Abstract
A rapid, sensitive, low-cost device to detect trimethylamine was presented in this paper. The preparation of water soluble polyaniline was firstly studied. Then the polyaniline was characterized via Fourier transform infrared spectroscopy (FTIR), UV-visible spectroscopy and scanning electron microscopy (SEM). Based on the water soluble polyaniline film, a quartz crystal microbalance (QCM) sensor for trimethylamine detection was fabricated and its characteristics were examined. The sensor consisted of one quartz crystal oscillator coated with the polyaniline film for sensing and the other one for reference. Pretreated with trimethylamine, the QCM sensor had an excellent linear sensitivity to trimethylamine. Easily recovered by N2 purgation, the response of the sensor exhibited a good repeatability. Responses of the sensor to trimethylamine, ethanol and ethyl acetate were compared, and the results showed that the response was related to the polarity of the analyte vapor. Experimental result also showed that the sensitivity of the sensor was relatively stable within one month. The simple and feasible method to prepare and coat the polyaniline sensing film makes it promising for mass production.
Keywords: QCM, Gas Sensor, Conducting Polymer, Polyaniline, Trimethylamine
1. Introduction
Biogenic amines are formed by the activity of bacterial amino acid decarboxylase during the degradation processes of proteins, which have a carcinogenic effect on human body and can be used to indicate bacterial contamination. Being one of biogenic volatile amines, trimethylamine is a good target for the detection of biogenic amines to evaluate the quality of meat food products [1-3]. In human body, trimethylamine is derived from diet either directly from the consumption of foods containing trimethlamine, or by the intake of food containing precursors to trimethylamine such as trimethylamine-N-oxide, choline and L-carnitine. Normally, the flavin-containing monooxygenase isoform 3 (FMO3) enzyme converts fishy-smelling trimethylamine into another molecule that has no odor. If the enzyme is deficient or its activity is reduced, trimethylamine is not processed properly and can build up in the body. As excess trimethylamine is released in a person's sweat, urine, and breath, it causes the strong odor characteristic of trimethylaminuria [4]. Trimethylamine is also of clinical interest because of its potential to contribute to neurological toxicity and ‘uraemic breath’ in patients with end-stage renal disease [5]. Together with triethylamine, trimethylamine is found to be emitted by building materials degraded by microbial growth [6]. Trimethylamine has an unpleasant smell, and is irritant to the skin, eyes, mucous membranes and respiratory tract [7].In a word, a rapid, sensitive, low-cost method to detect trimethylamine is meaningful.
Various analytical methods have been reported in literature to detect trimethylamine [1, 2, 8-11]. However, all these methods (photometry, gas chromatography, colorimetric analysis, high performance liquid chromatography, ion mobility spectrometry, etc.) are relatively cumbersome, time consuming and require experienced operators.
Polyaniline, a kind of conductive conjugated polymer, has been regarded as a good sensing material due to its advantages of gas sensing ability and optimum performance at room temperature. It has been exploited in thin film sensors for several gas molecules as well as volatile organic compounds [12-16]. In spite of the various advantages of polyaniline based gas sensors, some fundamental problems, such as long-time instability and irreversibility and low selectivity, still persist [17]. Usually solubility of polyaniline is a problem to fabrication quality films. We have recently investigated the method to prepare water-soluble polyaniline based on oxidative polymerization. Moreover, the effect of trimethylamine on the conductivity of polyaniline was investigated [18].
QCM sensor can be constructed by coating the surface of a quartz crystal electrode with a film capable of interaction with the environment of interest. The operating principle of QCM sensors is based on the interaction between the surface of a quartz crystal coated with the sensing materials and the target materials. The Sauerbrey equation was developed for oscillation in air and only applies to rigid mass attached to the crystal [19]. It gives the change in the oscillation frequency of piezoelectric quartz (Δf) as a function of the mass (Δm) added to the crystal:
| (1) |
where Δf is the observed frequency change (Hz), f0 is the fundamental resonant frequency of crystal, A is the active area of the crystal (between electrodes), ρq is the density of quartz and υq is the shear wave velocity in the quartz. Quartz crystal microbalance (QCM) sensors were widely investigated due to their high sensitivity, durability and linearity for mass of the target materials [20-24].
Chemiresistors are the most popular device configuration of gas sensors based on conducting polymer. However, the resistance of chemiresistor is influenced by many ambient factors, and not only determined by the resistance of the conducting polymer sensing film, but also the contact resistance of electrodes. Moreover, other disadvantages for use of chemiresistors are low limit of detection and sensitivity, compared with sensors based on other configurations such as transistors, optic devices and QCMs. For transistor, its preparation and characterization is slightly complicate. The sensitivity of optic sensors is high, but the detecting procedure is complicate. Because QCM is rather sensitive to mass change, adsorbing a very small amount of analyte can be detected through frequency change. Although a number of polymers have been successfully employed in the coating of QCM sensors [20, 24], polyaniline-coated QCM gas sensor has seldom been investigated. Therefore, we attempted to coat a polyaniline film on a quartz crystal surface, to make a low-cost, high-sensitivity and rapid-response QCM trimethlamine gas sensor. The performance of the sensor was investigated.
2. Experiment
2.1. Materials
Ammonium peroxydisulfate (AR), hydrochloric acid (AR), poly(sodium-p-styrenesulfonate), 33% trimethylamine solution, aniline (AR), ethyl acetate (AR), enthanol (AR) are commercially available. Aniline was vacuum distilled prior to use. AT-cut 6.000 MHz (HC-49/U) quartz crystals with silver electrodes on both sides were purchased from Hosonic International (Hangzhou) Ltd, China. The crystals are rinsed by ethanol and then deionized water prior to use.
2.2. Preparation and characterization of water soluble polyaniline
Polyaniline was prepared by polymerization of aniline with chemical oxide method as described previously [18]. A small amount of poly(sodium- p-styrenesulfonate) was added in a 0.1 M hydrochloric acid solution containing 0.01 M aniline and ammonium peroxydisulfate. The mixture was left undisturbed for 24 h, and then blue-black aqueous solution of polyaniline was obtained. The FTIR spectra of water-soluble polyaniline were recorded in silicon disks with an IFS66 V/S Fourier transform infrared spectrometer (Bruker Company). The UV-vis absorption of water-soluble polyaniline on the quartz glass was recorded by a CARY Bio100 spectrophotometer. The SEM image of soluble polyaniline composites coated on the glass was studied by an S-4800 (Hitachi Company) electron scanning microscope.
2.3. Preparation and pretreatment of coated sensor
A drop-coating [25] method was used to form the water-soluble polyaniline film on the surface of the electrode. An AT-cut 6.000 MHz crystal was rinsed repeatedly into ethanol and deionized water and dried in air at room temperature. Then two microliters of polyaniline aqueous solution was dispensed onto the surface of the electrode using a micropipette. The device was then dried at room temperature.
The pretreatment of polyaniline-coated sensor is described as following. It was put into a sealed chamber (500 mL), and desorbed by high-purity N2. Then the sensor was exposed to 200 ppm trimethylamine in N2 for deprotonation.
2.4. Gas sensing experiments
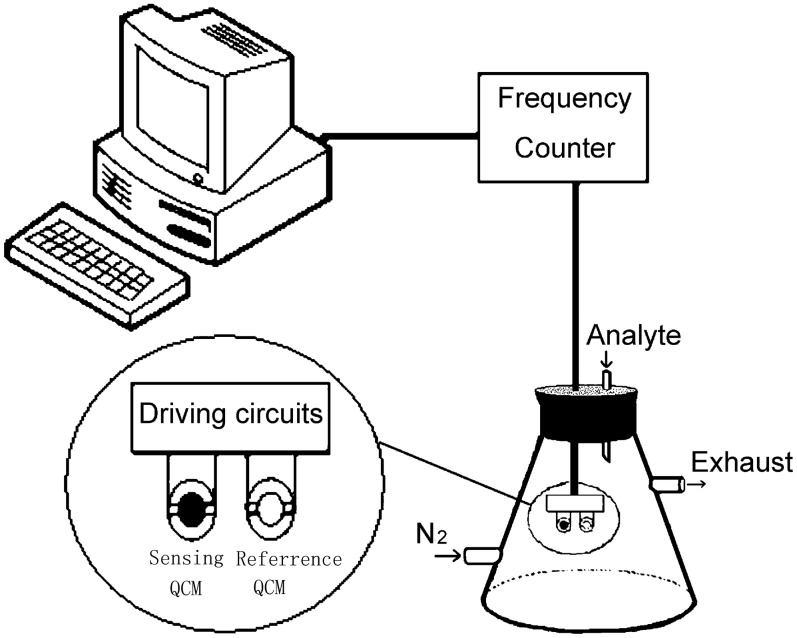
Fig. 1 shows a schematic view of the experimental setup. Two same type QCM devices were set in the sealed chamber. One coated with polyaniline film was used as the sensing device while the other without polyanine was used as the reference. The frequency difference between two QCMs was determined by mass changes of the polyaniline coated on the sensing QCM. The instrumentation utilized consisted of driving circuits and a frequency counter. The driving circuits made the QCMs oscillate and output the frequency difference. The frequency difference was measured by the frequency counter and sent to an IBM compatible computer via a RS-232 serial communication port.
Figure 1.
Schematic view of the experimental setup.
Experiments were carried out at room temperature and atmospheric pressure. The gases used during the investigation were trimethylamine, ethanol and ethyl acetate. For each vapour, 10 mL solution rested on the bottom of a 1000 mL glass container for at least 20 min, so that saturated volatile organic compounds could be extracted in the headspace of the container as analyte. High-purity N2 gas passed through the sensor chamber until the frequency difference became stable. Appropriate analyte concentrations were obtained in the test chamber by injecting known volumes of headspace gas via a gas tight syringe. The sensors were exposed to each vapour for 5 min, and then were purged by high-purity N2. The frequency difference was recorded every 1 s in the system. The computer is used to store this data and process it later.
When the analyte was introduced to the sensor chamber, the polyaniline film absorbed the analyte and the sensing QCM's frequency decreased. The frequency decrease of the sensing QCM resulted in an increase of the frequency difference. Change of the frequency difference was defined as the sensor's response to gases.
3. Results and discussion
3.1. Water soluble polyaniline film characteristics
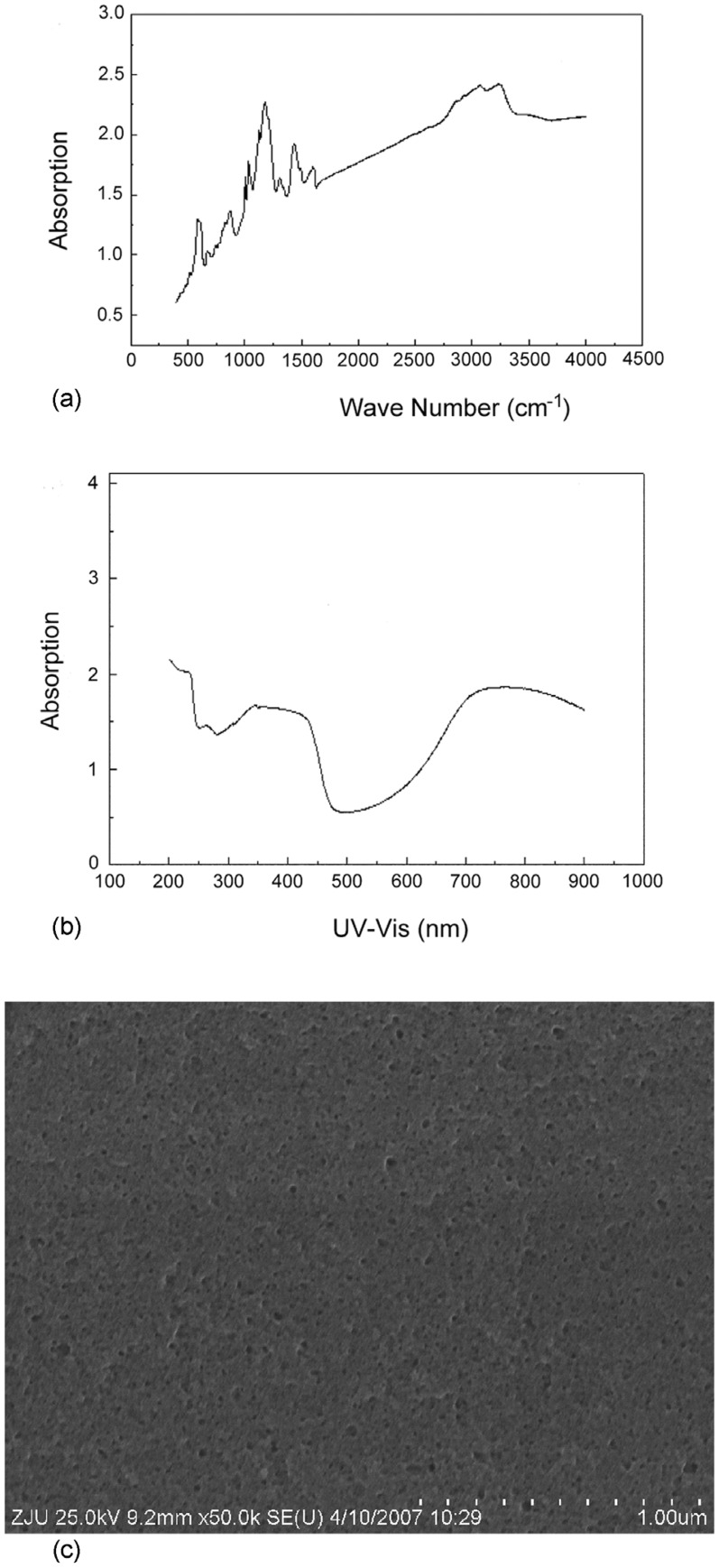
Figure 2a shows the FTIR spectra of polyaniline film. It illustrates that the FTIR spectra contains the N-H stretching peak (3248 cm-1), the C=C stretching peak (1594 cm-1) of quinoid ring in emeraldine base and emeraldine salt, the C-N stretching peak (1318 cm-1) and the C=N stretching peak (1171 cm-1). This shows that this composite film contained polyaniline. The characteristic peaks of polyaniline at 340, 440 and 800 nm appeared in the UV-vis spectra of composite film (as shown in Figure 2b). This result is consistent with previous work [26]. The morphology of the polyaniline film is shown in Figure 2c. It is clear that the surface morphology of polyaniline film is homogeneous.
Figure 2.
(a) FTIR spectra of polyaniline composite film; (b) UV-visible spectra of polyaniline composite film; (c) SEM image of a film of polyaniline on glass
3.2. Pretreatment of the polyaniline-coated sensor
There are some possible interactions, such as chemical bonding, hydrogen bonding and van der Waals force, between the polyaniline film and adsorbed gas molecule. Generally, conductivity sensors made of polyaniline are based on the reversible reaction of acid/base. For this mechanism, the conductivity response of polyaniline is increased when it is exposed to acid atmosphere, and decreased when it is exposed to base atmosphere. However, previous work [27] shows that the conductivity response of deprotonated polyaniline film is increased with the quantity of adsorbed basic gas. The unusual electrical condutivity response of polyaniline pretreated with trimethylamine was attributed to van der Waals' interactions. The essential of conductivity responses is that the polyaniline film adsorb gas molecule. In this way, the frequency of polyaniline-coated QCM will decrease when it is exposed to trimethylamine and the frequency difference will increase accordingly. When the van der Waals absorption is the main interaction between polyaniline and gas molecules, the responses of the sensor will can be recovered just with high-purity N2 purgation.
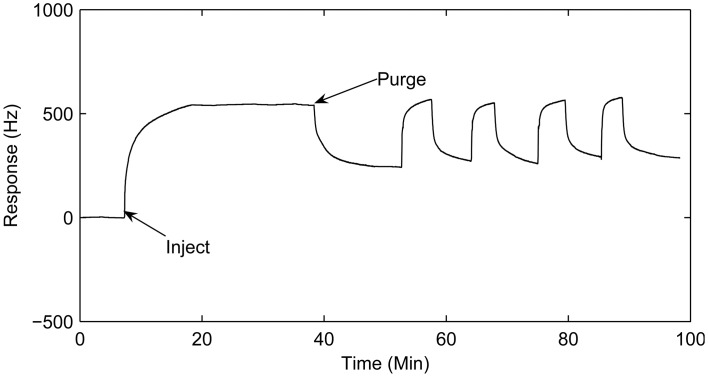
In order to confirm this hypothesis, the sensor was exposed to 200 ppm of trimethylamine for deprotonation, and then the responses of the sensor to 200 ppm of trimethylamine were investigated. Figure 3 illustrates the responses difference during the deprotonation process and after pretreatment. During the deprotonation process, the polyaniline-coated QCM responded to trimethylamine highly and persistently. This response did not recover completely though the frequency difference decreased due to trimethylamine desorption by purity N2. So the response of the sensor without pretreatment was irreversible. Although the responses of pretreated sensor were smaller than before, they were reversible. These different responses characters show that the mechanisms of the two processes were different. For the deprotonation process, there were the acid/base reaction and van der Waals' interactions between the polyaniline and trimethylamine molecule. The frequency drift caused by the acid/base reaction couldn't be recovered only by purging with N2, while the frequency drift caused by van der Waals interactions could be easily recovered by N2 purgation. When the pretreated polyaniline had already been deprotonated by the trimethylamine, the main interaction between polyaniline and trimethylamine molecule was the van der Waals interactions. Consequently, the responses of the polyaniline-coated sensor were reversible. The pretreated polyaniline-coated QCM sensor absorbing base gas could be recovered completely with high-purity N2 purgation at room termperature so that it can be utilized repeatedly.
Figure 3.
Pretreatment of polyaniline-coated QCM sensor and it's responses to 200 ppm of trimethylamine in N2 after pretreatment.
3.3. Repeatability
To investigate the response repeatability of the sensor, a cycle test was conducted while a pretreated polyaniline-coated QCM sensor was exposed to trimethylamine. To recover the sensing QCM, high-purity N2 was purged through the chamber until full desorption was achieved. Figure 3 depicts the frequency difference changing alternatively with trimethylamine injection and high-purity N2 purgation. The result shows that the adsorption-desorption process on the polyaniline film was highly reversible for trimethylamine molecules. The time to reach 95% of the plateau response is about 60s, which is faster than the conductivity response reported previously [18].
3.4. Responses to different vapors
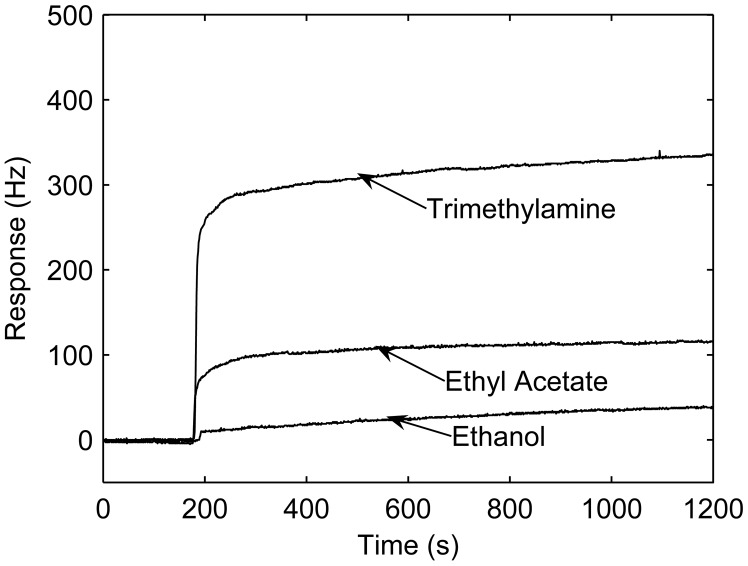
The sensor was exposed to trimethylamine, ethanol and ethyl acetate of same concentration (200 ppm), respectively. Plots of the responses for the sensor upon the introduction of trimethylamine, ethanol and ethyl acetate in N2 is shown in Figure 4. This figure indicates that response of the sensor after pretreatment strongly depends on the polarity of adsorbed vapors. Since the electronegativity of the nitrogen of polyaniline base is very large, the radius of the nitrogen atom is small, and nitrogen atom has a lone electron, the interaction between the surface of the polyaniline base and polar vapors will contain hydrogen bonding and a strong van der Waals force. Therefore, the stronger the polarity of vapor, the response is higher.
Figure 4.
Polyaniline-coated QCM sensor's responses to 200 ppm of trimethylamine,ethanol and ethyl acetate in N2.
3.5. Sensitivity of the responses
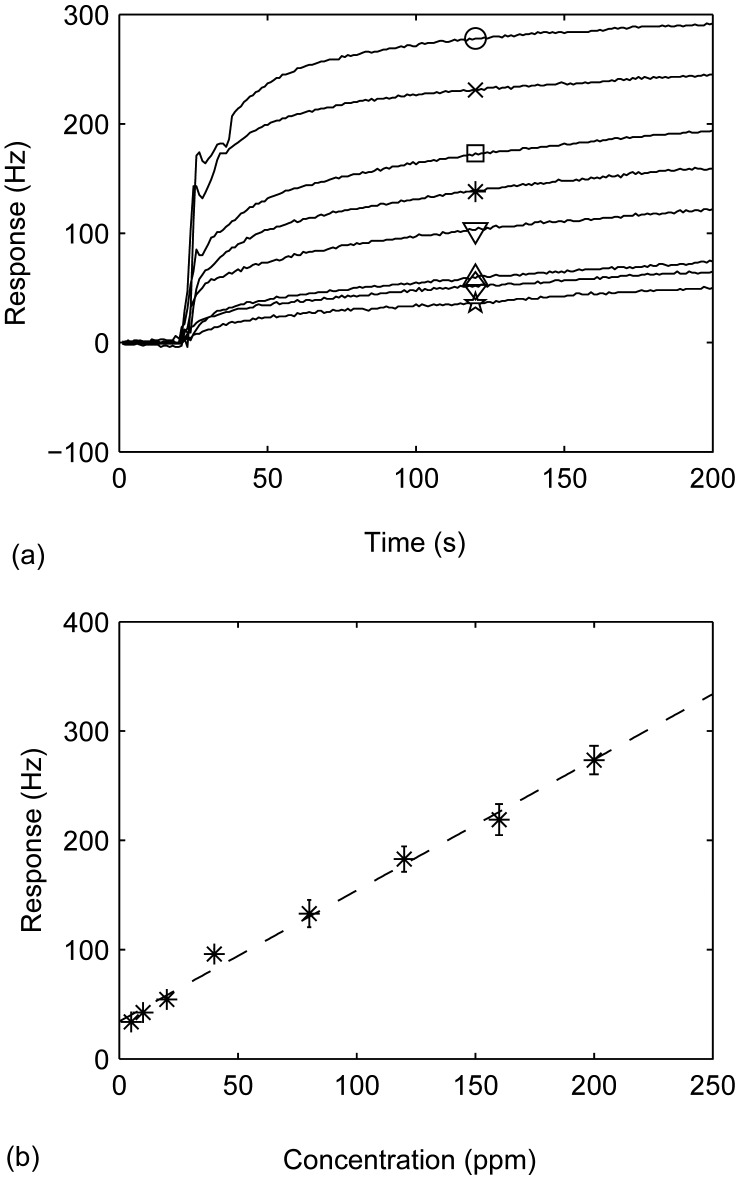
A fabricated sensor was exposed to various concentration of trimethylamine ranged 0 to 200 ppm to examine the sensitivity. Figure 5a shows the change of frequency difference versus time for various concentration of trimethylamine. It is obvious from the graph that as the concentration of trimethylamine increased, the magnitude of the response also increased. For each concentration, five responses were measured and recorded. The relation between the frequency difference and trimethylamine concentration is shown in Figure 5b. Response (R) of the sensor was almost linear proportional to trimethylamine concentration (C) in the range of 5-200 ppm. The regression equation is R = 1.198C+34.267 with a correlation coefficient of 0.997.
Figure 5.
(a) Polyaniline-coated QCM sensor's responses to a 5(☆), 10(◇), 20(△), 40(▽), 80(﹡),120(□), 160(×) and 200(○) ppm (v/v) of trimethylamine in N2; (b) Polyaniline-coated QCM sensor's responses versus concentration of trymethylamine in N2
3.6. Durability
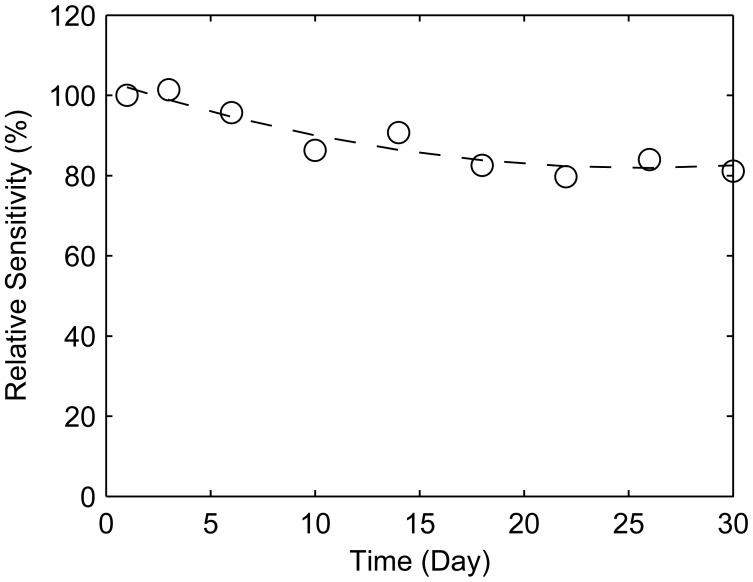
In order to investigate the durability of the sensor, further experiments were carried out. The response of polyaniline-coated QCM sensor to 120 ppm trimethylamine in N2 was recorded over 1 month. The response change over one month period is shown in Figure 6. The response after one month dropped about 20% of the initial sensitivity. The sensor's responses decreased with storing time at first due to the aging of polyaniline, and then remained stable at about 80% of the initial sensitivity.
Figure 6.
Long-term durability of Polyaniline-coated QCM sensor during 1 month.
4. Conclusion
Water-soluble polyaniline based QCM trimethylamine sensors were developed and characterized. The preparation of the composite polyaniline film on QCM was rather simple. Pretreated with trimethylamine, the sensors exhibited a good linear response to trimethylamine. Experimental results showed that responses of the sensors depended on the polarity of vapors absorbed by polyaniline. Purged by N2, the sensing film could be easily recovered, so that the sensors could be repeatedly utilized. The method used to prepare the sensor was very simple and feasible. It has potential for mass production at a low cost. As a sensing film for QCM trimethlamine sensor, thin polyaniline films have not been fully exploited. Although the sensor's response was reasonable stable within one month, there are still lots of work to be done on the long term stability.
Acknowledgments
The work is supported by the National Creative Research Groups Science Foundation of China (NCRCSFC: 60421002).
References and Notes
- 1.Bota G. M., Harrington P. B. Direct detection of trimethylamine in meat food products using ion mobility spectrometry. Talanta. 2006;68:629–635. doi: 10.1016/j.talanta.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Chan S.T., Yao M.W.Y., Wong Y.C., Wong T., Mok C.S., Sin D.W.M. Evaluation of chemical indicators for monitoring freshness of food and determination of volatile amines in fish by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur. Food Res. Technol. 2006;224:67–74. [Google Scholar]
- 3.Periago M.J., Rodrigo J., Ros G., Rodriguez-Jerez J.J., Hernandez-Herrero M. Monitoring volatile and nonvolatile amines in dried and salted roes of tuna (Thunnus thynnus L.) during manufacture and storage. J. Food Prot. 2005;66:335–340. doi: 10.4315/0362-028x-66.2.335. [DOI] [PubMed] [Google Scholar]
- 4.Bain M.A., Fornasini G., Evans A.M. Trimethylamine: metabolic, pharmacokinetic and safety aspects. Curr. Drug Metab. 2005;6:227–240. doi: 10.2174/1389200054021807. [DOI] [PubMed] [Google Scholar]
- 5.Bain M.A., Faull R., Fornasini G., Milne R.W., Evans A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006;21:1300–1304. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 6.Claeson A.S., Sandström M., Sunesson A.L. Volatile organic compounds (VOCs) emitted from materials collected from buildings affected by microorganisms. J. Environ. Monit. 2007;9:240–245. doi: 10.1039/b614766f. [DOI] [PubMed] [Google Scholar]
- 7.Greim H., Bury D., Klimisch H.J., Oeben-Negele M., Ziegler-Skylakakis K. Toxicity of aliphatic amines: Structure-activity relationship. Chemosphere. 1998;36:271. doi: 10.1016/s0045-6535(97)00365-2. [DOI] [PubMed] [Google Scholar]
- 8.Kaniou I., Samouris G., Mouratidou T., Eleftheriadou A., Zantopoulos N. Determination of biogenic amines in fresh unpacked and vacuum-packed beef during storage at 4±°C. Food Chem. 2001;74:515–519. [Google Scholar]
- 9.Cháfer-Pericás C., Campíns-Falcó P., Herráez-Hernández R. Comparative study of the determination of trimethylamine in water and air by combining liquid chromatography and solid-phase microextraction with on-fiber derivatization. Talanta. 2006;69:716–723. doi: 10.1016/j.talanta.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Namieśnik J., Jastrzębska A., Zygmunt B. Determination of volatile aliphatic amines in air by solid-phase microextraction coupled with gas chromatography with flame ionization detection. J. Chromatogr. A. 2003;1016:1–9. doi: 10.1016/s0021-9673(03)01296-2. [DOI] [PubMed] [Google Scholar]
- 11.Adhoum N., Monser L., Sadok S., El-Abed A., Greenway G.M., Uglow R.F. Flow injection potentiometric detection of trimethylamine in seafood using tungsten oxide electrode. Anal. Chim. Acta. 2003;478:53–58. [Google Scholar]
- 12.Sadek A.Z., Wlodarski W., Shin K., Kaner R.B., Kalantar-zadeh K. A layered surface acoustic wave gas sensor based on a polyaniline/In2O3 nanofibre composite. Nanotechnology. 2006;17:4488–4492. [Google Scholar]
- 13.Athawale A.A., Bhagwat S.V., Katre P.P. Nanocomposite of Pd-polyaniline as a selective methanol sensor. Sens. Actutator B: Chem. 2006;114:263–267. [Google Scholar]
- 14.Prasad G.K., Radhakrishnan T.P., Kumar D.S., Ghanashyam Krishna M. Ammonia sensing characteristics of thin film based on polyelectrolyte templated polyaniline. Sens. Actutator B: Chem. 2005;106:626–631. [Google Scholar]
- 15.Lee Y.S., Song K.D., Huh J.S., Chung W.Y., Lee D.D. Fabrication of clinical gas sensor using MEMS process. Sens. Actutator B: Chem. 2005;108:292–297. [Google Scholar]
- 16.Dixit V., Misra S.C.K., Sharma B.S. Carbon monoxide sensitivity of vacuum deposited polyaniline semiconducting thin films. Sens. Actutator B: Chem. 2005;104:90–93. [Google Scholar]
- 17.Bai H., Shi G. Gas sensors based on conducting polymers. Sensors. 2007;7:267–307. [Google Scholar]
- 18.Ma X., Wang M., Chen H., Li G. J. Sun, Bai R. Preparation of water soluble poly(aniline) and its gas-sensitivity. Green Chem. 2005;7:507–513. [Google Scholar]
- 19.Sauerbrey G. The use of quartz oscillators for weighing layers and for micro-weighing. Z. Phys. 1959;155:206–222. [Google Scholar]
- 20.Matshuguchi M., Kadowaki Y., Tanaka M. A QCM-based NO2 gas detector using morpholine-functional cross-linked copolymer coatings. Sens. Actutator B: Chem. 2005;108:572–575. [Google Scholar]
- 21.Koshets I.A., Kazantseva Z.I., Shirshov Y.M., Cherenok S.A., Kalchenko V.I. Calixarene films as sensitive coating for QCM-based sensors. Sens. Actutator B: Chem. 2005;106:177–181. [Google Scholar]
- 22.Vilaseca M., Yagüe C., Coronas J., Santamaria J. Development of QCM sensors modified by AlPO4-18 films. Sens. Actutator B: Chem. 2006;117:143–150. [Google Scholar]
- 23.Sasaki I., Tshuchiya H., Nishioka M., Sadakata M., Okubo T. Gas sensing with zeolite-coated quartz crystal microbalances—principal component analysis approach. Sens. Actutator B: Chem. 2002;86:26–33. [Google Scholar]
- 24.Mirmohseni A., Oladegaragoze A. Construction of a sensor for determination of ammonia and aliphatic amines using polyvinylpyrrolidone coated quartz crystal microbalance. Sens. Actutator B: Chem. 2003;89:164–172. [Google Scholar]
- 25.Stahl U., Rapp M., Wessa T. Adhesives: a new class of polymer coating for surface acoustic wave sensors for fast and reliable process control applications. Anal. Chim. Acta. 2001;450:27–36. [Google Scholar]
- 26.Ayad M.M., Shenashin M.A. Polyaniline film deposition from the oxidative polymerization of aninie using K2Cr2O7. Eur. Polym. J. 2004;40:197–202. [Google Scholar]
- 27.Ma X., Li G., Wang M., Bai R., Yang F., Chen H. Unusual electrical response of a poly(aniline) composite film on exposure to a basic atmosphere and its application to sensors. Green Chem. 2006;8:63–691. [Google Scholar]