Abstract
In light of postmortem human studies showing extensive degeneration of the center median (CM) and parafascicular (Pf) thalamic nuclei in Parkinson's disease patients, the present study assessed the extent of neuronal loss in CM/Pf of non-human primates that were rendered parkinsonian by repeated injections of low doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In order to determine the course of CM/Pf degeneration during the MPTP intoxication, motor-asymptomatic animals with partial striatal dopamine denervation were also used. The Cavalieri's principle for volume estimation and the unbiased stereological cell count method with the optical dissector technique were used to estimate the total number of neurons in the CM/Pf. We found substantial neurons loss in the CM/Pf in both, motor-symptomatic MPTP-treated monkeys in which the striatal dopamine innervation was reduced by more than 80 %, and in motor-asymptomatic MPTP-treated animals with 40–50 % striatal dopamine loss. In MPTP-treated parkinsonian monkeys, 60 and 62 % neurons loss was found in CM and Pf, respectively, while partially dopamine-depleted asymptomatic animals displayed 59 and 52 % neurons loss in the CM and Pf, respectively. Thus, our study demonstrates that the CM/Pf neurons loss is an early phenomenon that occurs prior to the development of parkinsonian motor symptoms in these animals. In contrast, the neighboring mediodorsal nucleus of the thalamus was only mildly affected (18 % neurons loss) in the parkinsonian monkeys. Together with recent findings about the possible role of the CM/Pf-striatal system in cognition, our findings suggest that the pathology of the thalamostriatal system may precede the development of motor symptoms in PD, and may account for some of the cognitive deficits in attentional set-shifting often seen in these patients. Future studies in this animal model, and in monkeys with selective lesion of CM or Pf, are needed to further elucidate the role of the CM/Pf-striatal system in normal and parkinsonian conditions.
Keywords: Centromedian, Parafascicular, Thalamostriatal, Thalamus, Stereology, Monkey, MPTP
Introduction
It has long been known that Parkinson's disease (PD) is associated with massive degeneration of the dopaminergic nigrostriatal system, and that the lack of striatal dopamine accounts for the cardinal motor symptoms of PD (resting tremor, bradykinesia, rigidity, and akinesia; Gelb et al. 1999). However, postmortem studies in human patients with PD have also shown that the pathology extends beyond the dopaminergic nigrostriatal system to include cell loss in other ascending monoaminergic and cholinergic systems as well as degeneration of non-monoaminergic cell groups (Hirsch et al. 1987; Jellinger 1988; Halliday et al. 1990; Gai et al. 1991; Marien et al. 1993; Fornai et al. 1997a, b, 2007; Braak et al. 2003; Rommelfanger et al. 2007; Rommelfanger and Weinshenker 2007; Masilamoni et al. 2010, 2011). It is likely that the degeneration of these non-dopaminergic cell groups contributes to PD motor symptoms, as well as the many autonomic, psychiatric, and cognitive deficits experienced by PD patients (Cools et al. 2001; Williams-Gray et al. 2006).
The caudal intralaminar thalamic nuclei, made up of the center median and parafascicular nuclei (CM/Pf), are one of the neuron groups in the brain that are profoundly affected by the degenerative process in PD (Henderson et al. 2000a, b, 2005; Brooks and Halliday 2009; Halliday 2009; Halliday et al. 2011). This thalamic degeneration, which was found to be relatively specific to CM/Pf, occurs even in early diagnosed patients with mild parkinsonian motor symptoms (Henderson et al. 2005).
The CM/Pf nuclear complex is a key component of the ascending reticular activating system (Kinomura et al. 1996). These nuclei are also known to receive topographically organized projections from the basal ganglia, and to send a massive glutamatergic projection to the striatum (Sadikot et al. 1992; Sadikot and Rymar 2009; Sidibe and Smith 1999; Raju et al. 2006, 2008; Smith et al. 2004, 2009; Galvan and Smith 2011). Through these projections, the CM/Pf complex is part of functionally segregated basal ganglia-thalamostriatal loops that process, motor, associative, and limbic-related information (Smith et al. 2004, 2009, 2011; Galvan and Smith 2011).
A deeper understanding of the significance of CM/Pf degeneration in PD pathophysiology has been limited by the lack of reliable PD animal models that display CM/Pf cell loss. Although attempts have been made at characterizing Pf degeneration in rodent models of PD, results have been inconsistent and controversial across laboratories (Freyaldenhoven et al. 1997; Sedaghat et al. 2009, but see also Henderson et al. 2005; Aymerich et al. 2006; Kusnoor et al. 2012). In the present study, we demonstrate a major loss of CM/Pf neurons in a non-human primate model of PD, induced by chronic systemic administration of low doses of MPTP over a 4–6 months period (Masilamoni et al. 2010, 2011). In order to assess the progression of CM/Pf neurons loss during the course of MPTP intoxication, we compared the degree of CM/Pf degeneration in asymptomatic and symptomatic monkeys. Some results of this study have been presented in abstract forms (Villalba et al. 2011, 2012).
Materials and methods
Animals
We used nine adult female rhesus macaque monkeys (Macaca mulatta, 4.5–8.5 kg; Yerkes National Primate Research Center colony) in these studies (3 control: C1–C3 and 6 MPTP-treated: M1–M3 and M4–M6). The brain tissue from animals M1–M3 has been used in previous studies (Masilamoni et al. 2010, 2011). The housing, feeding, and experimental conditions used in these studies followed guidelines by the National Institutes of Health, and were approved by the Chemical Safety and Animal Care and Use Committees of Emory University.
MPTP injections and parkinsonism
Before the beginning of the MPTP treatment, the monkeys were first habituated to a behavioral observation cage, and a baseline of motor behavior was established. Three monkeys (M1–M3) received intramuscular injections of MPTP (Sigma-Aldrich, St. Louis, MO) once a week (0.2–0.5 mg/kg) for 21 weeks until they displayed stable parkinsonian motor symptoms (total dose 4.3–8 mg/kg) (more details in Masilamoni et al. 2010, 2011). The other three monkeys (M4–M6) received 0.2 mg/kg of MPTP per week for a period of 22–27 weeks (total dose 4.4–5.4 mg/kg) and had not developed parkinsonian motor signs at the time of euthanasia.
During the MPTP treatment, behavioral changes and parkinsonian motor signs were measured with quantitative methods that are routinely used in our laboratory (Wichmann et al. 2001; Soares et al. 2004; Wichmann and Soares 2006; Kliem et al. 2009; Galvan et al. 2010; Masilamoni et al. 2010, 2011; Bogenpohl et al. 2012). Briefly, animals were transferred to an observation cage equipped with eight infrared beams arranged in a square formation on the back and one side of the cage. A computer system was attached and logged the timing of beam crossings (Banner Engineering Corp., Minneapolis, MN). In addition, the animal's spontaneous behavior was also videotaped and a computer-assisted observation method was used to quantify limbs, head, and trunk movements, within a 20-min period. As in our previous studies (Wichmann et al. 2001), the video records we also used to score parkinsonian motor signs with a rating scale similar to that described by Kurlan et al. (1991). This scale assesses key parkinsonian motor signs including gross motor activity, balance, posture, bradykinesia, and hypokinesia.
Three of the animals (M1–M3) displayed moderate parkinsonian motor signs that remained stable for a period of at least 6 weeks before killing (see Masilamoni et al. 2011 for more details). The other three monkeys (M4–M6) showed no significant reduction in motor activity compared with baseline up to 3 weeks after last MPTP injection.
Animal perfusion and tissue preparation
Animals were deeply anesthetized with an overdose of pentobarbital (100 mg/kg, iv) and six of them (C1–C3 and M1–M3) perfused transcardially with cold oxygenated Ringer's solution, followed by 2 L of fixative containing 4 % paraformaldehyde and 0.1 % glutaraldehyde in phosphate buffer (PB; 0.1 M, pH 7.4). After perfusion, the brains were removed from the skull, cut into 10 mm-thick blocks in the frontal plane, and sectioned in 50 μm-thick sections with a vibrating microtome. The three other MPTP-treated monkeys (M4–M6) were also deeply anesthetized as described above, and killed by decapitation. Their brain was removed from the skull and immersion-fixed in paraformaldehyde 4 % for 1 week, followed by a 1 week immersion in a 30 % sucrose solution in PB (0.1 M, pH 7.4) before being cut in 20 mm-thick blocks that were then sectioned in 50 μm-thick sections with a freezing microtome. Sections were collected in cold phosphate-buffered saline (PBS; 0.01 M, pH 7.4), or an anti-freeze solution until further processing.
Tissue staining
Depletion of the dopaminergic nigrostriatal system in MPTP-treated animals was confirmed by staining sections at the level of the striatum and the substantia nigra with mouse anti-tyrosine hydroxylase (TH) antibody (Table 1; Fig. 1).
Table 1.
Antibody information
| Antigen | Immunogen | Immunizing species | Vendor | Antibodies dilution used |
|---|---|---|---|---|
| Tyrosine hydroxylase (TH) | Clone LNCI; tyrosine hydroxylase purified from PC12 cells | Mouse | Millipore, Temecula, CA, USA; catalog no. MAB318 | 1 in 1,000 |
| Calbindin D28k (Cb) | BALB/c mice immunized with a purified bovine kidney calbindin-D-28K | Mouse | Sigma, catalog no. C9848 | 1 in 4,000 |
Fig. 1.
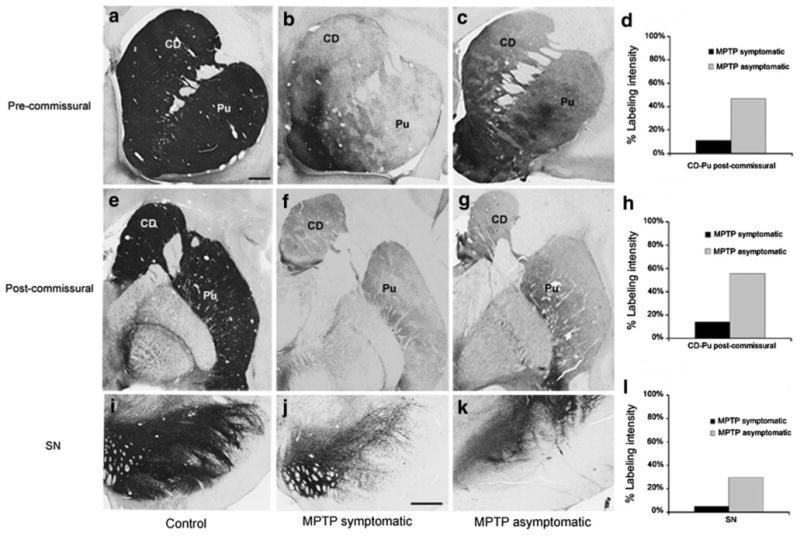
Tyrosine hydroxylase immunoreactivity (TH-IR) in the striatum and substantia nigra (SN). a–k Coronal sections at the level of the pre-commissural striatum (a–c), post-commissural striatum (e–g) and SN (i–k) to illustrate TH-IR in control (a, e, i), symptomatic MPTP-treated monkeys (b, f, j) and asymptomatic MPTP-treated animals (c, g, k). d, h, l Intensity of TH-IR in the pre- and post-commissural striatum and SN in symptomatic and asymptomatic MPTP-treated monkeys, expressed as the proportion of the intensity of TH-IR in controls (100 %). The data in d and h are based on averages of the labeling intensity in both the caudate nucleus and putamen. CD caudate nucleus, Pu putamen. Scale bars in a (applies to b, c, e, f, g, i) and in j (applies to k) correspond to 2 mm
For stereological analysis of the CM/Pf, every twelfth section (7–9 sections per animal) was Nissl-stained (Fig. 2a), and used for unbiased cell counting. Adjacent sections were stained for calbindin (Cb) (Fig. 2b) or acetylcholinesterase (Fig. 2c) to help delineate the external border of the CM/Pf complex (Cb), and to trace the limit between CM and Pf (acetylcholinesterase staining).
Fig. 2.
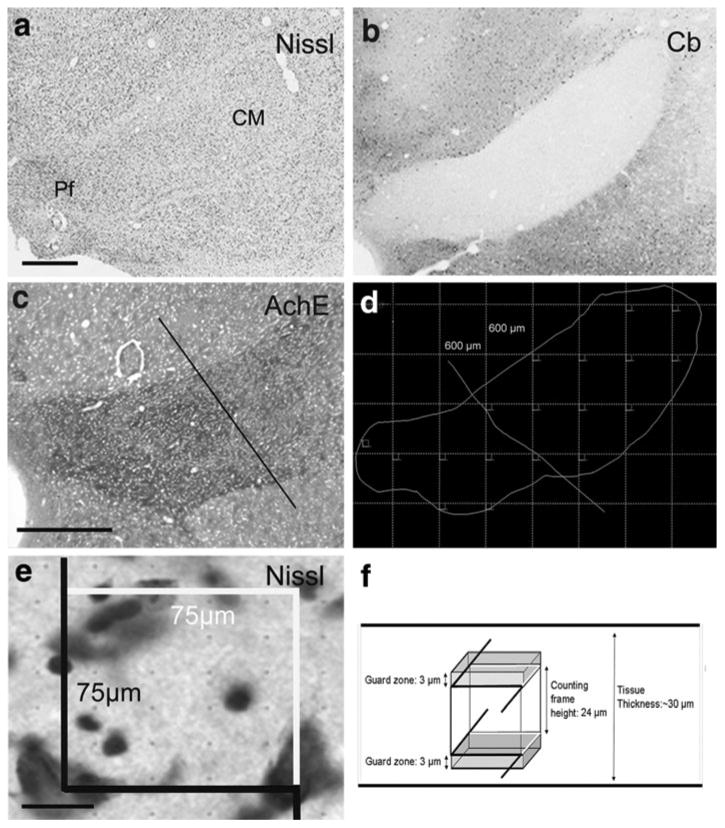
Stereological estimation of the total number of neurons within the centromedian (CM) and parafascicular (Pf) thalamic nuclei. a–c Low magnification photomicrographs of a representative Nissl-stained section used in the stereological neuronal count (a), an adjacent section immunostained for calbindin (Cb), used to delineate the external border of the CM/Pf nuclear complex (b), and a serial section histochemically stained for acetylcholinesterase (AchE) (c), to delineate the limit between the CM and Pf (bar in c). d Schematic outline of CM/Pf complex from the Nissl-stained section shown in (a), with a superimposed virtual grid (grid spacing, 600 × 600 μm). The dissectors (see e, f) were sampled in the corners of each virtual square. Based on the AchE staining (c), a line was traced in each section to separate CM and Pf, and the dissectors for each thalamic nucleus. e High magnification of the Nissl-stained section shown in (a). A single focal plane of the microscopic field of the 3D dissector probe (see f) is shown as overlay. The different colors of the dissector borders indicate the included (white) and excluded (black) edges of the frame. Only the neuronal cell bodies inside the square that did not touch the black lines were counted. f Diagram of the virtual 3D square dissector probe (75 × 75 μm), placed “inside” the section with a height of 24 and 3 μm guard zone above and below the counting frame. Objects intersecting the bottom of the ‘brick’ were excluded, as were objects intersecting with the front or left side of the brick. Scale bars in a (applies to b) and c = 1 mm and in e = 20 μm
Immunoperoxidase labeling
Primary antibodies
All commercially available antibodies used in this study have been well characterized using immunoblots on brain tissue or transfected cells, peptide preadsorption, and omission of primary antibodies. The source and dilution of these antibodies are shown in Table 1.
Cb and TH immunoperoxidase labeling
Sections of control and MPTP-treated monkeys were immunostained with specific Cb or TH antibodies (Table 1) that were localized with the avidin–biotin-per-oxidase complex method (ABC, Vectastain Standard kit), and the chromogen diaminobenzidine (DAB) was used for the peroxidase reaction (Smith and Bolam 1991; Raju et al. 2006; Villalba et al. 2006, 2009; Villalba and Smith 2011). Sections were mounted onto gelatin-coated slides, dehydrated, and then coverslipped with Permount. The tissue was examined with a Leica DMRB light microscope (Leica Microsystems, Inc., Bannockburn, IL) and images were taken with a CCD camera (Leica DC 500; Leica IM50 software). For low magnification images, slides were scanned at 20× using a ScanScope CS scanning system (Aperio Technologies, Vista, CA). Digital representations of the slides were saved and analyzed using ImageScope software (Aperio Technologies).
Control experiments
In a series of control experiments, sections were processed as described above, but without primary antibodies (as a control for the specificity of secondary antibodies). The resulting sections were completely devoid of immunostaining.
Striatal TH immunostaining intensity measurements
Using the ImageScope viewer software (Aperio), 0.7× magnification digital images of the stained tissue slides containing the caudate nucleus, putamen or SN were selected and imported into ImageJ (National Institutes of Health, Rasband, 1997–2009) for additional processing. For optical density measurements, the images were converted into 16-bit grayscale format and inverted. To control for differences in background staining, the optical density measurement in the internal capsule (for caudate and putamen) was subtracted from that obtained in striatal measurements, and the intensity measurement values at the level of the cerebral peduncle were used as background measurements for the SN analysis. Measurements of the intensity of labeling were obtained in two sections at the striatal and nigral levels from the three groups of animals (control, MPTP-treated symptomatic and MPTP-treated asymptomatic). In each section, the intensity of TH-staining was measured in four representative areas in each structure. The resulting values were averaged in each animal, and compared with data obtained in controls to determine the percent of TH immunostaining loss in the striatum (caudate nucleus + putamen) and the SN. (Fig. 1d, h, l).
Stereological estimation of volume and the total number of CM-Pf and MD neurons
We used Cavalieri's principle to estimate the volume, and quantified the total number of neurons with the unbiased optical dissector technique (Gundersen and Osterby 1981; West et al. 1991; West 1999; Schmitz and Hof 2005). The delineation of CM-Pf and MD (regions of interest, ROIs) and the volume estimation were performed at low magnification (Fig. 2a–d), while the counts of cells (profiles) were generated using a 100× oil-immersion objective (Fig. 2e). The volume was determined by multiplying the sum of the ROI areas (CM/Pf or MD) by the distance between sections. Because of tissue shrinkage, the mean section thickness was estimated from at least six measurements/section, obtained by moving the focus from the top to the bottom surface of the tissue at each microscopic field with the z-axis position encoder of the stereology system. The dissectors were sampled (after a random start) in a superimposed virtual grid placed over the ROI area, with individual array elements separated by 600 μm (Fig. 2d) for CM/Pf complex and 700 μm for MD nucleus. Using the acetylcholinesterase staining, a straight line was traced in each section at approximately the same locations to separate CM and Pf, and the dissectors placed in each thalamic nucleus (Fig. 2d). In the corners of each virtual square, we used a square dissector frame (75 × 75 μm), with a height of 24 μm, allowing 3 μm guard zone both above and below the counting frame (Fig. 2e, f). A source of bias in neuron counts with unbiased virtual counting spaces are the so-called “lost-caps”, i.e. loss of neurons at the section surfaces due to damage during tissue sectioning. This bias was reduced through the use of adequate histological techniques and the consideration of guard zones at the upper and the lower surfaces of the sections. This grid spacing and dissector dimensions let us place about 7–10 dissectors per ROI, and 3–4 profiles per dissector probe. A minimum of 200 cells were sampled according with the rules of the optical dissector (West et al. 1991; West 1999; Schmitz and Hof 2005). The software also controlled the position of the x–y stage of the microscope, so that the entire brain region could be scanned by successively meandering between counting frames. The precision and efficiency of the estimates of the total number of cells (N) was evaluated by computing the coefficient of error (CE), an estimate of the degree of variability one would encounter if counts were repeated numerous times (Schmitz and Hof 2005; Slomianka and West 2005). Our sampling was designed to obtain a CE (Gundersen, m = 1) between 0.04 and 0.07.
Photomicrographs production
Pictures were digitally acquired and imported in TIFF format to Adobe Photoshop (version 7.0; Adobe Systems, San Jose, CA) and adjusted only for brightness and contrast, to optimize the quality of the images for analysis. Micrographs were then compiled into figures in Adobe Illustrator 12.0.
Results
Nigrostriatal dopamine denervation in symptomatic and asymptomatic MPTP-treated monkeys
Three groups of monkeys were used in this study: control, MPTP-symptomatic and MPTP-asymptomatic animals. In each of the MPTP-treated animals, the depletion of striatal dopamine innervation was determined by decreases in the extent of TH immunoreactivity (TH-IR) in representative coronal sections of the pre-commissural, commissural, and post-commissural levels of the striatum (Fig. 1). In the three MPTP-treated symptomatic animals, an almost complete and uniform depletion of TH-IR was observed at all striatal levels, except for the ventral striatum in which sparse TH-positive processes remained (Fig. 1b, f). However, in the three MPTP-treated asymptomatic monkeys (M4–M6), that did not display any significant parkinsonian motor symptoms, only a partial TH-IR depletion was observed in the lateral sector of the pre- (Fig. 1c) and post-commissural (Fig. 1g) striatum. The quantification of the intensity of TH immunostaining (ImageJ) in the pre- and post-commissural striatal levels showed a 85–90 % decrease in symptomatic MPTP-treated monkeys (Fig. 1d, h), while in asymptomatic animals, the decrease of TH immunostaining intensity was between 40 and 50 % of control values in both pre- and post-commissural striatal levels (Fig. 1d, h). In each of these monkeys, a corresponding decrease of TH-positive cell bodies was seen in the ventral midbrain, being more prominent in the symptomatic than asymptomatic MPTP-treated monkeys. The analysis of the TH immunostaining intensity showed a >90 % decrease in the SN of symptomatic MPTP-treated monkeys, while in MPTP-treated asymptomatic animals the intensity of the TH labeling decreased by about 60–70 % (Fig. 11). The extent of neuronal loss in the ventral midbrain of the MPTP-treated symptomatic animals was also determined using stereological cell counts in a previous study from our group (see Masilamoni et al. 2011 for more details).
Neuronal loss and volume reduction of the CM and Pf nuclei in MPTP-treated monkeys
In order to determine the extent and time course of the CM/Pf neuronal loss in monkeys chronically treated with MPTP, we used unbiased stereological cell counts to compare the total number of neurons in the CM and Pf of MPTP-treated symptomatic (N = 3) and MPTP-treated asymptomatic (N = 3) monkeys with control animals (N = 3). A qualitative analysis of Nissl-stained coronal sections of CM and Pf showed an obvious neuronal loss in these nuclei in MPTP-treated symptomatic (Fig. 3b, e, h) and asymptomatic (Fig. 3c, f, i) monkeys compared with controls (Fig. 3a, d, g). To further support these qualitative observations, we used the unbiased optical dissector technique to perform stereological neuronal counts in CM/Pf. For each animal, the number of neurons in the CM and Pf nuclei was counted either separately or together as part of the CM/Pf nuclear complex. In cases in which the CM and Pf were analyzed separately, the border between the two nuclei was determined by using two complementary criteria, i.e., the more compact arrangement of Pf cells compared with CM in Nissl-stained sections (Figs. 2a, 3a–c), and the stronger AchE staining in Pf than CM (Fig. 2c). The lack of calbindin immunostaining was used as a highly reliable marker to delineate the borders of the CM/Pf complex (Fig. 2b).
Fig. 3.
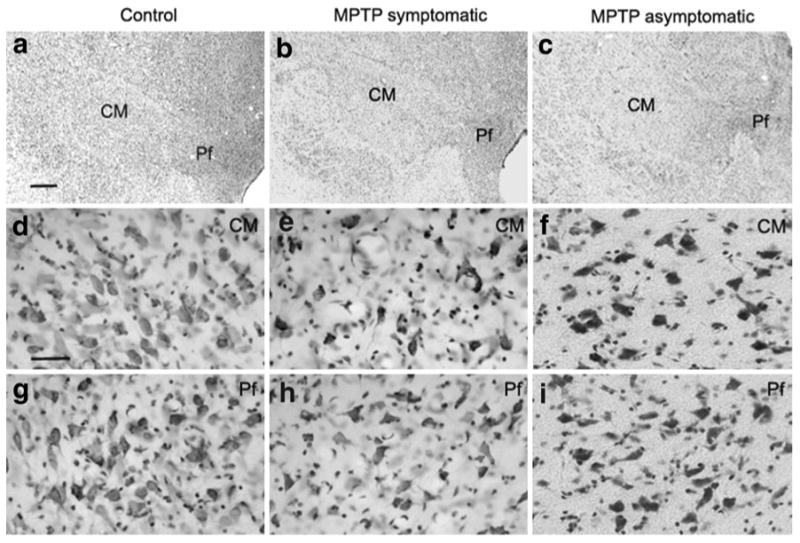
Cell loss in CM/Pf of MPTP-treated monkeys. Nissl-stained coronal sections of the CM/Pf complex in control (a, d, g), MPTP-treated symptomatic (b, e, h) and asymptomatic (c, f, i) monkeys. The high power images (d–i) were taken from the CM or Pf (a–c) in each group. Notice the significant loss of neuronal cell bodies in the CM and Pf of both groups of MPTP-treated monkeys compared with controls. Scale bar in a (applies to b and c) = 1 mm, and in d (applies to e–i) = 50 μm
Treatment with MPTP resulted in a substantial decrease in the total number of Nissl-stained neurons in both the CM and Pf nuclei compared with control animals (Table 2; Fig. 4a). In MPTP-treated monkeys with stable parkinsonian motor features (i.e. MPTP-treated symptomatic group, N = 3, M1–M3), an extensive reduction of 60 and 62 % neuronal loss was found in CM and Pf. Interestingly, MPTP-treated asymptomatic animals (N = 3; M4– M6) also displayed a marked neuronal loss in CM (59 % loss) and Pf (52 % loss) compared with controls (Table 2; Fig. 4a). When neuronal counts were performed in the whole CM/Pf complex, MPTP-treated symptomatic animals displayed a 56 % neuronal loss, which was also closely related to percent cell loss estimated for the MPTP-treated asymptomatic monkeys (52 %). The Cavalieri analysis (Table 2; Fig. 4b) of the CM/Pf volume in control and MPTP-treated monkeys showed a decrease of 18 % in asymptomatic MPTP-treated monkeys (N = 3; M4–M6), and a 33 % reduction in symptomatic MPTP-treated monkeys (N = 3; M1–M3) compared with controls (N = 3,C1–C3) (Fig. 4b). These results demonstrate that the CM/Pf complex in non-human primates displays a 50–60 % neurons loss early in the course of chronic MPTP administration, and that prominent CM/Pf cell loss can be seen in animals that do not display typical parkinsonian motor features.
Table 2.
Summary of the results of stereological analysis in CM-Pf
| Total estimated neuronal number | Total estimated volume (mm3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| CM | CE | Pf | CE | CM + Pf | CE | CM-Pf | CE | CM-Pf | CE | ||
| Control | Control | ||||||||||
| Mean | 752,924 | 0.04 | 617,529 | 0.04 | 1,370,454 | 0.03 | 1,438,318 | 0.04 | Mean | 31.4 | 0.03 |
| SE | 39,908 | 44,571 | 42,240 | 25,791 | SE | 2.3 | |||||
| SD | 69,122 | 77,199 | 73,161 | 44,671 | SD | 3.9 | |||||
| MPTP-asymptomatic | MPTP-asymptomatic | ||||||||||
| Mean | 306,347 | 0.06 | 296,960 | 0.06 | 603,307 | 0.05 | 689,746 | 0.05 | Mean | 25.7 | 0.04 |
| SE | 4,751 | 18,870 | 11,811 | 8,773 | SE | 1.4 | |||||
| SD | 8,229 | 32,684 | 20,457 | 15,195 | SD | 2.4 | |||||
| Decrease in neurons number | 59 % | 52 % | 56 % | 52 % | Decrease in volume | 18 % | |||||
| MPTP-symptomatic | MPTP-symptomatic | ||||||||||
| Mean | 300,800 | 0.06 | 235,008 | 0.06 | 535,808 | 0.04 | 630,413 | 0.05 | Mean | 21.1 | 0.02 |
| SE | 25,419 | 9,725 | 17,572 | 32,595 | SE | 1.8 | |||||
| SD | 44,027 | 16,844 | 30,436 | 56,456 | SD | 2.8 | |||||
| Decrease in neurons number | 60 % | 62 % | 61 % | 56 % | Decrease in volume | 33 % | |||||
CM centromedian nucleus, Pf parafascicular nucleus, CE coefficient of error, SE standard error, SD standard deviation
Fig. 4.
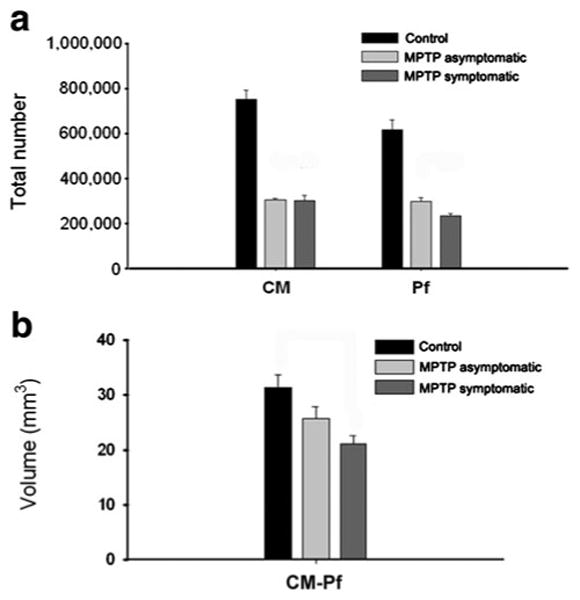
Stereological analysis of the neuronal loss in CM/Pf of MPTP-treated monkeys. a Histograms comparing the extent of neuronal loss in CM or Pf between control (N = 3), symptomatic MPTP-treated monkeys (N = 3), and asymptomatic MPTP-treated monkeys (N = 3). In MPTP-treated parkinsonian monkeys, a robust reduction of 60 and 62 % neuronal loss was found in CM and Pf, respectively, while in the partially dopamine-depleted asymptomatic MPTP-treated monkeys, there was a 59 % loss in CM and 52 % in Pf. b The Cavalieri analysis of the volume of CM/Pf complex between control and MPTP-treated monkeys show a 33 % decrease in symptomatic MPTP-treated parkinsonian monkeys (N = 3), but no significant reduction in asymptomatic MPTP-treated monkeys (N = 3). Data are expressed as mean ± SE
Neuronal count and volume changes of the mediodorsal thalamic nucleus between normal and MPTP-treated parkinsonian monkeys
In order to determine if the major neurons loss seen in CM/Pf was specific for that nuclear group, we assessed the extent of degeneration in the mediodorsal nucleus (MD), located in close vicinity of CM/Pf. Stereological cell count and volume analyses of MD were performed using serial Nissl-stained coronal sections (Fig. 5a, b) in three control (C1–C3) and three MPTP-treated symptomatic monkeys (M1–M3). The delineation of the MD nucleus was based on nuclear borders used in stereotaxic atlases of the rhesus monkey thalamus (Jones 1985; Ilinsky and Kultas-Ilinsky 2002; Lanciego and Vazquez 2012) (Fig. 5a). In Nisslstained sections, only large neuronal cell bodies, with visible nuclei were counted (Fig. 5b-asterisks). A small 18 % reduction in the overall number of MD neurons and 19 % reduction in MD volume were found in the three MPTP-treated symptomatic monkeys compared with controls (Table 3; Fig. 5a, b).
Fig. 5.
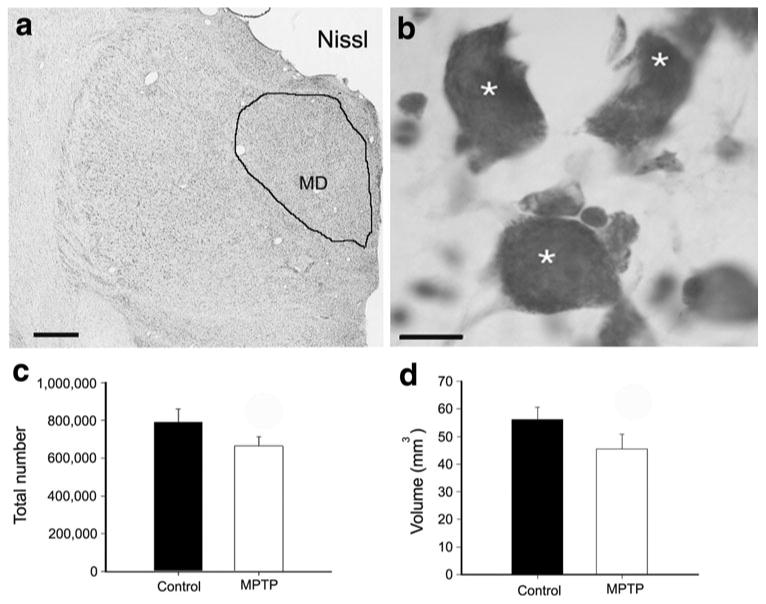
Number of neurons and volume of the mediodorsal nucleus (MD) between control and MPTP-treated symptomatic monkeys. Low (a) and high (b) magnification photomicrographs of a Nissl-stained section showing a representative coronal section the MD nucleus and the large neurons (asterisks) counted in the stereological quantitative analysis. Histograms comparing the total number (mean ± SE) of neurons (c) and the volume (mean ± SE) (d) of MD between control (N = 3) and MPTP-treated parkinsonian (N = 3) animals. There is a small decrease of 18 % in the total number of neurons in MD (c) and a 19 % reduction of the MD volume (d) in MPTP-treated parkinsonian monkeys compared with controls. Scale bars in a = 2 mm and in b = 10 μm
Table 3.
Summary of the results of stereological analysis in MD
| Total estimated neuronal number | Total estimated volume (mm3) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Number of neurons | CE | Volume | CE | ||
| Control | Control | ||||
| Mean | 789,933 | 0.05 | Mean | 56.2 | 0.02 |
| SE | 41,457 | SE | 2.5 | ||
| SD | 71,806 | SD | 4.4 | ||
| MPTP complete DA depletion | |||||
| Mean | 646,419 | 0.06 | Mean | 45.5 | 0.02 |
| SE | 23,938 | SE | 2.7 | ||
| SD | 47,875 | SD | 5.3 | ||
| Decrease in neurons number | 18 % | Decrease in volume | 19 % | ||
MD mediodorsal nucleus, CE coefficient of error, SE standard error, SD standard deviation
Discussion
The present study demonstrates that non-human primates chronically treated with low doses of MPTP display significant neuronal degeneration in CM and Pf, a pathological feature that parallels similar findings reported in postmortem studies of PD patient brains (Xuereb et al. 1991; Heinsen et al. 1996; Henderson et al. 2000a, b; Halliday et al. 2005; Brooks and Halliday 2009; Halliday 2009, 2011). Our findings also show that this cell loss occurs early, prior to the appearance of parkinsonian motor symptoms, in MPTP-treated monkeys. Furthermore, in line with postmortem human data (Halliday et al. 2011), our results demonstrate that this thalamic degeneration is far more pronounced in CM/Pf than in neighboring thalamic nuclei. In light of recent behavioral studies showing the key role of CM/Pf in the transmission of attention-related salient stimuli to the striatum, and its importance in regulating the physiological responses of striatal cholinergic interneurons to reward-related stimuli (Matsumoto et al. 2001; Smith et al. 2011), it seems likely that CM/Pf degeneration contributes to cognitive- and limbic-related deficits in PD (Galvan and Smith 2011; Smith et al. 2011). Our study confirms that the neurodegeneration observed in chronically MPTP-treated non-human primates is not confined to SNc dopaminergic cell loss, but also affects non-dopaminergic cell groups known to be degenerated in PD (see also Schneider 1990; Hantraye et al. 1993; Masilamoni et al. 2011). In addition, our data show that chronically MPTP-treated monkey is a reliable model to study the impact of CM/Pf degeneration on cognition and other functions in PD.
CM/Pf pathology in PD
Our findings provide further evidence that the thalamus may be more strongly involved in the pathophysiology of PD than previously thought. The data reveal that the CM and Pf, projecting to the putamen and the caudate nucleus, respectively (Smith et al. 2004, 2009; Galvan and Smith 2011), undergo the same degree of degeneration in chronically MPTP-treated monkeys, confirming previous postmortem studies showing a major cell loss in the CM/Pf complex of PD patients (Henderson et al. 2000a, b; Halliday et al. 2005; Halliday 2009). Although this was not specifically characterized in our monkeys, there was no apparent evidence for significant heterogeneity in the pattern of cell death throughout the nuclear complex (Fig. 3). In humans, a report suggests that subpopulations of parvalbumin-containing neurons are mainly affected in Pf, while non-parvalbumin/non-calbindin neurons are more specifically targeted in CM (Henderson et al. 2000b). We found that the CM and Pf degeneration occurs early during the course of MPTP treatment, prior to the appearance of parkinsonian motor symptoms and severe degeneration of the nigrostriatal dopaminergic system. Although pre-symptomatic CM/Pf neuronal loss has not been reported in PD patients, the extent of CM/Pf degeneration has also been shown to not be correlated with the severity of parkinsonian motor signs, i.e., both early diagnosed and advanced PD patients display corresponding CM/Pf damage (Henderson et al. 2000a, b).
To our knowledge, our data represent the first report of CM/Pf cell loss in a non-human primate model of PD. However, Pf cell loss has been demonstrated in rodent models of PD, but the outcome of these studies was somewhat discrepant. While some authors did not find evidence for Pf degeneration 3 months after unilateral 6-hydroxydopamine (6-OHDA) nigrostriatal dopaminergic lesion in rats (Henderson et al. 2005; Kusnoor et al. 2012), other studies reported significant Pf cell loss in the same animal model (Aymerich et al. 2006; Sedaghat et al. 2009), or after systemic MPTP administration in mice (Freyaldenhoven et al. 1997). Some authors also showed that intrastriatal administration of 1-methyl-4-phenylpyridinium ion (MPP+) induces significant Pf cells damage in rats (Ghorayeb et al. 2002). It remains to be determined whether these discrepancies were the result of differences in the neurotoxin exposure protocols, animal strains or other technical differences between these studies. In contrast to these data, the extent of CM/Pf cell loss in all MPTP-treated monkeys used in our study was consistent and comparable across individuals. Therefore, the use of the chronically MPTP-treated monkey model of parkinsonism offers unique opportunities to further characterize the importance of thalamostriatal degeneration in the basal ganglia pathophysiology, and in the development of early non-motor cognitive impairments in PD (see below; see also Schneider and Kovelowski 1990; Schneider 2006).
CM/Pf cell loss and striatal denervation of thalamic inputs
The prominent CM/Pf cell loss reported in this study is consistent with our recent data showing a significant decrease in the relative prevalence of vGluT2-positive axo-dendritic synapses in the monkey putamen (Raju et al. 2008). Knowing that as much as 70 % of CM terminals form axo-dendritic synapses in the monkey striatum, and that inputs from other thalamic nuclei terminate almost exclusively on spines (Sadikot et al. 1992; Smith et al. 2004, 2009; Raju et al. 2006), CM/Pf neuronal loss is, indeed, the most likely source of these synaptic changes in the striatum of MPTP-treated monkeys. Because both interneurons and projection neurons receive significant CM/Pf inputs to the striatum (Sidibe and Smith 1996, 1999; Raju et al. 2008),a detailed analysis of the specific impact of CM/Pf cell loss on the synaptic denervation of different neuron populations in the striatum is warranted to better understand the functional consequences of thalamostriatal degeneration in PD. Stereological synapse counting studies are also needed to estimate the total number of vGluT2-immunoreactive terminals, and the total number of asymmetric axo-spinous and axo-dendritic synapses in the striatum of MPTP-treated parkinsonian monkeys.
Potential mechanisms of MPTP-induced CM/Pf cell death in monkeys
The analysis of possible mechanisms by which CM/Pf undergoes degeneration in MPTP-treated monkeys was outside the scope of this study. We suggest, however, that the thalamic pathology is a consequence of the direct toxic effects of the MPTP-derivative, MPP+ on the thalamus, independent from its effects on the nigrostriatal system. Although there is evidence that MPP+ can mediate toxic effects upon all monoaminergic neurons (Altar et al. 1986; Singer et al. 1988; Herkenham et al. 1991; Johannessen 1991; Przedborski et al. 2000; Watanabe et al. 2005; Fornai et al. 2005; Masilamoni et al. 2011), the mechanisms by which MPP+ may induce death of non-monoaminergic cells are less well understood, although other glutamatergic cell groups, specifically the cerebellar granule cells, are also highly sensitive to MPP+ toxicity (Altar et al. 1986; Marini et al. 1989; Storey et al. 1992; Camins et al. 1997; Gonzalez-Polo et al. 2004; Alvira et al. 2007; Harbison et al. 2011). In addition, it is noteworthy that intrastriatal injection of MPP+ resulted in significant neuronal loss in Pf, without any significant effect on cortical neurons in mice (Ghorayeb et al. 2002), suggesting that CM/Pf neurons display unique features that make them particularly sensitive to MPTP toxicity. Their lack of calbindin, and preferential expression of different proteins such as cerebellin 1, leucine-rich repeat containing GPCR8 (LGR8) and Frizzled5 compared with other thalamic neurons (Shen et al. 2005; Liu et al. 2008; Sedaghat et al. 2009; Kusnoor et al. 2010) may confer to them such particular properties.
Functional consequences of CM/Pf cell loss in parkinsonism
It appears that the loss of this thalamic input to the striatum may have a significant impact upon the physiological activity of cholinergic interneurons in the rat and monkey striatum. Functional data from Kimura et al. have demonstrated that the silencing of CM/Pf neurons alters significantly the response pattern of cholinergic interneurons to reward-related salient stimuli in monkeys, thereby indicating that CM/Pf inputs are key regulators of cholinergic interneurons activity in primates (Matsumoto et al. 2001; Kimura et al. 2004). These findings are supported by neurochemical studies showing that manipulation of CM/Pf activity alters the extracellular levels of acetylcholine in the striatum (Zackheim and Abercrombie 2005; Nanda et al. 2009). Furthermore, recent studies have suggested that the thalamostriatal system may gate corticostriatal activity through regulation of cholinergic interneurons (Ding et al. 2010). It appears, therefore, that the degeneration of the CM/Pf-striatal system may have major functional consequences upon transmission, processing and integration of information at the striatal level. Finally, recent studies have shown that the Pf-striatal system may be involved in the control of behavioral flexibility and sensory cue-dependent associative learning in rats (Kato et al. 2011; Brown et al. 2010). Taken together, these different lines of evidence suggest that the CM/Pf-striatal system may help to regulate cognitive functions related to attentional shift, behavioral reinforcement, and action selection (Galvan and Smith 2011; Smith et al. 2011). In line with this hypothesis, neuropsychological testing in a patient with unilateral infarct restricted to the CM/Pf revealed that this person suffered of attentional defects that made her susceptible to distraction, and unable to carry out simultaneous cognitive processes (Mennemeier et al. 1992, 1997). Interestingly, population-based studies of newly diagnosed PD patients have also reported cognitive deficits related to attention and associative learning (Foltynie et al. 2004; Levin and Katzen 2005; Muslimovic et al. 2005; Aarsland et al. 2009; Elgh et al. 2009), two features that may be accounted for by an early degeneration of the CM/Pf-striatal system (Matsumoto et al. 2001; Galvan and Smith 2011; Kato et al. 2011; Smith et al. 2011).
Concluding remarks
This study provides the first direct evidence for early CM/Pf neurons loss in the MPTP-treated non-human primate model of PD. The use of this model will help us to better understand the potential role of the thalamostriatal degeneration in the pathophysiology of PD and other neurological diseases, specifically with regard to the development of non-motor cognitive impairments related to attentional set shifting and behavioral reinforcement.
Acknowledgments
The authors thank Jean-Francois Pare and Susan Jenkins for technical assistance. Thanks are also due to Dr. Gunasingh Masilamoni and the Yerkes Center Animal Resources Division for the MPTP-treatment and care of the monkeys. The authors also thank to Dr. Adriana Galvan for critical reading of the manuscript. Special thanks are due to Professor Carlos Avendaño (Anatomy Department of the School of Medicine, University Auto-noma in Madrid, Spain) for his generous help with the initial design of stereological analysis. This work was supported by research grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants R01NS062876 and P50-NS071669 (TW), and by funding from the National Center for Research Resources P51RR000165 and the Office of Research Infrastructure Programs/OD P51OD011132 to the Yerkes National Primate Research Center.
Abbreviations
- AchE
Acetylcholinesterase
- Cb
Calbindin
- CD
Caudate nucleus
- CM
Center median nucleus
- MD
Mediodorsal nucleus
- MPP+
1-Methyl-4-phenylpyridinium ion
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson's disease
- Pf
Parafascicular nucleus
- Pu
Putamen
- ROI
Regions of interest
- SN
Substantia nigra
- TH
Tyrosine hydroxylase
- vGluT2
Vesicular glutamate transporter type 2
- 6-OHDA
6-Hydroxydopamine
Contributor Information
R. M. Villalba, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd. NE, Atlanta, GA 30329, USA, rvillal@emory.edu, Udall Center of Excellence for Parkinson's Disease Research, Emory University, Atlanta, GA, USA
T. Wichmann, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd. NE, Atlanta, GA 30329, USA, rvillal@emory.edu, Udall Center of Excellence for Parkinson's Disease Research, Emory University, Atlanta, GA, USADepartment of Neurology, Emory University, Atlanta, GA, USA
Y. Smith, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd. NE, Atlanta, GA 30329, USA, rvillal@emory.edu, Udall Center of Excellence for Parkinson's Disease Research, Emory University, Atlanta, GA, USA, Department of Neurology, Emory University, Atlanta, GA, USA
References
- Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson's disease. Mov Disord. 2009;24:2175–2186. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Heikkila RE, Manzino L, Marien MR. 1-Methyl-4-phenylpyridine (MPP+): regional dopamine neuron uptake, toxicity, and novel rotational behavior following dopamine receptor proliferation. Eur J Pharmacol. 1986;131:199–209. doi: 10.1016/0014-2999(86)90573-x. [DOI] [PubMed] [Google Scholar]
- Alvira D, Tajes M, Verdaguer E, de Arriba SG, Allgaier C, Matute C, Trullas R, Jimenez A, Pallas M, Camins A. Inhibition of cyclin-dependent kinases is neuroprotective in 1-methyl-4-phenylpyridinium-induced apoptosis in neurons. Neuroscience. 2007;146:350–365. doi: 10.1016/j.neuroscience.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci. 2006;23:2099–2108. doi: 10.1111/j.1460-9568.2006.04741.x. [DOI] [PubMed] [Google Scholar]
- Bogenpohl JW, Galvan A, Hu X, Wichmann T, Smith Y. Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultrastructural localization and electro-physiological effects of activation in the striatopallidal complex. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.05.017. (In press), Corrected proof available online 22 May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brooks D, Halliday GM. Intralaminar nuclei of the thalamus in Lewy body diseases. Brain Res Bull. 2009;78:97–104. doi: 10.1016/j.brainresbull.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–14398. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camins A, Sureda FX, Gabriel C, Pallas M, Escubedo E, Camarasa J. Effect of 1-methyl-4-phenylpyridinium (MPP+) on mitochondrial membrane potential in cerebellar neurons: interaction with the NMDA receptor. J Neural Transm. 1997;104:569–577. doi: 10.1007/BF01291876. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: a population-based study. Eur J Neurol. 2009;16:1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127:550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Fornai F, Bassi L, Bonaccorsi I, Giorgi F, Corsini GU. Noradrenaline loss selectivity exacerbates nigrostriatal toxicity in different species of rodents. Funct Neurol. 1997a;12:193–198. [PubMed] [Google Scholar]
- Fornai F, Alessandri MG, Torracca MT, Bassi L, Corsini GU. Effects of noradrenergic lesions on MPTP/MPP+ kinetics and MPTP-induced nigrostriatal dopamine depletions. J Pharmacol Exp Ther. 1997b;283:100–107. [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci USA. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, di Poggio AB, Pellegrini A, Ruggieri S, Paparelli A. Noradrenaline in Parkinson's disease: from disease progression to current therapeutics. Curr Med Chem. 2007;14:2330–2334. doi: 10.2174/092986707781745550. [DOI] [PubMed] [Google Scholar]
- Freyaldenhoven TE, Ali SF, Schmued LC. Systemic administration of MPTP induces thalamic neuronal degeneration in mice. Brain Res. 1997;759:9–17. doi: 10.1016/s0006-8993(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Gai WP, Halliday GM, Blumbergs PC, Geffen LB, Blessing WW. Substance P-containing neurons in the mesopontine tegmentum are severely affected in Parkinson's disease. Brain. 1991;114:2253–2267. doi: 10.1093/brain/114.5.2253. [DOI] [PubMed] [Google Scholar]
- Galvan A, Smith Y. The primate thalamostriatal system: anatomical organization, functional roles and possible involvement in Parkinson's disease. Basal Ganglia. 2011;1:179–189. doi: 10.1016/j.baga.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. Localization and function of GABA transporters in the globus pallidus of parkinsonian monkeys. Exp Neurol. 2010;223:505–515. doi: 10.1016/j.expneurol.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Hervier L, Labattu B, Bioulac B, Tison F. A ‘single toxin-double lesion’ rat model of striatonigral degeneration by intrastriatal 1-methyl-4-phenylpyridinium ion injection: a motor behavioural analysis. Neuroscience. 2002;115:533–546. doi: 10.1016/s0306-4522(02)00401-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Soler G, Fuentes JM. MPP+: mechanism for its toxicity in cerebellar granule cells. Mol Neurobiol. 2004;30:253–264. doi: 10.1385/MN:30:3:253. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Osterby R. Optimizing sampling efficiency of stereological studies in biology: or ‘do more less well!’. J Microsc. 1981;121:65–73. doi: 10.1111/j.1365-2818.1981.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Halliday GM. Thalamic changes in Parkinson's disease. Parkinsonism Relat Dis. 2009;15(S3):S153–S155. doi: 10.1016/S1353-8020(09)70804-1. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Gai WP, Blessing WW, Geffen LB. Substance P-containing neurons in the pontomesencephalic tegmentum of the human brain. Neuroscience. 1990;39:81–96. doi: 10.1016/0306-4522(90)90223-q. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Macdonald V, Henderson JM. A comparison of degeneration in motor thalamus and cortex between progressive supranuclear palsy and Parkinson's disease. Brain. 2005;128:2272–2280. doi: 10.1093/brain/awh596. [DOI] [PubMed] [Google Scholar]
- Halliday G, Lees A, Stern M. Milestones in Parkinson's disease-clinical and pathologic features. Mov Disord. 2011;26:1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- Hantraye P, Varastet M, Peschanski M, Riche D, Cesaro P, Willner JC, Maziere M. Stable parkinsonian syndrome and uneven loss of striatal dopamine fibres following chronic MPTP administration in baboons. Neuroscience. 1993;53:169–178. doi: 10.1016/0306-4522(93)90295-q. [DOI] [PubMed] [Google Scholar]
- Harbison RA, Ryan KR, Wilkins HM, Schroeder EK, Loucks FA, Bouchard RJ, Linseman DA. Calpain plays a central role in 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in cerebellar granule neurons. Neurotox Res. 2011;19:374–388. doi: 10.1007/s12640-010-9172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsen H, Rub U, Gangnus D, Jungkunz G, Bauer M, Ulmar G, Bethke B, Schuler M, Bocker F, Eisenmenger W, Gotz M, Strik M. Nerve cell loss in the thalamic centromedian-parafascicular complex in patients with Huntington's disease. Acta Neuropathol. 1996;91:161–168. doi: 10.1007/s004010050408. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson's disease: clinical and therapeutic implications. Brain. 2000a;123:1410–1421. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson's disease. Ann Neurol. 2000b;47:345–352. [PubMed] [Google Scholar]
- Henderson JM, Schleimer SB, Allbutt H, Dabholkar V, Abela D, Jovic J, Quinlivan M. Behavioural effects of parafascicular thalamic lesions in an animal model of parkinsonism. Behav Brain Res. 2005;162:222–232. doi: 10.1016/j.bbr.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Little MD, Bankiewicz K, Yang SC, Markey SP, Johannessen JN. Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: an in vivo autoradiographic study. Neuroscience. 1991;40:133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA. 1987;84:5976–5980. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinsky IA, Kultas-Ilinsky K. Motor thalamic circuits in primates with emphasis on the area targeted in treatments of movement disorders. Mov Disord. 2002;17(Suppl 3):S9–S14. doi: 10.1002/mds.10137. [DOI] [PubMed] [Google Scholar]
- Jellinger K. The pedunculopontine nucleus in Parkinson's disease, progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1988;51:540–543. doi: 10.1136/jnnp.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen JN. A model of chronic neurotoxicity: long-term retention of the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) within catecholaminergic neurons. Neurotoxicology. 1991;12:285–302. [PubMed] [Google Scholar]
- Jones EG. The thalamus. Plenum Press; New York: 1985. [Google Scholar]
- Kato S, Kuramochi M, Kobayashi K, Fukabori R, Okada K, Uchigashima M, Watanabe M, Tsutsui Y, Kobayashi K. Selective neural pathway targeting reveals key roles of thalamo-striatal projection in the control of visual discrimination. J Neurosci. 2011;31:17169–17179. doi: 10.1523/JNEUROSCI.4005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N, Hori Y. Monitoring and switching of cortico-basal ganglia loop functions by the thalamo-striatal system. Neurosci Res. 2004;48:355–360. doi: 10.1016/j.neures.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas J, Roland PR. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Pare JF, Khan ZU, Wichmann T, Smith Y. Comparative ultrastructural analysis of D1 and D5 dopamine receptor distribution in the substantia nigra and globus pallidus of monkeys. Adv Behav Biol. 2009;58:239–253. doi: 10.1007/978-1-4419-0340-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlan R, Kim MH, Gash DM. Oral levodopa dose-response study in MPTP-induced hemiparkinsonian monkeys: assessment with a new rating scale for monkey parkinsonism. Mov Disord. 1991;6:111–118. doi: 10.1002/mds.870060205. [DOI] [PubMed] [Google Scholar]
- Kusnoor SV, Parris J, Muly EC, Morgan JI, Deutch AY. Extracerebellar role for Cerebellin 1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol. 2010;518:2525–2537. doi: 10.1002/cne.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnoor SV, Bubser M, Deutch AY. The effects of nigrostriatal dopamine depletion on the thalamic parafascicular nucleus. Brain Res. 2012;1446:46–55. doi: 10.1016/j.brainres.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Vazquez A. The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. Brain Struct Funct. 2012;217:613–666. doi: 10.1007/s00429-011-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Katzen HL. Early cognitive changes and nonde-menting behavioral abnormalities in Parkinson's disease. Adv Neurol. 2005;96:84–94. [PubMed] [Google Scholar]
- Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled 5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien M, Briley M, Colpaert F. Noradrenaline depletion exacerbates MPTP-induced striatal dopamine loss in mice. Eur J Pharmacol. 1993;236:487–489. doi: 10.1016/0014-2999(93)90489-5. [DOI] [PubMed] [Google Scholar]
- Marini AM, Schwartz JP, Kopin IJ. The neurotoxicity of 1-methyl-4-phenylpyridinium in cultured cerebellar granule cells. J Neurosci. 1989;9:3665–3672. doi: 10.1523/JNEUROSCI.09-10-03665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamoni G, Votaw J, Howell L, Villalba RM, Goodman M, Voll RJ, Stehouwer J, Wichmann T, Smith Y. (18)F-FECNT: validation as PET dopamine transporter ligand in parkinsonism. Exp Neurol. 2010;226:265–273. doi: 10.1016/j.expneurol.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134:2057–2073. doi: 10.1093/brain/awr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Yoshida M, Watanabe S, Yamamoto T. Involvement of cholinergic and glutamatergic functions in working memory impairment induced by interleukin-1 beta in rats. Eur J Pharmacol. 2001;430:283–288. doi: 10.1016/s0014-2999(01)01374-7. [DOI] [PubMed] [Google Scholar]
- Mennemeier M, Fennell E, Valenstein E, Heilman KM. Contributions of the left intralaminar and medial thalamic nuclei to memory. Comparisons and report of a case. Arch Neurol. 1992;49:1050–1058. doi: 10.1001/archneur.1992.00530340070020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennemeier M, Crosson B, Williamson DJ, Nadeau SE, Fennell E, Valenstein E, Heilman KM. Tapping, talking and the thalamus: possible influence of the intralaminar nuclei on basal ganglia function. Neuropsychologia. 1997;35:183–193. doi: 10.1016/s0028-3932(96)00062-0. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Nanda B, Galvan A, Smith Y, Wichmann T. Effects of stimulation of the centromedian nucleus of the thalamus on the activity of striatal cells in awake rhesus monkeys. Eur J Neurosci. 2009;29:588–598. doi: 10.1111/j.1460-9568.2008.06598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G. The parkinsonian toxin MPTP: action and mechanism. Restor Neurol Neurosci. 2000;16:135–142. [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci USA. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot AF, Rymar V. The primate centromedian-parafascicular complex: anatomical organization with a note on neuro-modulation. Brain Res Bull. 2009;78:122–130. doi: 10.1016/j.brainresbull.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315:137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Schneider JS. Chronic exposure to low doses of MPTP. II. Neurochemical and pathological consequences in cognitively-impaired, motor asymptomatic monkeys. Brain Res. 1990;534:25–36. doi: 10.1016/0006-8993(90)90108-n. [DOI] [PubMed] [Google Scholar]
- Schneider JS. Modeling cognitive deficits associated with parkinsonism in chronic-low-dose MPTP-treated monkey. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. CRC Press; Boca Raton: 2006. (Chapter 9, Frontiers in Neuroscience) [PubMed] [Google Scholar]
- Schneider JS, Kovelowski CJ., 2nd Chronic exposure to low doses of MPTP. I. Cognitive deficits in motor asymptomatic monkeys. Brain Res. 1990;519:122–128. doi: 10.1016/0006-8993(90)90069-n. [DOI] [PubMed] [Google Scholar]
- Sedaghat K, Finkelstein DI, Gundlach AL. Effect of unilateral lesion of the nigrostriatal dopamine pathway on survival and neurochemistry of parafascicular nucleus neurons in the rat-evaluation of time-course and LGR8 expression. Brain Res. 2009;1271:83–94. doi: 10.1016/j.brainres.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Shen PJ, Fu P, Phelan KD, Scott DJ, Layfield S, Tregear GW, Bathgate RA, Gundlach AL. Restricted expression of LGR8 in intralaminar thalamic nuclei of rat brain suggests a role in sensorimotor systems. Ann NY Acad Sci USA. 2005;1041:510–515. doi: 10.1196/annals.1282.076. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Differential synaptic innervation of striatofugal neurones projecting to the internal or external segments of the globus pallidus by thalamic afferents in the squirrel monkey. J Comp Neurol. 1996;365:445–465. doi: 10.1002/(SICI)1096-9861(19960212)365:3<445::AID-CNE8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sidibe M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–1208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- Singer TP, Ramsay RR, McKeown K, Trevor A, Castagnoli NE., Jr Mechanism of the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), the toxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicology. 1988;49:17–23. doi: 10.1016/0300-483x(88)90169-2. [DOI] [PubMed] [Google Scholar]
- Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labeling study. Neuroscience. 1991;44:45–73. doi: 10.1016/0306-4522(91)90250-r. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Surmeier DJ, Redgrave P, Kimura M. Thalamic contributions to basal ganglia-related behavioral switching and reinforcement. J Neurosci. 2011;31:16102–16106. doi: 10.1523/JNEUROSCI.4634-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey E, Hyman BT, Jenkins B, Brouillet E, Miller JM, Rosen BR, Beal MF. 1-Methyl-4-phenylpyridinium produces excito-toxic lesions in rat striatum as a result of impairment of oxidative metabolism. J Neurochem. 1992;58:1975–1978. doi: 10.1111/j.1471-4159.1992.tb10080.x. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J Comp Neurol. 2011;519:989–1005. doi: 10.1002/cne.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Raju DV, Hall RA, Smith Y. GABA(B) receptors in the centromedian/parafascicular thalamic nuclear complex: an ultrastructural analysis of GABA(B)R1 and GABA(B)R2 in the monkey thalamus. J Comp Neurol. 2006;496:269–287. doi: 10.1002/cne.20950. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Wichmann T, Smith Y. Neuronal loss in the caudal intralaminar nuclear group, CM/Pf, in MPTP-treated parkinsonian monkeys. Society for Neuroscience. 2011 (Abstracts) [Google Scholar]
- Villalba RM, Wichmann T, Smith Y. Early neuronal loss in the centre median and parafascicular thalamic nuclei prior to the development of parkinsonian motor symptoms in MPTP-treated monkeys. Society for Neuroscience. 2012 (Abstracts) [Google Scholar]
- Watanabe Y, Himeda T, Araki T. Mechanisms of MPTP toxicity and their implications for therapy of Parkinson's disease. Med Sci Monit. 2005;11:RA17–RA23. [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Lewis SJ, Barker RA. Cognitive deficits and psychosis in Parkinson's disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20:477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- Xuereb JH, Perry RH, Candy JM, Perry EK, Marshall E, Bonham JR. Nerve cell loss in the thalamus in Alzheimer's disease and Parkinson's disease. Brain. 1991;114:1363–1379. [PubMed] [Google Scholar]
- Zackheim J, Abercrombie ED. Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience. 2005;131:423–436. doi: 10.1016/j.neuroscience.2004.11.006. [DOI] [PubMed] [Google Scholar]


