Abstract
Introduction
Fish and omega-3 fatty acids are reported to be beneficial in pediatric nonalcoholic fatty liver disease (NAFLD), but no studies have assessed their relation to histological severity. The objectives of this study were to evaluate the dietary intake of fish and omega-3 fatty acids in children with biopsy-proven NAFLD, and examine their association with serological and histological indicators of disease.
Materials and Methods
This was a cross-sectional analysis of 223 children (6–18 years) that participated in the Treatment of Nonalcoholic Fatty Liver Disease in Children trial or the NAFLD Database study conducted by the Nonalcoholic Steatohepatitis Clinical Research Network. The distribution of fish and omega-3 fatty acid intake were determined from responses to the Block Brief 2000 Food Frequency Questionnaire, and analyzed for associations with serum alanine aminotransferase, histological features of fatty liver disease, and diagnosis of steatohepatitis after adjusting for demographic, anthropometric and dietary variables.
Results
The minority of subjects consumed the recommended eight ounces of fish per week (22/223 (10%)) and 200 mg of long-chain omega-3 fatty acids per day (12/223 (5%)). Lack of fish and long-chain omega-3 fatty acid intake was associated with greater portal (p=0.03 and p=0.10, respectively) and lobular inflammation (p=0.09 and p=0.004, respectively) after controlling for potential confounders.
Discussion
Fish and omega-3 fatty acid intake were insufficient in children with NAFLD, which may increase susceptibility to hepatic inflammation. Patients with pediatric NAFLD should be encouraged to consume the recommended amount of fish per week.
Keywords: Adolescents, Fatty Acid, Omega-3, Fish, Nonalcoholic Fatty Liver Disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common complication of pediatric obesity, which is characterized by altered lipid metabolism resulting in macrovesicular liver steatosis (1). Many children with NAFLD have concomitant inflammation and/or fibrosis of the liver termed nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis (2–3). There is emerging evidence that ectopic fat deposition in the liver may be a risk factor for development of other metabolic disorders (4). Similar to other obesity-related conditions, successful weight loss attempts are effective at treating NAFLD in the short-term, but generally fail beyond one year, resulting in recrudescence (5). Consequently, there is considerable interest in identifying dietary factors that affect NAFLD pathogenesis independently of weight loss.
The long-chain omega-3 fatty acids found in fish, eicosapentaenoic acid (EPA; 20:5 ω-3) and docosahexaenoic acid (DHA; 22:6 ω-3) are thought to have a protective role in the development and progression of NAFLD (6–7). This is most clearly demonstrated in animal models of obesity where EPA and DHA are able to prevent and reverse liver disease (6). In humans, obesity and NAFLD are negatively associated with the long-chain omega-3 fatty acid content of cell membranes, which has been linked to altered hepatic lipid metabolism (8–9). Moreover, supplementation with long-chain omega-3 fatty acids has been shown to improve serological biomarkers of NAFLD and radiological measures of liver steatosis in several clinical trials, including one study in children, which found a marked reduction in ultrasound liver steatosis grade in subjects that received DHA supplements (10–11).
There is a paucity of research looking at the dietary intake of fish and omega-3 fatty acids in pediatric NAFLD. One study reported a low intake of omega-3 fatty acids, and a significant negative correlation between EPA and DHA intake and serum alanine aminotransferase (ALT) in 35 children with NAFLD (12). A more robust analysis with liver biopsy data would provide important insight into the role of dietary fish and omega-3 fatty acids in attenuating the progression of NAFLD. The purpose of this study was to evaluate the dietary intake of fish and omega-3 fatty acids, and their relation to serum ALT and histological features of liver disease in pediatric NAFLD. We hypothesized that most pediatric NAFLD patients would report fish and omega-3 fatty acids intakes that were below the recommended levels for children, and that lower intakes of fish and omega-3 fatty acids would be associated with higher serum ALT values and more severe histological indicators of liver disease.
MATERIALS AND METHODS
Study population
This study was a cross-sectional analysis of data that was collected as part of the Treatment of Nonalcoholic Fatty Liver Disease in Children (TONIC) trial and the NAFLD Database study (13–14). The design of the TONIC trial has been described previously (13,15). Briefly, children (8–17 years) with biopsy-proven NAFLD were recruited amongst unsolicited referrals from September 2005 to September 2007 to eight clinical centers of the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN, n = 229) including the University of California, San Diego, University of California, San Francisco, University of Washington, St. Louis University (collaborated with Texas Children's Hospital), Duke University Medical Center (collaborated with Johns Hopkins University), Indiana University, Case Western Reserve University and Virginia Commonwealth University (collaborated with Children's National Medical Center). Subjects that had liver cirrhosis, diabetes mellitus, other liver diseases, or who were pregnant were excluded from the study (n = 56) (15). The NAFLD Database study included NAFLD patients age 2 years or older who were being treated at one of the NASH CRN clinical centers (n = 218) from October 2004 to February 2008, and contained 23 patients who participated in the TONIC trial.
Of the 368 children enrolled in TONIC or the NAFLD Database, 238 completed the Block Brief 2000 food frequency questionnaire (FFQ, 16) within six months of the liver biopsy. Three subjects did not meet the definition of NAFLD because they had a liver steatosis grade indicating <5% macrovesicular steatosis, and were excluded from the study. Additional subjects were excluded because they were missing one or more variables of interest (n = 7), or did not report intake of fried and/or non-fried fish (n = 4). Finally, there was a two-year-old that was identified as an outlier with respect to age, and was therefore not included in the study sample. Consequently, 223 (61%) children from the initial study population were deemed eligible and included in the study sample. Sensitivity analysis was performed for demographic and histological characteristics of children eligible and included in the study compared to children that were excluded; study subjects were more likely to be male, and to have more severe steatosis and hepatocyte ballooning (p <0.05).
Assessment of fish and omega-3 fatty acid intake
Participants completed the FFQ at baseline and annually for the TONIC trial and NAFLD Database study. This semi-quantitative FFQ approximates usual dietary intake based on reported consumption of 77 food items in the previous year. The food items are a composite of food lists developed for Whites, Hispanics and African Americans based on 24-hour dietary recall data from the National Health and Nutrition Examination Survey III (NHANES III) (16). Visual displays of portion sizes were included in the FFQ to assist in estimation of quantity (16). This FFQ has been used previously to assess the relationship between other dietary variables and histological features of pediatric NAFLD (17).
As part of the Block Brief 2000 FFQ, subjects reported their intake of fried and non-fried fish across nine frequencies ranging from never to daily, and four serving sizes ranging from 1/4 cup to 2 cups. The responses for fried and non-fried fish were pooled together to estimate the overall frequency and amount of fish consumed. The nutrient content of the diet was calculated by NutritionQuest using the USDA Food and Nutrient Database for Dietary Studies, which provide a population-weighted nutrient composition for each food item based on national dietary intake data (16). Total omega-3 fatty acid consumption was analyzed as a nutrient density (g / 1,000 kcal) and in relation to omega-6 fatty acid intake. The ratio of omega-6 fatty acids to omega-3 fatty acids was used because the synthesis of long-chain omega-3 fatty acids (EPA and DHA) from alpha-linolenic acid (ALA; 18:3 ω-3), which makes up approximately 95% of total omega-3 fatty acids in the diet of children and adolescents in the US, is thought to be dependent on omega-6 fatty acid intake due to shared metabolic pathways (18–19). Although nutrient density is often preferred over absolute intakes when using FFQ data, absolute intakes of EPA, DHA and long-chain omega-3 fatty acids were used in statistical analyses since they are concentrated in a limited number of sporadically consumed foods that are represented in the Block Brief 2000 FFQ (20).
Assessment of nonalcoholic fatty liver disease
Liver disease was evaluated using serum alanine aminotransferase (ALT) and histological features of NAFLD. For the histological parameters, liver biopsies were graded centrally by NASH CRN pathologists for steatosis (<5%, 5% to 33%, >33% to 66% or >66% macrovesicular steatosis), portal inflammation (none, mild, more than mild), lobular inflammation (<2 foci, 2–4 foci or >4 foci at 20x field), hepatocellular ballooning (none, few, many), and fibrosis (none [0], zone 3 perisinusoidal, delicate [1A], zone 3 perisinusoidal, dense [1B], portal/periportal [1C], perisinusoidal and portal/periportal [stage 2], bridging fibrosis [stage 3] or cirrhosis [stage 4]) using standard scoring criteria (21). Zone 3 perisinusoidal [1A] and [1B], and portal/periportal [1C] fibrosis were collapsed for analysis as stage 1, as were bridging and cirrhosis, as stage 3–4. Finally, subjects biopsies were diagnosed as “not NASH”, “borderline zone 1 pattern”, “borderline zone 3 pattern”, or “definite NASH”, as described previously (14).
Other Variables
Demographic and anthropometric data were obtained from participants in the TONIC trial and NAFLD Database study through structured interviews and questionnaires conducted at each of the NASH CRN clinical centers. For ethnicity, subjects were asked to identify themselves as American Indian, Asian, Pacific Islander, Black or White, and as Hispanic or non-Hispanic. After examining the ethnicity distribution, responses were assigned as Hispanic, non-Hispanic White and Other due to a limited representation from most ethnic groups. Height and weight were measured in duplicate to the nearest 0.1 cm and 0.1 kg, respectively, with subjects wearing lightweight clothing and no shoes. The average of height and weight were used to calculate BMI (kg/m2), and these values were converted in z-scores for age and gender based on the Center for Disease Control and Prevention (CDC) growth charts (22). Laboratory data including serum triglycerides, total-, LDL- and HDL-cholesterol, glucose, insulin and c-reactive protein (CRP) were available for a subset of subjects. The homeostatic model of insulin resistance (HOMA-IR) index was calculated as fasting glucose (mmol/L) \m=x\ fasting insulin (μU/mL) / 22.5, to provide an estimate of insulin resistance (23).
Statistical analysis
Demographic and dietary characteristics of subjects were summarized as frequency and percentage for categorical variables (gender and ethnicity), mean ± standard deviation for normally distributed continuous variables (age), and median and interquartile range for non-normally distributed continuous variables (energy intake, total, fried and non-fried fish consumption, omega-3 fatty acid density and omega-6 to omega-3 fatty acid ratio of the diet, and EPA, DHA and long-chain omega-3 fatty acid intake). Additionally, the proportion of subjects consuming the recommended amount of fish (≥2 serving and ≥8 oz / week), and long-chain omega-3 fatty acids (≥200 mg / d) was determined and reported as frequency and percentages (24–25). Continuous variables were designated as normally distributed based on Shapiro-Wilk test, and visual analysis of frequency distribution graphs. The association between age and dietary fish and omega-3 fatty acid intake variables were analyzed using Spearman's rank correlation coefficient. Differences in subject characteristics by gender and ethnicity were evaluated using chi-square analysis, independent two-sample t-test and Wilcoxon rank sum test for categorical, and normally and non-normally distributed continuous variables, respectively. A positive correlation has been observed between how often a food is consumed, and the serving size that is eaten (26). To determine whether this pattern was present for dietary fish in this population, the relationship between the frequency and the quantity of fried and non-fried fish intake was assessed. Subjects were grouped according to the reported frequency of fried and non-fried fish intake as a few times a year, monthly (1× per month and 2–3× per month), and weekly (1× per week, 2× per week, 3–4× per week, 5–6× per week and daily), and the corresponding portion size of fried and non-fried fish were compared across the frequency groups using the Mantel-Haenszel test for trend. Only a few subjects reported eating 2-cup servings of fried (n = 3) and non-fried (n = 4) fish, so a combined 1–2 cups serving was used in this analysis.
The association of serum ALT with total, fried and non-fried fish consumption, omega-3 fatty acid density and omega-6 to omega-3 fatty acid ratio of the diet, and EPA, DHA and long-chain omega-3 fatty acid intake was assessed using Spearman's rank correlation coefficient, and linear regression analysis adjusting for age, gender, BMI z-score, and daily intake of energy, carbohydrates, protein, total, saturated, monounsaturated and polyunsaturated fat, sugar, fiber, cholesterol, vitamins A, C and E, β-carotene, betaine and choline. For the histological features of NAFLD, the differences in fish, long-chain omega-3 fatty acid and omega-6 to omega-3 fatty acid ratio were examined across histology levels using the Kruskal-Wallis test for steatosis, portal and lobular inflammation, hepatocyte ballooning and fibrosis, and the Wilcoxon's rank-sum test to compare subjects diagnosed with isolated steatosis to those with definite NASH. The associations with histological features and NASH diagnosis were also assessed using multivariate analysis of variance (MANOVA) adjusting for age, gender, ethnicity, BMI z-score, and daily intake of energy, carbohydrates, protein, total, saturated, monounsaturated and polyunsaturated fat, sugar, fiber, cholesterol, vitamins A, C and E, β-carotene, betaine and choline. In the linear regression and MANOVA, serum ALT and all dietary variables were log-transformed because they were non-normally distributed as per Shapiro-Wilk test and analysis of frequency distribution graphs. Total, fried and non-fried fish consumption were entered as log (fish intake + 1) prior to transformation to accommodate zero values.
All statistical tests were carried out using SAS version 9.2, and graphs were constructed using Microsoft Excel for Mac 2011 v. 14.2.5. The University of Hawaii Committee on Human Studies approved this research study.
RESULTS
The 223 subjects included in the study were mostly male (171 (77%)), and Hispanic (128 (57%)) or non-Hispanic White (79 (35%)) with a mean age of 12.6 ± 2.6 years old (Table 1). Consistent with the diagnosis of pediatric NAFLD, both BMI z-score (2.31 ± 0.36) and serum ALT (85 U/L (IQR 64–130 U/L)) were well above the normal ranges. The dietary variables of interest are summarized in Table 1. The median daily intake of 1616 kcal (IQR 1185–2351 kcal) was lower than would be expected based on the energy needs for this population, which is a common issue of dietary assessment using FFQs with moderately sized food lists. However, the macronutrient distribution appears probable (50.4 ± 8.3% carbohydrates, 16.2 ± 3.4% protein, 35.3 ± 6.9% fat), and was similar to what has been reported in other pediatric NAFLD studies (12,27).
Table 1.
Demographic and dietary characteristics of subjects (n = 223)
| Parameter | Total |
|---|---|
| Age (y) | 12.6 ± 2.5 |
| Gender (M) | 171 / 223 (77%) |
| Ethnicity | |
| White, non-Hispanic | 79 / 223 (35%) |
| Hispanic | 128 / 223 (57%) |
| Other | 16 / 223 (7%) |
| Energy (kcal / d) | 1616 (1186–2367) |
| Fish (oz / mo) | 4.4 (0.7–13.6) |
| (≥8 oz / wk) | 22 / 223 (10%) |
| (≥2 times / wk) | 33 / 223 (15%) |
| Fish, not fried (oz / mo) | 1.4 (0.0–12.1) |
| Fish, fried (oz / mo) | 1.5 (0.0–3.0) |
| Omega-3 Fatty Acid (g / 1,000 kcal) | 0.71 (0.59–0.93) |
| Omega-6: Omega-3 Fatty Acid | 9.0 (7.4–10.2) |
| Eicosapentaenoic Acid (mg / d) | 9 (5–22) |
| Docosahexaenoic Acid (mg / d) | 27 (16–48) |
| Long-Chain Omega-3 Fatty Acid (mg / d) | 43 (26–80) |
| (≥200 mg / d) | 12 / 223 (5%) |
Legend: Other ethnicities include non-Hispanic American Indian, Asian, Pacific Islander, Black and Mixed. Categorical variables (gender, ethnicity, and proportion of subjects consuming ≥8 oz and ≥2 servings of fish per week, and ≥200 mg of long-chain omega-3 fatty acids per day) are presented as frequency (percent), normally distributed continuous variables (age, BMI, and proportion of calories from carbohydrates, protein and fat) are presented as mean ± standard deviation, non-normally distributed continuous variables (energy intake, total, fried and non-fried fish consumption, omega-3 fatty acid density and omega-6 to omega-3 fatty acid ratio of the diet, and eicosapentaenoic acid, docosahexaenoic acid and long-chain omega-3 fatty acid intake) are presented as median (interquartile range). Continuous variables were determined to be normally distributed based on Shapiro-Wilk test and analysis of frequency distribution graph.
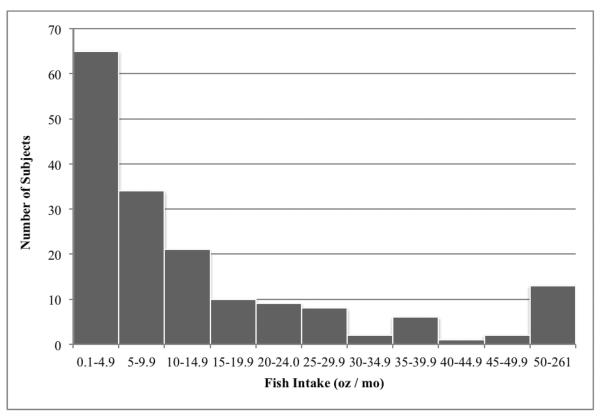
Although there is no specific recommendation for fish intake in pediatric NAFLD patients, most guidelines suggest consuming at least two servings, or eight ounces of fish per week (25). Only 33 / 223 (15%) of subjects reported consumption of fish two or more times per week, and less than 10% (22 / 223) achieved the target of eight ounces per week (Table 1). Furthermore, nearly one-quarter (52 / 223, 23%) indicated that they never ate fish (Figure 1). As seen in Figure 1, the distribution of fish consumption was typical of sporadically consumed foods with the mode located at the lowest level of intake, and a large right skew. This pattern can be partly attributed to the larger portion sizes consumed by regular fish eaters (supplemental Fig. 1 [http://links.lww.com/MPG/A242] and Fig. 2 [http://links.lww.com/MPG/A243]). Among subjects that reported eating fish (n = 171), there was no correlation between fried and non-fried sources (Spearman's rho = −0.05, p = 0.55), suggesting that the two food items were distinct in the mind of the respondents (data not shown). As expected, fish intake was a major determinant of the long-chain omega-3 fatty acid content of the diet (Spearman's rho 0.73, p<0.0001, data not shown). Given the relatively limited amount of fish consumed, it is not surprising that the diets were low in long-chain omega-3 fatty acids with only 12 / 223 (5%) of subjects consuming more than 200 mg / d (Table 1).
Figure 1. Frequency distribution of fish intake among fish consumers (n = 171).
Fish intake is sum of fried and non-fried fish intake calculated from semi-quantitative food frequency questionnaire responses. An additional 52 (23.3%) subjects reported never consuming fish.
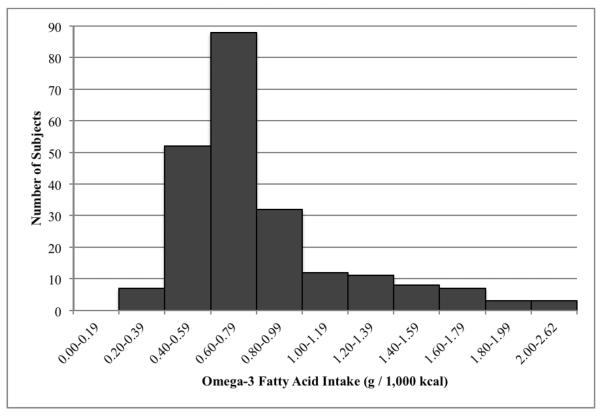
The omega-3 fatty acid density of the diet was largely reflective of alpha-linolenic acid (ALA) intake, as ALA constituted 96% (IQR 94–98%) of omega-3 fatty acid intake (data not shown). According to the dietary reference intakes (DRIs), the acceptable macronutrient distribution range (AMDR) for ALA in children and adolescents is 0.6–1.2% of energy, which equates to 0.67–1.33 g per 1,000 kcal of omega-3 fatty acids when applying an Atwater factor of 9 kcal per g of ALA (25). The omega-3 fatty acid density of the diets observed here (0.72 g / 1,000 kcal (IQR 0.59–0.93 g / 1,000 kcal)) was concentrated around the lower end of the AMDR (Figure 2, Table 1). The omega-3 fatty acid intake was also low in relation to omega-6 fatty acids (median omega-6 to omega-3 ratio = 9.0 (IQR 7.4–10.2) (Table 1). While a desirable omega-6 to omega-3 fatty acid ratio has not been determined for pediatric NAFLD, the observed ratio of 9:1 greatly exceeds most references for disease prevention, which range from approximately 3:1 to 6:1 (19). Despite greater reported energy intake in boys (1772 kcal (IQR 1369–2406 kcal)) compared to girls (1220 kcal (IQR 949–1860 kcal)) (p <0.001), there were no significant differences in total, fried and non-fried fish consumption by gender (supplemental Table 1 [http://links.lww.com/MPG/A244]). Girls appeared to consume a more omega-3 fatty acid rich diet than boys in relation to energy intake (0.77 g / 1,000 kcal (0.61–0.98 g / 1,000 kcal vs 0.71 g / 1,000 kcal (0.58–0.88 g / 1,000 kcal), p = 0.05), and omega-6 fatty acid intake (omega-6 to omega-3 ratio 8.6 (7.1–10.0) vs 9.1 (7.6–10.3), p = 0.05) (supplemental Table 1). Larger disparities in fish and omega-3 fatty acid intake were observed between ethnic groups than between genders (supplemental Table 2 [http://links.lww.com/MPG/A245]). Subjects from the “Other” ethnicity group consumed greater amount of fish (12.8 oz / month (3.7–31.3 oz / month)) than Hispanics (4.4 oz / month (0.0–13.0 oz / month), p = 0.03) and non-Hispanic Whites (3.3 oz / month (0.7–13.6 oz / month), p = 0.02). Additionally, the non-Hispanic Whites reported diets that were lower in omega-3 fatty acid content compared to subjects in the Hispanic and “Other” ethnicity groups (supplemental Table 2). Age was not associated with any of the fish or omega-3 fatty acid parameters measured (data not shown).
Figure 2.
Frequency distribution of omega-3 fatty acid intake density (g / 1,000 kcal).
Higher fish and omega-3 fatty acid intakes were generally associated with lower ALT values, although none of the correlations were strong or statistically significant (p >0.05). However, when additional factors including demographic, anthropometric and dietary variables were accounted for in a linear regression model, the relationships between serum ALT and long-chain omega-3 fatty acid intake tended towards significance (p = 0.08, data not shown).
The dietary fish and long-chain omega-3 fatty acid intake, and omega-6 to omega-3 fatty acid intake ratio were also examined in relation to histological features of NAFLD. The main significant findings of these analyses were related to inflammation (Table 2). There appeared to be a protective effect of fish intake on hepatic inflammation that was significant for portal (p = 0.02), and tended towards significance for lobular inflammation (p = 0.08) (Table 2). Fish and long-chain omega-3 fatty acid consumption, and omega-6 to omega-3 fatty acid ratio were not associated with the other histological parameters or a diagnosis of definite NASH (data not shown). Adjustment for demographic, anthropometric and dietary variables did not influence the findings for fish intake and hepatic inflammation, but resulted in a stronger association between long-chain omega-3 fatty acid intake and lobular inflammation (p = 0.004, Table 2).
Table 2.
The relationship between dietary fish and long-chain omega-3 fatty acid intake, and histological features of nonalcoholic fatty liver disease
| Histological Parameter | n | Fish (oz / mo) | LCn-3 (mg / d) |
|---|---|---|---|
| Diagnosis | |||
| Steatosis | 55 | 4.4 (0.7–13.6) | 45 (23–83) |
| Borderline, Zone 1 | 42 | 3.3 (0.0–8.0) | 48 (27–72) |
| Borderline, Zone 3 | 50 | 3.0 (0.0–25.0) | 40 (27–117) |
| Definite NASH | 76 | 6.0 (1.1–14.4) | 40 (26–80) |
| Crude | p = 0.39 | p = 0.90 | |
| Adjusted | p = 0.38 | p = 0.97 | |
| Portal Inflammation | |||
| None | 20 | 11.7 (4.7–19.8) | 57 (36–110) |
| Mild | 181 | 4.3 (0.7–13.1) | 44 (26–78) |
| More than Mild | 22 | 2.9 (0.0–5.9) | 33 (23–102) |
| Crude | p = 0.02 | p = 0.22 | |
| Adjusted | p = 0.03 | p = 0.10 | |
| Lobular Inflammation | |||
| <2 Foci / 200x Field | 116 | 4.4 (0.7–16.1) | 48 (26–81) |
| 2–4 Foci / 200x Field | 94 | 5.3 (1.4–13.0) | 43 (28–80) |
| >4 Foci / 200x Field | 13 | 0.0 (0.0–5.8) | 29 (11–41) |
| Crude | p = 0.08 | p = 0.13 | |
| Adjusted | p = 0.09 | p = 0.004 |
Legend: Zones 1 and 3 refer to the periportal and centrilobular zones of the liver, respectively. Intake of fish and long-chain omega-3 fatty acids are reported for each level of the histological parameters as a median with interquartile range in parentheses. Crude analysis using Kruskal-Wallis test to assess differences in dietary variables across levels of portal and lobular inflammation, and Wilcoxon's rank-sum test to compare dietary variables of subjects diagnosed with steatosis and definite nonalcoholic steatohepatitis. Multivariate analysis of variance (MANOVA) controlling for age, gender, ethnicity, BMI z-score, and intake of energy, carbohydrates, protein, total, saturated, monounsaturated and polyunsaturated fat, sugar, fiber, cholesterol, vitamins A, C and E, β-carotene, betaine and choline was used for the adjusted analysis. Dietary variables were determined to be non-normally distributed using Shapiro-Wilk test and analysis of frequency distribution graphs, and were log-transformed for the MANOVA. Fish intake was entered as log (fish intake + 1) to accommodate zero values. LCn3 = long-chain omega-3 fatty acids, NASH = nonalcoholic steatohepatitis.
DISCUSSION
There is emerging evidence that long-chain omega-3 fatty acids may be important mediators of NAFLD pathogenesis (7). The findings from this registry-based study offer insight into the dietary intake of fish and omega-3 fatty acids in children with documented NAFLD in the United States. This provides valuable contextual information, and a useful perspective from which to examine the diet-disease relationship.
Most of the subjects in this sample were not consuming the recommended amounts of fish and omega-3 fatty acids (Table 1) (24–25). The observed low intake of omega-3 fatty acids are consistent with the results of 3-day food records collected from 35 pediatric NAFLD patients in Toronto, which reported average intakes of ALA that were less than two-thirds the adequate intake level (12). Together, these findings indicate that diet may be contributing to the low EPA and DHA content of cell membranes that has been observed in patients with NAFLD (9). Although this study did not have a control group for comparison, the frequency of fish consumption was less than that reported from a sample of more than 1,000 adolescents from five public schools in Rhode Island, which had 36% of subjects who indicated eating fish at least once per week (vs 26%), and 17% who reported never eating fish (vs 23%) (28). Of interest, the “Other” ethnicity group reported approximately three times greater fish consumption than the Hispanic and non-Hispanic White subjects; however, this was a diverse group of non-Hispanic Indian, Asian, Pacific Islander, Black and Mixed ethnicities that contained only 16 subjects, which precluded meaningful subgroup analysis (Supplementary Table 2).
Although NAFLD risk could not be determined from this analysis, it was possible to explore the relationship of fish and omega-3 fatty acid consumption to serological and histological indicators of disease. The Toronto study noted a strong inverse relationship between EPA and DHA intake, and ALT (12). A similar, though non-significant, inverse association between fish and long-chain omega-3 fatty acid intake and ALT was observed in this sample. More importantly, lack of fish and long-chain omega-3 fatty acid consumption was associated with greater portal and lobular inflammation (Table 2). Although fish and long-chain omega-3 fatty acid intake were not associated with a diagnosis of definite NASH, inflammation is known to predispose to fibrosis and progressive liver disease (29). The failure to detect a relationship between fish intake and definite NASH may be related to the fact that histologic parameters for steatohepatitis in pediatric biopsies are not as well understood as they are in adults (30).
There are several anti-inflammatory and pro-resolution mechanisms of EPA and DHA in fish that would support the observation of protective effects on both portal and lobular inflammation. Historically, the effects of long-chain omega-3 fatty acids on inflammation have been largely attributed to the shift from omega-6 to omega-3 fatty acid-derived eicosanoids (19). In recent years, additional EPA and DHA-derived lipid mediators, including protectins and resolvins, have been identified, and are thought to be important anti-inflammatory mediators (31). A series of experiments by Oh et al. (2010) also demonstrated that DHA suppresses activation of the nuclear factor-kappaB (NF-κB) inflammatory pathway in macrophages through a G-protein coupled receptor (GPR 120) (32). C-reactive protein (CRP), an indicator of systemic inflammation, was measured in a subset of subjects in this study (n = 151). While CRP was not associated with fish consumption, there was a weak inverse correlation with long-chain omega-3 fatty acid intake (Spearman's rho = −0.155, p = 0.058, data not shown).
Subjects consuming greater amounts of fish and long-chain omega-3 fatty acids did not have lower levels of hepatic steatosis (Table 2). The profound effects of EPA and DHA on lipid metabolism that have been reported in experimental animal models of obesity have been given as a major rationale for their importance in NAFLD (6). Moreover, this finding conflicts with the results of a double-blind, randomized trial of pediatric NAFLD patients in Italy, which noted dramatic reductions in ultrasound liver steatosis grade among subjects that were receiving DHA compared to germ oil (11). One possible explanation for this discrepancy may be the dose of long-chain omega-3 fatty acids required to induce an effect. The DHA supplements in the Italian study were 5-times greater than the median intake of long-chain omega-3 fatty acids that was reported by our subjects (11). Alternatively, the effect of omega-3 fatty acids on hepatic steatosis may have been confounded by genetic factors. Recently, a study of obese adolescents noted that hepatic fat fraction detected by magnetic resonance imaging was associated with the omega-6 to omega-3 fatty acid ratio of the diet, but only among participants that were homozygous for the G allele of rs738409 in the patatin-like phospholipase 3 gene, which codes for adiponutrin (33). In interpreting the results of this study, there are limitations that should be considered. The diet was assessed using a FFQ that was administered after subjects were diagnosed with NAFLD. There was differential inclusion of subjects by gender, and by level of steatosis and hepatocyte ballooning, which may have overestimated the association of including a more diseased population. The relatively low intake of sugar-sweetened beverages observed in the analysis of children in the NASH CRN database by Vos et al. (2012) suggests that respondents may have already modified their diets prior to filling out the FFQ, and/or were misreporting their intake to appear healthier (17). Although the NASH CRN Standards of Care for Pediatric Patients with Fatty Liver Disorders makes no reference to fish consumption, there are recommendations to limit fried food, which may have prompted subjects to reduce the amount of fried fish they were eating or reporting. At the same time, most adolescents consider fish to be healthy, so overall consumption of fish may have actually increased (28). The validity of the Block Brief FFQ has not been evaluated in adolescents, but a moderate amount of error can be inferred based on the subject's low calorie intakes (Table 1). However, for the size and purpose of this study, a FFQ may be superior to short-term dietary assessment instruments such as 24-hour dietary recalls or food diaries for episodically consumed foods such as fish. While data were collected on both fried and non-fried fish intake, the long-chain omega-3 fatty acid content varies considerably by species (34). Furthermore, no data were available on the use of supplements containing omega-3 fatty acids. Finally, care must be taken when attempting to extrapolate these findings to other pediatric NAFLD populations, as there appeared to be some selection bias.
The results of this study show that pediatric NAFLD patients consume less than the recommended amount of fish and omega-3 fatty acids (24–25). Promoting the intake of fish may help to reduce both portal and lobular inflammation, but further research is needed to test this hypothesis and to determine the necessary amount and best sources. Based on the current fish consumption, dietary supplements may be a good option for increasing long-chain omega-3 fatty acid intake to recommended levels (25). Advances in food biotechnology may offer opportunities for alternative sources of omega-3 fatty acids in the future, but this remains to be seen (35). Until additional clinical trials evaluating the effectiveness of long-chain omega-3 fatty acid supplements on pediatric NAFLD are conducted, patients should be encouraged to increase fish intake to meet general health recommendations.
Supplementary Material
Acknowledgments
Sources of Support: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD).
Several clinical centers use support from the National Center for Advancing Translational Sciences (NCATS) in conduct of NASH CRN Studies (grants UL1TR000439, UL1TR000077, UL1TR000436, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058, UL1TR000067, UL1TR000454).
CW received a grant from the National Oceanic and Atmospheric Administration (NOAA).
Footnotes
Conflicts of Interest: All other authors have no conflicts of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2009;48(1):1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: A multicenter clinicopathological study. Hepatology. 2009;50:1113–20. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohli R, Boyd T, Lake K, et al. Rapid progression of NASH in childhood. JPGN. 2009;50(4):453–6. doi: 10.1097/MPG.0b013e3181a9387b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U. S. A. 2009;106(36):15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut. 2009;58:1538–44. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro H, Tehilla M, Attal-Singer J, et al. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin Nutr. 2011;30(1):6–19. doi: 10.1016/j.clnu.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Masterton GS, Plevris JN, Hayes PC. Review article: Omega-3 fatty acids – a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31(7):679–92. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 8.Araya J, Rodrigo R, Pettinelli P, et al. Decreased liver fatty acid Δ-6 and Δ-5 desaturase activity in obese patients. Obesity. 2010;18:1460–3. doi: 10.1038/oby.2009.379. [DOI] [PubMed] [Google Scholar]
- 9.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106(6):635–43. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 10.Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 2012;56(4):944–51. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: Double-blind randomized controlled clinical trial. Arch Dis Child. 2011;96(4):350–3. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 12.Mager DR, Patterson C, So S, et al. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr. 2010;64:628–35. doi: 10.1038/ejcn.2010.35. [DOI] [PubMed] [Google Scholar]
- 13.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA. 2011;305(16):1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patton H, Lavine JE, Van Natta ML, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis (NASH) Gastroenterology. 2008;135(6):1961–71. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavine JE, Schwimmer JB, Molleston JP, et al. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials. 2010;31(1):62–70. doi: 10.1016/j.cct.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NutritionQuest Assessment and analysis services. [Accessed January 19, 2012];NutritionQuest Web site. http://nutritionquest.com/assessment/. Published 2009.
- 17.Vos MB, Colvin R, Belt P, et al. Correlation of vitamin E, uric acid, and diet composition with histological features of pediatric NAFLD. JPGN. 2012;54:90–6. doi: 10.1097/MPG.0b013e318229da1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ervin RB, Wright JD, Wang C, et al. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data. 2004;348:1–6. [PubMed] [Google Scholar]
- 19.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 20.Blasbalg TL, Hibbeln JR, Ramsden CE, et al. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Natta MV, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Percentile data files with LMS values. [Accessed August 25, 2012];Centers for Disease Control and Prevention Web site. http://www.cdc.gov/growthcharts/percentile_data_files.htm. Published August 4, 2009.
- 23.Keskin M, Kurtoglu S, Kendirci M, et al. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 24.Koletzko B, Uauy R, Palou A, et al. Dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in children – a workshop report. Br J Nutr. 2010;103(6):923–8. doi: 10.1017/S0007114509991851. [DOI] [PubMed] [Google Scholar]
- 25.Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):99–104. doi: 10.1016/j.plefa.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Tooze JA, Midthune D, Dodd KW, et al. A new method for estimating the usual intake of episodically-consumed foods with application to the distribution. J Am Diet Assoc. 2006;106(10):1575–87. doi: 10.1016/j.jada.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quiros-Tejeira RE, Rivera CA, Ziba TT, et al. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI ≥95th percentile. JPGN. 2007;44:228–36. doi: 10.1097/MPG.0b013e31802d4acc. [DOI] [PubMed] [Google Scholar]
- 28.Harel Z, Riggs S, Vaz R, et al. Omega-3 polyunsaturated fatty acids in adolescents: Knowledge and consumption. J Adolesc Health. 2001;28(1):10–5. doi: 10.1016/s1054-139x(00)00179-8. [DOI] [PubMed] [Google Scholar]
- 29.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD) – Clinicopathologic correlations from the Nonalcoholic Steatohepatitis Clinical Research Network. Hepatology. 2009;49:809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: Pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Periz A, Horrillo R, Ferre N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009;23(6):1946–57. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santoro N, Savoye M, Kim G, et al. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS ONE. 2012;7(5):e37827. doi: 10.1371/journal.pone.0037827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watters CA, Edmonds CM, Rosner LS, et al. A cost analysis of EPA and DHA in fish, supplements and foods. J Nutr Food Sci. 2012;2(8) [Google Scholar]
- 35.Lemke SL, Vicini JL, Su H, et al. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: Randomized, double-blind clinical study of efficacy and safety. Am J Clin Nutr. 2010;92:766–75. doi: 10.3945/ajcn.2009.29072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.