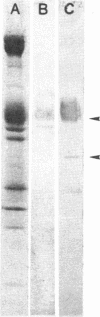
Abstract
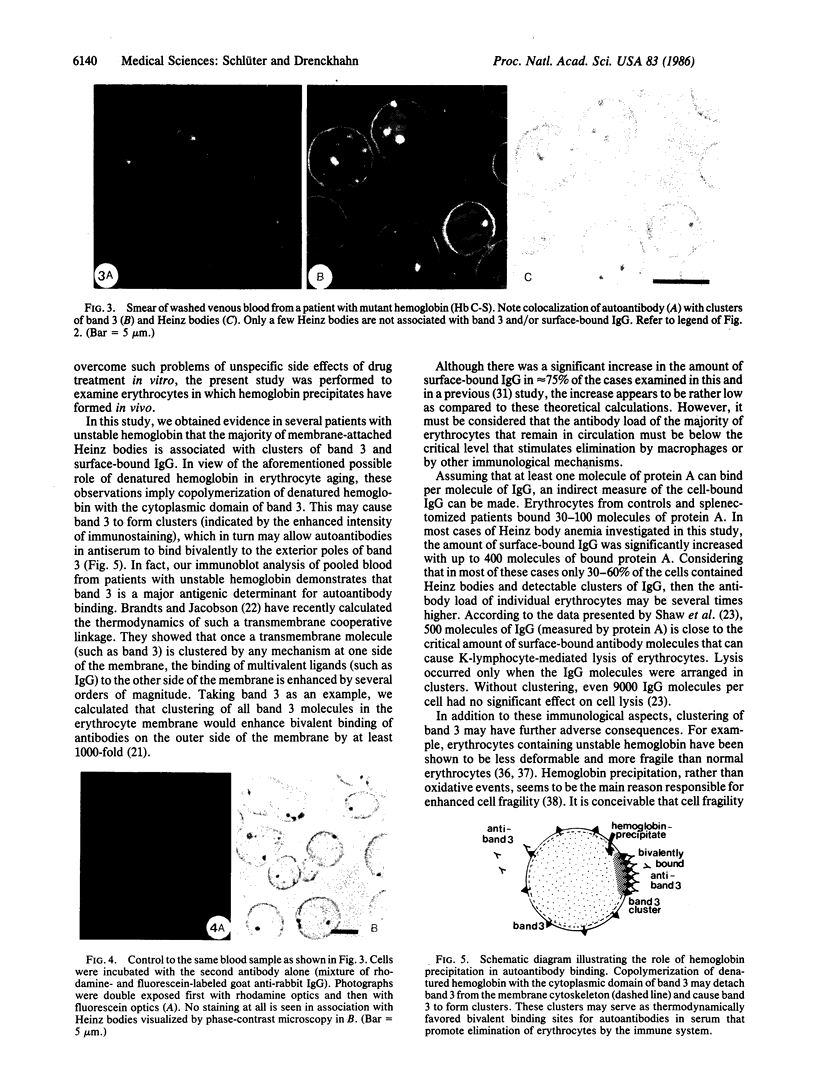
Precipitates of hemoglobin, termed Heinz bodies, occur in a fraction of erythrocytes after removal of the spleen and are also observed in aged erythrocytes. This implies that precipitates of hemoglobin might play a particular role in senescent cell recognition. By using immunofluorescence microscopy, evidence is presented in splenectomized patients and in several patients with unstable (mutant) hemoglobins that membrane-attached Heinz bodies are associated with both clusters of the anion channel, band 3, and clusters of surface-bound immunoglobulins (IgG). In 75% of the cases of unstable hemoglobin, such as sickle cell anemia or hemoglobin Köln disease, the level of cell-bound IgG (measured by 125I-labeled staphylococcal protein A) was increased severalfold above the level found in healthy controls. Immunoblot analysis identified the major fraction of cell-bound IgG to be directed to band 3. These observations indicate copolymerization of denatured hemoglobin with the cytoplasmic domain of band 3, which may cause band 3 to form clusters. These clusters probably serve as thermodynamically favored binding sites for autoantibodies in serum, which promote elimination of the erythrocytes by the immune system. Thus, erythrocytes may be removed from circulation when hemoglobin begins to denature and the cells begin to fail in their main function of oxygen transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman E. M., Fudenberg H. H., Lovins R. E. Binding of immunoglobulin classes to subpopulations of human red blood cells separated by density-gradient centrifugation. Blood. 1980 May;55(5):817–822. [PubMed] [Google Scholar]
- Bates D. A., Winterbourn C. C. Haemoglobin denaturation, lipid peroxidation and haemolysis in phenylhydrazine-induced anaemia. Biochim Biophys Acta. 1984 Mar 22;798(1):84–87. doi: 10.1016/0304-4165(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Chen L. T., Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973 Apr;41(4):529–537. [PubMed] [Google Scholar]
- Choy Y. M., Wong S. L., Lee C. Y. Changes in surface carbohydrates of erythrocytes during in vivo aging. Biochem Biophys Res Commun. 1979 Nov 28;91(2):410–415. doi: 10.1016/0006-291x(79)91537-7. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Ekholm J. E., Luthra M. G., Hanahan D. J. Biochemical characterization of density-separated human erythrocytes. Biochim Biophys Acta. 1976 Jan 21;419(2):229–242. doi: 10.1016/0005-2736(76)90349-7. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Drenckhahn D., Wagner J. Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol. 1986 May;102(5):1738–1747. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Zinke K., Schauer U., Appell K. C., Low P. S. Identification of immunoreactive forms of human erythrocyte band 3 in nonerythroid cells. Eur J Cell Biol. 1984 May;34(1):144–150. [PubMed] [Google Scholar]
- Durocher J. R., Payne R. C., Conrad M. E. Role of sialic acid in erythrocyte survival. Blood. 1975 Jan;45(1):11–20. [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG F. Das Schicksal toxisch veränderter roter Blutzellen in der Milz. Klin Wochenschr. 1958 Jan 15;36(2):63–66. doi: 10.1007/BF01486245. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Winterhalter K. H. The role of hemoglobin heme loss in Heinz body formation: studies with a partially heme-deficient hemoglobin and with genetically unstable hemoglobins. J Clin Invest. 1970 Nov;49(11):2008–2016. doi: 10.1172/JCI106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubowski M., Agutter P. S. Changes in the activities of some membrane-associated enzymes during in vivo ageing of the normal human erythrocyte. Br J Haematol. 1977 Sep;37(1):111–125. [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Sorensen K., Wong P., Bolton P. Antigenicity, storage, and aging: physiologic autoantibodies to cell membrane and serum proteins and the senescent cell antigen. Mol Cell Biochem. 1982 Nov 26;49(2):65–85. doi: 10.1007/BF00242486. [DOI] [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Lubin A., Desforges J. F. Effect of Heinz bodies on red cell deformability. Blood. 1972 May;39(5):658–665. [PubMed] [Google Scholar]
- Lutz H. U. Elimination alter Erythrozyten aus der Zirkulation: Freilegung eines zellater-spezifischen Antigens auf alternden Erythrozyten. Schweiz Med Wochenschr. 1981 Oct 10;111(41):1507–1517. [PubMed] [Google Scholar]
- Lutz H. U., Fehr J. Total sialic acid content of glycophorins during senescence of human red blood cells. J Biol Chem. 1979 Nov 25;254(22):11177–11180. [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- Petz L. D., Yam P., Wilkinson L., Garratty G., Lubin B., Mentzer W. Increased IgG molecules bound to the surface of red blood cells of patients with sickle cell anemia. Blood. 1984 Jul;64(1):301–304. [PubMed] [Google Scholar]
- Schneider R. G., Takeda I., Gustavson L. P., Alperin J. B. Intraerythrocytic precipitations of haemoglobins S and C. Nat New Biol. 1972 Jan 19;235(55):88–90. doi: 10.1038/newbio235088a0. [DOI] [PubMed] [Google Scholar]
- Sears D. A., Friedman J. M., White D. R. Binding of intracellular protein to the erythrocyte membrane during incubation: the production of Heinz bodies. J Lab Clin Med. 1975 Nov;86(5):722–732. [PubMed] [Google Scholar]
- Shaw G. M., Aminoff D., Balcerzak S. P., LoBuglio A. F. Clustered IgG on human red blood cell membranes may promote human lymphocyte antibody-dependent cell-mediated cytotoxicity. J Immunol. 1980 Aug;125(2):501–507. [PubMed] [Google Scholar]
- WESTERMAN M. P., PIERCE L. E., JENSEN W. N. ERYTHROCYTE LIPIDS: A COMPARISON OF NORMAL YOUNG AND NORMAL OLD POPULATIONS. J Lab Clin Med. 1963 Sep;62:394–400. [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Waugh S. M., Low P. S. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry. 1985 Jan 1;24(1):34–39. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Batt R. D. Lipid composition of human red cells of different ages. Biochim Biophys Acta. 1970 Feb 10;202(1):1–8. doi: 10.1016/0005-2760(70)90212-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Studies of hemoglobin denaturation and Heinz body formation in the unstable hemoglobins. J Clin Invest. 1974 Sep;54(3):678–689. doi: 10.1172/JCI107806. [DOI] [PMC free article] [PubMed] [Google Scholar]