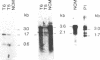Abstract
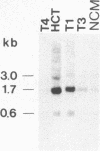
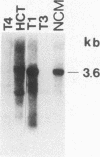
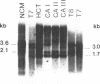
Type C-related human endogenous retroviral sequences have been previously discovered and characterized [Martin, M. A., Bryan, T., Rasheed, S. & Khan, A.S. (1981) Proc. Natl. Acad. Sci. USA 78, 4892-4896]. We investigated the transcriptional pattern of these sequences to determine whether and to what extent their expression is altered in colon tumors and colon cancer cell lines as compared to normal colon mucosa (NCM). Of two long terminal repeat (LTR)-specific transcripts [3.6 and 2.1 kilobases (kb)], the 3.6-kb RNA was particularly abundant in NCM but strikingly decreased in most primary colon cancers tested. In NCM we identified three envelope gene (env)-related transcripts--namely, 3.0, 1.7, and 0.6 kb. Colon tumors appeared to express higher levels of these transcripts, especially the 1.7-kb species. In one case of dysplasia and in one benign tumor, this 1.7-kb transcript was clearly increased. We also examined the pattern of transcription in colon cancer cell lines HCT and Caco2. The LTR-homologous 3.6-kb transcript, very abundant in NCM, was decreased in primary tumors and in HCT cells and virtually absent in Caco2 cells. The latter, however, appeared to produce the transcript when growing exponentially, indicating that Caco2 cultures provide an inducible system susceptible to in vitro manipulation. Both cell lines also contained higher amounts of the 1.7-kb env-related transcript. The decrease of the 3.6-kb RNA in colon tumors versus NCM may be the result of an altered pattern of differentiation, whereas the increase of the 1.7-kb RNA in tumors may represent an early marker of colon neoplasia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R. W., Bishop D. T., Cannon L. A., Dowdle M. A., Lee R. G., Skolnick M. H. Dominant inheritance of adenomatous colonic polyps and colorectal cancer. N Engl J Med. 1985 Jun 13;312(24):1540–1544. doi: 10.1056/NEJM198506133122403. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Dragani T. A., Manenti G., Della Porta G., Gattoni-Celli S., Weinstein I. B. Expression of retroviral sequences and oncogenes in murine hepatocellular tumors. Cancer Res. 1986 Apr;46(4 Pt 2):1915–1919. [PubMed] [Google Scholar]
- Gattoni-Celli S., Hsiao W. L., Lambert M., Kirschmeier P., Weinstein I. B. Genetic targets in multistage carcinogenesis. Carcinog Compr Surv. 1985;9:29–40. [PubMed] [Google Scholar]
- Gattoni S., Kirschmeier P., Weinstein I. B., Escobedo J., Dina D. Cellular Moloney murine sarcoma (c-mos) sequences are hypermethylated and transcriptionally silent in normal and transformed rodent cells. Mol Cell Biol. 1982 Jan;2(1):42–51. doi: 10.1128/mcb.2.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Effects of 5-azacytidine on expression of endogenous retrovirus-related sequences in C3H 10T1/2 cells. J Virol. 1986 Mar;57(3):1119–1126. doi: 10.1128/jvi.57.3.1119-1126.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Effects of 5-azacytidine on the progressive nature of cell transformation. Mol Cell Biol. 1985 Jul;5(7):1800–1803. doi: 10.1128/mcb.5.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmeier P., Gattoni-Celli S., Dina D., Weinstein I. B. Carcinogen- and radiation-transformed C3H 10T1/2 cells contain RNAs homologous to the long terminal repeat sequence of a murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2773–2777. doi: 10.1073/pnas.79.9.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. B., Hamagishi Y., Steele P. E., Tykocinski M., Martin M. A. Characterization of human endogenous retroviral envelope RNA transcripts. J Virol. 1985 Oct;56(1):176–182. doi: 10.1128/jvi.56.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. B., Steele P. E., Garon C. F., Martin M. A. mRNA transcripts related to full-length endogenous retroviral DNA in human cells. Nature. 1983 Dec 8;306(5943):604–607. doi: 10.1038/306604a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]