Abstract
Objective
Physical activity may reduce the risk of cognitive decline in the elderly, but its effects among the oldest old are not well known. Our study assessed the association between very late life physical activity and 5-year risk of mild cognitive impairment (MCI) or dementia and neuropsychological test performance among oldest old women.
Design
Prospective.
Setting
Three study sites.
Participants
1249 women (mean age 83.3 ± 2.8).
Measurements
Baseline physical activity was measured by self-reported blocks walked per week and analyzed by tertile. Five years later, surviving participants who were 85 and older (oldest old) completed neuropsychological testing and underwent adjudication of clinical cognitive status (normal, MCI, or dementia). All analyses were adjusted for baseline age, education, cognition, depression, body mass index, hypertension, smoking, and coronary artery disease.
Results
Comparedto women in the lowest tertile, women in the highest tertile were less likely to develop dementia compared to those in the lowest tertile (13.0% vs 23.2%, multivariate adjusted odds ratio, 0.54; 95% confidence interval, 0.36–0.82). However, risk of MCI was not associated with physical activity. Physical activity was also associated with higher performance 5 years later on tests of global cognition, category fluency, and executive function but not phonemic fluency, memory, or attention.
Conclusions
Higher level of very late-life physical activity was associated with a lower risk of subsequent dementia in oldest old women. These findings support future studies for late-life physical activity interventions for the prevention of dementia among oldest old women.
Keywords: Cognitive aging, physical activity, cohort studies, dementia, oldest old
OBJECTIVE
Several studies have reported that greater physical activity during the life course, starting as early as the teenage years through late life, is associated with a reduced risk of cognitive decline and cognitive impairment in the elderly.1–6 Several randomized controlled trials support these findings, although others have been negative.6 It is not well known, though, whether very late-life physical activity continues to be protective in those 85 and over, a group often known as the oldest old. The oldest old is the fastest growing segment of the elderly population7 and may be distinct because of survival effects and the etiology of dementia in this age group, as suggested by the 90+ Study which found that cardiovascular risk factors were not as strongly associated with cognitive impairment as seen in the young old.8 Studies investigating the oldest old have estimated the prevalence of mild cognitive impairment (MCI) to be at least 20–30%8,9 and dementia to be 40%.9–14 Given the rising health care costs and caregiving needs due to cognitive impairment,15 interventions that may reduce the risk of cognitive decline in the oldest old population would be valuable. In this study, we tested our hypothesis that higher levels of very late-life physical activity would be associated with lower rates of cognitive impairment, as measured by the presence of MCI or dementia 5 years later, among women 85 and over. We also hypothesized that very late-life physical activity would be associated with better cognitive test performance 5 years later.
METHODS
Sample
Participants in this study were recruited from the Study of Osteoporotic Fractures (SOF). SOF is a prospective cohort study of 9704 community-dwelling, mostly Caucasian women from four sites (Baltimore, Md., Minneapolis, MN; Monongahela Valley, PA; and Portland, OR).16 Baseline eligibility criteria included participants being able to ambulate without assistance from another person and no bilateral hip replacements. Since the initial study visit, follow-up visits were conducted approximately every 2 to 4 years. All women provided written informed consent; IRB approval for the use of human subjects at each study site and at the coordinating center, the University of California at San Francisco was obtained.
We studied women from the original SOF cohort (mean age 83.3 ± 2.8) who participated in Women Cognitive Impairment Study of Exceptional Aging (WISE), an ancillary study looking at clinical cognitive status.9 SOF WISE was conducted at all the sites except at the Baltimore site at the Year 20 visit, and as part of this study, women completed a neuropsychological battery and underwent adjudication of clinical cognitive status. Of the 1299 women who were 85 years or older at follow-up and participated in SOF WISE, 50 were excluded because at our study baseline (Year 16 visit), they did not report their physical activity status (n = 41) or reported being diagnosed with dementia including Alzheimer’s disease (AD) or taking medications for AD (n = 9). The remaining 1249 non-demented women made up our cohort. For our study, Year 16 was defined as our baseline, and Year 20 was our follow-up, with a mean interval of 5 years between these two visits.
We collected women’s demographic information upon enrollment including age, educational level, ethnicity and marital status. At our baseline and follow-up visits, participants also reported their medical history, including whether a health care provider had informed them that they had various medical conditions such as hypertension, angina, myocardial infarction, diabetes, stroke, and dementia. Coronary artery disease (CAD) was defined as the presence of angina or myocardial infarction. Participants also completed questionnaires about their lifestyle and physical activity. Medication use over the past 30 days was confirmed by examination of pill bottles (medication and dosage) which participants brought in. Depressive symptoms were measured using the Geriatric Depression Scale 15-item (GDS-15). We defined depression as being present if participants had GDS-15 scores of 6 or over (an established cutoff for significant depressive symptoms).17 Informants also completed the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), which queries about changes in participants’ cognition and function.18 Body mass index (BMI) was calculated based on measured height and weight.
Physical Activity Measurement
Women were also asked, on average, about the number of city blocks or equivalent (10 blocks ≈ 1 mile) they typically walked each day for exercise or as part of their normal routine.
Cognitive Assessments
At baseline, a modified Mini-Mental State Examination (mMMSE), a test of global cognition which is shortened version (score 0 to 26) of the Mini-Mental State Examination (MMSE) and Trails B, a test of executive function and psychomotor speed, were administered. At the 5-year follow-up, a neuropsychological testing battery was administered, including the Modified Mini-Mental State Examination (3MS),19 a measure of global cognition (0–100 points); digit span backwards,20 a test of working memory or attention (0–14 points); the California Verbal Learning Test II Short Form (CVLT II),21 a test of verbal learning and memory, including a 10 minute delayed recall portion (0–9 points); verbal fluency tests in which participants had to name as many different words starting with the letter “f” within a minute (phonemic fluency) and as many different vegetables within a minute (category fluency) as possible;22 and Trails B.
Clinical cognitive adjudication at follow-up
Clinical cognitive adjudication consisted of two phases: 1) a screening process and 2) a panel evaluation of those women who screened positive. Women screened positive if they met of any of the following criteria: 1) a score less than 88 on the 3MS;23 2) a score less than 4 on the CVLT-II delayed recall;21 3) a score of 3.6 or greater on the IQCODE;18 4) a diagnosis of dementia as per the medical history; and 5) residence in a nursing home. Those who did not meet any of these criteria were considered to be cognitively normal. In the second phase, women who screened positive underwent adjudication by one randomly selected member of a panel of experts (neuropsychologists, geropsychologist, and neurologists) to determine whether the women were cognitively normal or met criteria for MCI or dementia. Decisions about cognitive status were based on the detailed neuropsychological tests, GDS-15 scores, cognitive test scores from current and previous visits, functional status, medications, and medical history. The diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria.24 The diagnosis of MCI was based on a modified version of the Petersen criteria,25 which is defined as cognitive impairment that does not meet the criteria for dementia and generally intact functioning. Cognitive impairment was defined as performance on neuropsychological testing that was 1.5 standard deviations or more below age-appropriate reference values. 25 Functional status was determined by the IQCODE. If women did not have an informant, functional status was based on self-reported performance on ADLs (activities of daily living) and instrumental ADLs. Those who did not meet the criteria for MCI or dementia were classified as cognitively normal.
Statistical analysis
Since the distribution of the number of blocks walked per week at baseline was significantly right-skewed, women were divided by tertile into low, moderate, and high activity according to the number of blocks walked per week at baseline. The number of subjects for each activity level varied slightly because the number of blocks reported was an integer, which resulted in some clustering around certain values. Differences by each activity level for baseline characteristics were compared using analysis of variance (ANOVA) (continuous variables) and χ2 tests (categorical variables). Although women were diagnosed with subtypes of MCI and dementia, subtypes for each cognitive diagnosis were combined because of insufficient power for subgroup analyses. To evaluate the association between baseline physical activity and the 5-year risk of cognitive impairment (MCI or dementia), we used logistic regression analyses. The reference group was women with normal cognition. Results were then adjusted for baseline age, education, and mMMSE; and further adjusted for baseline medical conditions with a p-value < 0.10 (BMI, smoking, hypertension, depression, and CAD). We also compared the effects of physical activity on neuropsychological performance at 5-year follow-up using generalized linear modeling (GLM). All GLM analyses, unless indicated otherwise, were multivariate (MV) adjusted for age, education, mMMSE, BMI, smoking, hypertension, depression, and CAD. Decline in performance for mMMSE and Trails B was measured by calculating the difference between performance at baseline and 5 year follow-up. To ensure our findings were not overly influenced by participants who led a highly active lifestyle, we performed a sensitivity analysis with the removal of those subjects who walked ≥3 miles per day (the top 5% of the cohort). For all analyses, P < 0.05 was considered to be statistical significant. SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina) was used for all analyses.
RESULTS
The average number ± standard deviation (SD) (range) of blocks walked per week at baseline for the 1249 women was 64.0 ± 72.6 (0–504) and for the low, moderate and high activity level, respectively, was 7.1 ± 5.9 (0–20); 36.2 ± 12.5 (21–69), and 138.9 ± 73.4 (70–504). About 10.9% of participants reported that they did not walk at all for recreation or as part of their daily routine, whereas the top 5% led a highly active lifestyle, walking 3 or more miles per day. Women with high activity level were younger and had higher education levels, fewer depressive symptoms, lower BMI, higher performance on baseline mMMSE and Trails B, and lower rates of hypertension, CAD, and smoking (Table 1).
Table 1.
Baseline Characteristics of 1249* Women According to Activity Level
| Mean ± SD or % | Low (n = 388) | Moderate (n = 413) | High (n = 448) | Test Statistic | P-value |
|---|---|---|---|---|---|
| Age (y) | 83.8 ± 3.2 | 83.2 ± 2.7 | 82.9 ± 2.5 | F = 11.83 | <0.001a,b |
| Education (y) | 12.7 ± 2.4 | 12.8 ± 2.5 | 13.1 ± 2.6 | F = 3.84 | 0.022b |
| Currently married | 27.8 | 31.2 | 33.0 | χ2 = 2.69 | 0.26 |
| GDS-15 score | 2.7 ± 2.5 | 2.0 ± 2.3 | 1.5 ± 2.0 | F = 30.06 | <0.001a,b,c |
| Medical History | |||||
| Diabetes mellitus | 9.3 | 9.4 | 7.6 | χ2 = 1.14 | 0.57 |
| Hypertension | 62.1 | 62.2 | 49.1 | χ2 = 20.07 | <0.001b,c |
| History of stroke | 12.6 | 9.0 | 9.8 | χ2 = 3.15 | 0.21 |
| Coronary artery disease | 22.7 | 18.6 | 14.5 | χ2 = 9.27 | 0.010b |
| BMI | 27.4 ± 4.5 | 27.1 ± 4.2 | 26.2 ± 4.0 | F = 8.97 | <0.001b,c |
| Currently smoking | 3.4 | 1.2 | 0.5 | χ2 = 11.73 | 0.003b |
| mMMSE score | 24.8 ± 1.4 | 24.9 ± 1.4 | 25.0 ± 1.3 | F = 3.57 | 0.029b |
| Trails B score | 140.5 ± 57.6 | 130.0 ± 52.9 | 128.0 ± 51.8 | F = 5.44 | 0.004a,b |
n =1243–1249 except for BMI (n = 1168), mMMSE (n = 1157), and Trails B (n = 1125).
Activity level was defined as tertiles (low, moderate, and high) based on the number of blocks the subjects walked each week at their baseline visit.
P-values calculated using ANOVA (df = 2) and χ2 tests (df = 2). For P < 0.05 for ANOVA and χ2 tests, post-hoc multiple group comparisons using Tukey Honestly Significant Difference indicated a significant difference between alow and moderate activity level, blow and high activity level, and cmoderate and high activity level.
The adjudicated cognitive diagnoses at follow-up for the 1249 women were: 750 women (60.0%) had normal cognition, 287 women (23.0%) had MCI, and 212 women (17.0%) had dementia. For women with low activity level, 212 subjects had a diagnosis of normal cognition, 86 had MCI, and 90 had dementia. For women with moderate activity level, 250 subjects had a diagnosis of normal cognition, 99 had MCI, and 64 had dementia. For women with high activity level, 288 subjects had a diagnosis of normal cognition, 102 had MCI, and 58 had dementia (overall χ2[4, N = 1249] = 17.2, p = 0.002). Compared to those with low activity level, women with high activity level were 53% less likely to have a diagnosis of dementia: 13.0% vs 23.2%, odds ratio (OR) = 0.47; 95% confidence interval (CI), 0.33–0.69, Wald χ2[1, N = 962] = 15.2, p < 0.001; adjustment for age, education and baseline cognitive scores were similar with an adjusted OR (AOR) = 0.57; 95% CI, 0.38–0.85, Wald χ2[1, N = 962] = 7.5, p = 0.006. Compared to women with low activity level, women with moderate activity level also had less diagnoses of dementia: 15.5% vs 23.2%, OR= 0.60; 95% CI, 0.42–0.87, Wald χ2[1, N = 962] = 7.2, p = 0.007, and AOR = 0.65; 95% CI, 0.44–0.97, Wald χ2[1, N = 962] = 4.6, p = 0.032. Further adjustment for baseline comorbidities did not appreciably change the results (Figure). Moreover, for every 10 blocks walked per day (approximately a mile), women had a 25% (multivariate OR = 0.75; 95% CI 0.62–0.91, Wald χ2[1, N = 962] = 8.8, p = 0.003) lower odds of a diagnosis of dementia. However, there was no difference by activity level for risk of MCI (22.8% for high, 24.0% for moderate, and 22.2 % for low activity level, Wald χ2[2, N = 1037] = 0.26, p = 0.88).
Figure 1.
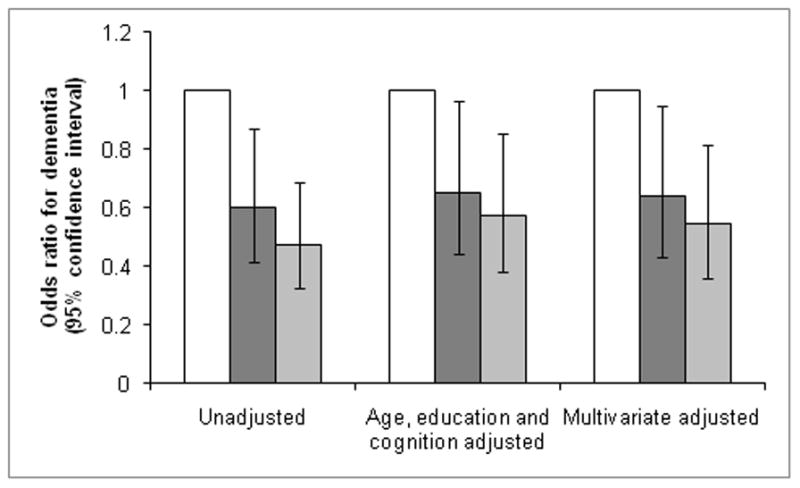
Association between risk of dementia at 5-year follow-up and baseline physical activity.
□ Lowest tertile
 Middle tertile
Middle tertile
 Highest tertile
Highest tertile
Vertical lines represent 95% confidence intervals. Multivariate (MV) logistic regression adjusted for baseline age, educational level, cognition, depressive symptoms, BMI, hypertension, smoking, and CAD.
Generalized linear modeling (GLM) regression was used to analyze the association between baseline physical activity and decline on MMSE and Trails B from baseline to 5-year follow-up and neuropsychological performance at 5-year follow-up. Baseline physical activity was positively associated with slower decline on the mMMSE (1.4 points for the high tertile and 2.2 points for the low tertile, t = 3.20, df = 1153, p = 0.001) and Trails B (31.1 for the high tertile and 41.5 seconds for the low tertile, t = −2.28, df = 935, p = 0.023) over the 5-year follow-up period. At the 5-year follow-up, baseline level of physical activity was positively associated with cognitive scores on the 3MS, CVLT delayed recall, phonemic fluency, category fluency, and Trails B but not on digit span backwards (Table 2). After adjustment for baseline age, education, cognition, depression, and medical conditions, differences in the rates of decline and neuropsychological performance at 5-year follow-up were unchanged, except for phonemic fluency (t = 1.60, df = 1196, p = 0.11) and CVLT delayed recall (t = 1.43, df = 1203, p = 0.15) which became insignificant (Table 2).
Table 2.
Neuropsychological Test Performance at 5-Year Follow-up by Baseline Activity Level
| Test, Mean ± SD | Low | Moderate | High | Test Statistica | Unadjusted P-valueb | Adjusted P-valuec |
|---|---|---|---|---|---|---|
| 3MS (points) | 85.4 ± 12.0 | 87.7 ± 9.7 | 88.9 ± 9.2 | 4.96 | <0.001 | <0.001 |
| CVLT delayed recall (words) | 4.8 ± 2.8 | 5.1 ± 2.6 | 5.3 ± 2.7 | 2.54 | 0.011 | 0.15 |
| Digits backward (number correct) | 5.4 ± 2.0 | 5.6 ± 2.0 | 5.7 ± 2.0 | 1.65 | 0.10 | 0.80 |
| Phonemic fluency (number correct) | 10.2 ± 4.2 | 10.6 ± 3.9 | 11.0 ± 4.3 | 2.79 | 0.005 | 0.11 |
| Category fluency (number correct) | 9.9 ± 3.6 | 10.7 ± 3.4 | 10.8 ± 3.6 | 3.43 | <0.001 | 0.009 |
| Trails B time (secs) | 175.9 ± 71.0 | 168.6 ± 71.6 | 155.4 ± 66.1 | −3.82 | <0.001 | 0.002 |
n = 1206–1247 except for Trails B at 5-year follow-up (n = 997).
Test statistics are from unadjusted generalized linear modeling (GLM) regression using a t-test with df = 1204 to 1245 except for Trails B, df = 995.
p-values are from unadjusted generalized linear modeling (GLM) regression using a t-test with df = 1204 to 1245 except for Trails B, df = 995.
p-values are from multivariate (MV) GLM regression adjusted for baseline age, educational level, cognition, depressive symptoms, BMI, hypertension, smoking, and CAD using a t-test with df = 1196 to 1237 except for Trails B, df = 987.
DISCUSSION
There are very few studies looking at the effects of very late-life physical activity on cognitive status in the oldest old. In this cohort of 1249 oldest old women, those with greater baseline physical activity (measured as blocks walked per week) were less likely to be diagnosed with dementia and had higher performance on most neuropsychological tests at 5-year follow-up. These differences remained even after adjustment for baseline age, education, cognition, depression, and medical conditions.
Several studies have shown that physical activity in various life stages can have an effect on late life cognition.1–6 Our results suggest that the benefits of physical activity may also extend to the oldest old, and that walking for either exercise or as part of a daily routine may provide cognitive benefits. Physical activity has also been shown to impact a wide range of cognitive function domains including processing speed, attention, executive functioning and delayed recall;6,26,27 these results, however, have not been consistently demonstrated, possibly due to variation in measurements of physical activity and cognition.6 The domain-specific effects of physical activity on cognition are not fully understood, but one functional MRI study in older adults found that physical activity resulted in better performance on executive functioning tasks by attenuating gray matter loss of the prefrontal cortex.28 In our study, physical activity was associated with higher cognitive performance, including on measures of global cognition and executive functioning, but not for attention and delayed verbal memory. While the clinical significance of these differences at follow-up testing among the activity levels is not known, these differences are consistent with our other finding that physical activity is associated with a lower risk of dementia.
Possible mechanisms by which physical activity may affect cognition in the young old include the reduction of beta amyloid deposition especially among APOE ε4 carriers,29 the lowering of cumulative vascular risk as well as individual cardiovascular risk factors,30 and modulation of cognitive plasticity via factors such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF-1).31 The applicability of these findings to the oldest old requires further study as the neuropathology, the role of APOE ε4, and plasticity in the cognitively impaired oldest old may be different from that of the young old. Another possible mechanism which may explain how physical activity affects cognition in the oldest old is that long-term depression may lead to decreased physical activity, and often heralds cognitive impairment. This mechanism is consistent with a number of studies which have shown depression is a risk factor for cognitive impairment in older adults, including in the oldest old.32 Although we controlled for baseline depressive symptoms, we still cannot exclude the possibility of the long-term effects of depressive symptoms on physical activity and, consequently, cognitive impairment. Furthermore, when depression is recognized and treated, cognitive impairment may still remain. Early cognitive impairment may lead to increased physical hesitancy and reduced physical activity, whereas structured physical activity (such as physical therapy) may benefit both depression and cognitive impairment.
About 21% (over 1.1 million) of AD cases in the US alone can be attributed to physical inactivity in the elderly.30,33 Age is a significant risk for decreased physical activity in the elderly. There is a noticeable drop off in leisure-time physical activity from 65–74 to those over 75 and over, and in the U.S.; only about 17% of adults 75 and older participated in regular leisure time activity, 40% engaged in some leisure activity, and 60% did none.33 In contrast, 67% of SOF-WISE participants had a moderately active or active lifestyle, and only 11% were completely sedentary. The highest tertile generally walked about one to two miles per day (the equivalent of walking briskly about 25–50 minutes per day), which is consistent with the CDC recommendations for healthy older adults to engage in moderate vigorous exercise for an average of at least 20 minutes per day, and if possible, 40 or more minutes per day for additional health benefits.34 Even after exclusion of those women who walked 3 or more miles a day (more than an hour of brisk walking per day), the most physically active group still had lower rates of dementia, suggesting that exercising significantly beyond these guidelines was not necessary to observe lower rates of dementia. Furthermore, the middle tertile walked an average of half a mile per day (the equivalent of strolling about 30 minutes per day or walking briskly about 15 minutes per day). So although the SOF-WISE cohort was more active than the national average, even activity levels as low as 15 minutes per day was sufficient to observe a lower risk of dementia. These results were similar to the Leisure World cohort study, which showed that in women 80 and over, mortality rates were lower for those who engaged in as little as 15 minutes per day of moderately vigorous physical activity with a maximum benefit reached at 45 minutes per day.35 In light of the public health consequences of physical inactivity and evidence of cognitive and functional benefits of physical activity, a number of ongoing randomized clinical trials, such as the Lifestyle Interventions and Independence for Elders Study (LIFE-P) trial, are testing whether moderate-intensity physical activity programs in sedentary elderly adults can affect functional outcomes, including cognition.36
Our finding about physical activity being associated with a lower risk of dementia at 5-year follow-up in the oldest old is consistent with earlier work in which physical activity was associated with lower risk of dementia,3–5 although some studies did not find such an association.6 The data for physical activity and its association with MCI are even more mixed. One study found that late-life moderate exercise (but not light or vigorous exercise) was associated with a lower risk of MCI,37 whereas two other studies did not find an association between physical activity and MCI.38,39 Physical activity was not associated with risk of MCI in our study of the oldest old. Possible explanations for this variability include physical activity targeting mechanisms that are specific to dementia, or the dilution of possibly positive effects on certain subtypes since we looked at the heterogeneous MCI sample as one group.
The strengths of this study included its large sample size, the longitudinal design, and clinically adjudicated cognitive outcomes. There were some limitations to our study. First, we relied on participants’ self-report of their physical activity. Self-report questionnaires have not been shown to accurately capture very low intensity activities such as household chores and fidgeting; measurements including these very low intensity activities may more accurately reflect total energy expenditure level in the oldest old as compared to measurements consisting only of low intensity activities such as walking.40 Nevertheless, BMI was inversely correlated with physical activity, supporting the reliability of this self-reported measurement in our study. Second, while we excluded participants with a dementia at baseline, it is possible that some women had subtle cognitive symptoms at baseline. Despite this, our data suggest that physical activity may be associated with a slower cognitive decline, at least in the areas of general cognition and executive functioning. Third, as with other longitudinal studies, selection bias may have arisen because more cognitively and physically impaired subjects may have dropped out or died, and the women who participated in SOF had a higher level of physical activity compared to the national average. These biases may have influenced our results. Fourth, since the participants were mostly white women, the relevance of these results to men and other ethnicities require further investigation, since men and Caucasians tend to have higher levels of physical activity.33 Finally, since the sample was selected on their participation in SOF-WISE and walking was an observed (rather than an independently manipulated) predictor, our findings are correlational.
CONCLUSIONS
Future research is needed to determine whether interventions increasing physical activity in the oldest old can result in amelioration of cognitive decline. Nevertheless, given the relative benefits and minimal risks of physical activity, clinicians should consider recommending that their healthy older patients engage in activities appropriate for their age and functional capabilities such as 30 minutes walk in the mall, water aerobics, Tai Chai, or physical therapy.34
Acknowledgments
The authors thank all study participants and the SOF investigators and staff. Data collection and sharing for SOF and SOF-WISE are supported by grants AG05407, AR35582, AG05394, AR3354, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, 2R01 AG027574-22A1, and 5R01AG026720-04. The study is coordinated by the University of California, San Francisco. SOF data are disseminated by University of California, San Francisco. This study was also supported in part by a VA Neurosciences Research fellowship (SW), Mount Sinai Alzheimer’s Disease Research Center award P50AG005138-27 from the National Institute of Aging (NIA) (XL, MS), UL1RR029887 from the National Center for Research Resources’ Clinical and Translational Science Award (MS), grant K24 AG 031155 from NIA and an Independent Investigator Award from the Alzheimer’s Association (KY). SW also received the APA/Lilly Resident Research Award for this work. MS serves on a scientific advisory board for Medivation, Inc., and as a consultant for Bayer Schering Pharma, Bristol-Meyers Squibb, Elan Corporation, Genentech, Inc., Medivation, Inc., Medpace Inc., Pfizer Inc, Janssen, Takeda Pharmaceutical Company Ltd, and United Biosource Corporation and receives research support from the Jeffrey Mann Fund. KY serves or has served on data safety monitoring boards for Pfizer Inc., Medivation, Inc., Takeda, and the NIH (NIMH and NIA trials) and has received board membership fees and travel or accommodation expenses from the Beeson Scientific Advisory Committee, consultancy fees from Novartis, and research support from the NIH (NIA, NIDDK, NIMH), the Department of Defense, American Health Assistance Foundation, Anonymous Foundation, and the Alzheimer Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sophia Wang, Department of Psychiatry, University of California, San Francisco, CA; San Francisco VA Medical Center, San Francisco, CA
Xiaodong Luo, Department of Psychiatry, Mount Sinai School of Medicine, New York, New York; Veterans Affairs Medical Center, Bronx, New York
Deborah Barnes, Departments of Psychiatry and Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA; San Francisco VA Medical Center, San Francisco, CA
Mary Sano, Department of Psychiatry, Mount Sinai School of Medicine, New York, New York; Veterans Affairs Medical Center, Bronx, New York
Kristine Yaffe, Departments of Psychiatry, Neurology, and Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA; San Francisco VA Medical Center, San Francisco, CA
References
- 1.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 2.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58:1322–1326. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 4.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 6.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Hobbes FB. Population profile of the United States: the elderly population. U.S. Census Bureau; [Accessed February 24, 2012]. Available at: http://www.census.gov/population/www/pop-profile/elderpop.html. [Google Scholar]
- 8.Peltz CB, Corrada MM, Berlau DJ, Kawas CH. Cognitive impairment in nondemented oldest-old: Prevalence and relationship to cardiovascular risk factors. Alzheimers Dement. doi: 10.1016/j.jalz.2011.02.008. Epub 2011 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old: results from the Canadian Study of Health and Aging. Neurology. 1994;44:1593–1600. doi: 10.1212/wnl.44.9.1593. [DOI] [PubMed] [Google Scholar]
- 11.von Strauss E, Viitanen M, De Ronchi D, Winblad B, Fratiglioni L. Aging and the occurrence of dementia: findings from a population-based cohort with a large sample of nonagenarians. Arch Neurol. 1999;56:587–592. doi: 10.1001/archneur.56.5.587. [DOI] [PubMed] [Google Scholar]
- 12.Riedel-Heller SG, Busse A, Aurich C, Matschinger H, Angermeyer MC. Prevalence of dementia according to DSM-III-R and ICD-10: results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Part 1. Br J Psychiatry. 2001;179:250–254. doi: 10.1192/bjp.179.3.250. [DOI] [PubMed] [Google Scholar]
- 13.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 14.Heeren TJ, Lagaay AM, Hijmans W, Rooymans HG. Prevalence of dementia in the ‘oldest old’ of a Dutch community. J Am Geriatr Soc. 1991;39:755–759. doi: 10.1111/j.1532-5415.1991.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 15.Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Chicago: Alzheimer’s Association; 2011. [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, Nevitt MC, Browner WS, et al. Study of Osteoporotic Fractures Research Group. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 17.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients: a comparison for the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157(4):449–454. [PubMed] [Google Scholar]
- 18.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corp; 1997. [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Second Edition (CVLT-II) San Antonio. TX: Psychological Corp; 2000. [Google Scholar]
- 22.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 23.Espeland MA, Rapp SR, Robertson J, et al. Women’s Health Initiative Memory Study. Benchmarks for designing two-stage studies using Modified Mini-Mental State Examinations: experience from the Women’s Health Initiative Memory Study. Clin Trials. 2006;3:99–106. doi: 10.1191/1740774506cn140oa. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 25.Petersen RC, Doody R, Kurz E, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 26.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 27.Eggermont LH, Milberg WP, Lipsitz LA, Scherder EJ, Leveille SG. Physical activity and executive function in aging: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57:1750–1756. doi: 10.1111/j.1532-5415.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein AM, Voss MW, Prakash RS, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26:811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Head D, Bugg JM, Goate AM, et al. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol. doi: 10.1001/archneurol.2011.845. Epub 2012 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30(4):493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spira AP, Rebok GW, Stone KL, et al. Depressive symptoms in oldest-old women: risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry. 2012 Dec;20:1006–1015. doi: 10.1097/JGP.0b013e318235b611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. [Accessed March 5, 2012];Health Behavior of Adults: United States. 2005–2007 http://www.cdc.gov/nchs/data/series/sr_10/sr10_245.pdf.
- 34.Centers for Disease Prevention and Control. [Accessed March 5, 2012]; http://www.cdc.gov/physicalactivity/everyone/guidelines/olderadults.html.
- 35.Paganini-Hill A, Kawas CH, Corrada MM. Activities and mortality in the elderly: the Leisure World cohort study. J Gerontol A Biol Sci Med Sci. 2011;66:559–567. doi: 10.1093/gerona/glq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middleton L, Kirkland S, Rockwood K. Prevention of CIND by physical activity: different impact on VCI-ND compared with MCI. J Neurol Sci. 2008;269:80–84. doi: 10.1016/j.jns.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 39.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middleton LE, Manini TM, Simonsick EM, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]


