoints can be distinguished from each other with respect to the locus encoding the constant region of the Ig lambda light chain (C lambda) at 22q11 and the ETS1 locus at 11q23----q24; ETS1 has been called hu-ets-1 or human c-ets-1. The tumor-associated chromosome 11 breakpoint is also different from those of leukemias with t(9;11) and t(4;11) translocations. Southern-blot analysis showed no rearrangement of ETS1 in these disorders in the region detected by our probe. ETS1 has also been mapped more precisely to 11q23.3----q24 by in situ hybridization to cells from an individual with an 11q23.3----qter deletion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurias A., Rimbaut C., Buffe D., Zucker J. M., Mazabraud A. Translocation involving chromosome 22 in Ewing's sarcoma. A cytogenetic study of four fresh tumors. Cancer Genet Cytogenet. 1984 May;12(1):21–25. doi: 10.1016/0165-4608(84)90003-7. [DOI] [PubMed] [Google Scholar]
- Bechet J. M., Bornkamm G., Freese U. K., Lenoir G. M. The c-sis oncogene is not activated in Ewing's sarcoma. N Engl J Med. 1984 Feb 9;310(6):393–393. doi: 10.1056/NEJM198402093100618. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Cannizzaro L. A., Emanuel B. S. An improved method for G-banding chromosomes after in situ hybridization. Cytogenet Cell Genet. 1984;38(4):308–309. doi: 10.1159/000132079. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Thierfelder W., Erikson J., Nishikura K., Finan J., Lenoir G. M., Nowell P. C. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6922–6926. doi: 10.1073/pnas.80.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. O., Le Beau M. M., Pitha P., Rowley J. D. Interferon and c-ets-1 genes in the translocation (9;11)(p22;q23) in human acute monocytic leukemia. Science. 1986 Jan 17;231(4735):265–267. doi: 10.1126/science.3455787. [DOI] [PubMed] [Google Scholar]
- Emanuel B. S., Cannizzaro L. A., Seyer J. M., Myers J. C. Human alpha 1(III) and alpha 2(V) procollagen genes are located on the long arm of chromosome 2. Proc Natl Acad Sci U S A. 1985 May;82(10):3385–3389. doi: 10.1073/pnas.82.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel B. S., Nowell P. C., McKeon C., Croce C. M., Israel M. A. Translocation breakpoint mapping: molecular and cytogenetic studies of chromosome 22. Cancer Genet Cytogenet. 1986 Jan 1;19(1-2):81–92. doi: 10.1016/0165-4608(86)90375-4. [DOI] [PubMed] [Google Scholar]
- Emanuel B. S., Selden J. R., Wang E., Nowell P. C., Croce C. M. In situ hybridization and translocation breakpoint mapping. I. Nonidentical 22q11 breakpoints for the t(9;22) of CML and the t(8;22) of Burkitt lymphoma. Cytogenet Cell Genet. 1984;38(2):127–131. doi: 10.1159/000132044. [DOI] [PubMed] [Google Scholar]
- Erikson J., Griffin C. A., ar-Rushdi A., Valtieri M., Hoxie J., Finan J., Emanuel B. S., Rovera G., Nowell P. C., Croce C. M. Heterogeneity of chromosome 22 breakpoint in Philadelphia-positive (Ph+) acute lymphocytic leukemia. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1807–1811. doi: 10.1073/pnas.83.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Martinis J., Croce C. M. Assignment of the genes for human lambda immunoglobulin chains to chromosome 22. Nature. 1981 Nov 12;294(5837):173–175. doi: 10.1038/294173a0. [DOI] [PubMed] [Google Scholar]
- Francke U., Oliver N. Quantitative analysis of high-resolution trypsin-giemsa bands on human prometaphase chromosomes. Hum Genet. 1978 Dec 18;45(2):137–165. doi: 10.1007/BF00286957. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Canaani E. An 8-kilobase abl RNA transcript in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5648–5652. doi: 10.1073/pnas.81.18.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Iselius L., Lindsten J., Aurias A., Fraccaro M., Bastard C., Bottelli A. M., Bui T. H., Caufin D., Dalprà L., Delendi N. The 11q;22q translocation: a collaborative study of 20 new cases and analysis of 110 families. Hum Genet. 1983;64(4):343–355. doi: 10.1007/BF00292366. [DOI] [PubMed] [Google Scholar]
- Jhanwar S. C., Neel B. G., Hayward W. S., Chaganti R. S. Localization of the cellular oncogenes ABL, SIS, and FES on human germ-line chromosomes. Cytogenet Cell Genet. 1984;38(1):73–75. doi: 10.1159/000132033. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- McDermid H. E., Duncan A. M., Brasch K. R., Holden J. J., Magenis E., Sheehy R., Burn J., Kardon N., Noel B., Schinzel A. Characterization of the supernumerary chromosome in cat eye syndrome. Science. 1986 May 2;232(4750):646–648. doi: 10.1126/science.3961499. [DOI] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Sacchi N., Watson D. K., Guerts van Kessel A. H., Hagemeijer A., Kersey J., Drabkin H. D., Patterson D., Papas T. S. Hu-ets-1 and Hu-ets-2 genes are transposed in acute leukemias with (4;11) and (8;21) translocations. Science. 1986 Jan 24;231(4736):379–382. doi: 10.1126/science.3941901. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tilly H., Bastard C., Chevallier B., Halkin E., Monconduit M. Chromosomal abnormalities in secondary Ewing's sarcoma. Lancet. 1984 Oct 6;2(8406):812–812. doi: 10.1016/s0140-6736(84)90736-0. [DOI] [PubMed] [Google Scholar]
- Triche T. J., Askin F. B. Neuroblastoma and the differential diagnosis of small-, round-, blue-cell tumors. Hum Pathol. 1983 Jul;14(7):569–595. doi: 10.1016/s0046-8177(83)80202-0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C., Philip I., Berger M. P., Philip T., Lenoir G. M. Chromosome study of Ewing's sarcoma (ES) cell lines. Consistency of a reciprocal translocation t(11;22)(q24;q12). Cancer Genet Cytogenet. 1984 May;12(1):1–19. doi: 10.1016/0165-4608(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Douglass E. C., Israel M. A. Chromosome translocation in peripheral neuroepithelioma. N Engl J Med. 1984 Aug 30;311(9):584–585. doi: 10.1056/NEJM198408303110907. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Kao-Shan S., Tsai S., Israel M. A. Cytogenetic characterization of selected small round cell tumors of childhood. Cancer Genet Cytogenet. 1986 Apr 1;21(3):185–208. doi: 10.1016/0165-4608(86)90001-4. [DOI] [PubMed] [Google Scholar]
- Zackai E. H., Emanuel B. S. Site-specific reciprocal translocation, t(11;22) (q23;q11), in several unrelated families with 3:1 meiotic disjunction. Am J Med Genet. 1980;7(4):507–521. doi: 10.1002/ajmg.1320070412. [DOI] [PubMed] [Google Scholar]
- de Chadarevian J. P., Vekemans M., Seemayer T. A. Reciprocal translocation in small-cell sarcomas. N Engl J Med. 1984 Dec 27;311(26):1702–1703. [PubMed] [Google Scholar]
- de Taisne C., Gegonne A., Stehelin D., Bernheim A., Berger R. Chromosomal localization of the human proto-oncogene c-ets. Nature. 1984 Aug 16;310(5978):581–583. doi: 10.1038/310581a0. [DOI] [PubMed] [Google Scholar]
- van Kessel A. G., Turc-Carel C., de Klein A., Grosveld G., Lenoir G., Bootsma D. Translocation of oncogene c-sis from chromosome 22 to chromosome 11 in a Ewing sarcoma-derived cell line. Mol Cell Biol. 1985 Feb;5(2):427–429. doi: 10.1128/mcb.5.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Bruns G., Gerhard D. S., Pravtcheva D., Jones C., Housman D., Ruddle F. A., Orkin S., Terhorst C. Assignment of the gene coding for the T3-delta subunit of the T3-T-cell receptor complex to the long arm of human chromosome 11 and to mouse chromosome 9. Proc Natl Acad Sci U S A. 1985 May;82(9):2920–2924. doi: 10.1073/pnas.82.9.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]