Abstract
The A118G single nucleotide polymorphism (SNP) of the human µ fopioid receptor (MOPR) gene (OPRM1) was associated with heightened dopamine release by alcohol intake, better treatment outcome for nicotine and alcohol addiction and reduced analgesic responses to morphine. A mouse model that possesses the equivalent substitution (A112G) in the oprm1 gene was generated to delineate the mechanisms of the impact of the SNP. Mice homozygous for the G allele (G/G) displayed lower morphine-induced antinociception than A/A mice, similar to humans. In this study, we examined whether A112G SNP affected MOPR-mediated G protein activation in the mouse model. We compared A/A and G/G mice in the MOPR selective agonist DAMGO-stimulated [35S]GTPγS binding in brain regions by autoradiography. When the data of males and females were combined, G/G mice exhibited lower DAMGO-stimulated [35S]GTPγS binding in the VTA than A/A mice, in accord with previously reported reduced morphine-induced hyperactivity and locomotor sensitization in G/G mice. In the NAc core, female G/G mice displayed lower DAMGO-stimulated [35S]GTPγS binding than female A/A mice, which is consistent with previously reported deficiency in morphine-induced conditioned place preference in female G/G mice. In G/G mice, males showed higher DAMGO-stimulated [35S]GTPγS binding than females in the cingulate cortex, CPu, NAc core, thalamus and amygdala. Thus A112G SNP affects DAMGO-stimulated [35S]GTPγS binding in region- and sex-specific manners.
Keywords: A118G, mu opioid receptor, single nucleotide polymorphism, [35S]GTPγS binding autoradiography
Introduction
The A118G single nucleotide polymorphism (SNP) of the human µ opioid receptor gene (OPRM1) is the most common SNP in the coding region of the MOPR (Bergen, Kokoszka, Peterson et al., 1997; Bond, LaForge, Tian et al., 1998). This SNP results in a change of amino acid at position 40 from asparagine to aspartate (N40D) in the N-terminal domain, which removes one of five potential N-linked glycosylation sites of the receptor (Bergen et al., 1997; Bond et al., 1998). Recently we reported that this SNP reduced the N-glycosylation and stability of the MOPR protein (Huang, Chen, Mague et al., 2012). The A118G allele frequency varies among ethnic groups: 1–3% in African Americans, 10–14% in both Caucasians and Hispanics, 35–49% in Asians and 8–21% in other populations [reviewed in (Kreek, Bart, Lilly et al., 2005; Mague and Blendy, 2010)]. Clinical studies revealed that among alcohol dependent patients treated with naltrexone, those with one or two copies of the G118 allele had better outcome (Oslin, Berrettini, Kranzler et al., 2003; Kim, Kim, Choi et al., 2009). Among individuals using transdermal nicotine for smoke cessation, those of the A/G and G/G genotype had better responses (Lerman, Wileyto, Patterson et al., 2004). Ramchandani, Umhau, Pavon et al. (2011) reported that alcohol intake induced dopamine release in carriers of the G118 allele, but not in A118 homozygotes. In addition, subjects with G118 allele needed higher morphine doses to attain adequate pain control for acute postoperative pain compared to those homozygous for the A118 allele subjects (Chou, Wang, Liu et al., 2006; Chou, Yang, Lu et al., 2006; Sia, Lim, Lim et al., 2008; Hayashida, Nagashima, Satoh et al., 2008; Campa, Gioia, Tomei et al., 2008). Studies on the role of G118 allele in alcohol or drug dependence have reported positive, negative, or no associations [for reviews, see (Kreek et al., 2005; Mague and Blendy, 2010)].
Mague, Isiegas, Huang et al. (2009) generated a mutant mouse line that possessed the mouse equivalent (A112G, N38D) of the human SNP for studying the mechanisms underlying the changes associated with OPRM1 A118G SNP in humans. The mice homozygous for G112 allele (G/G mice) displayed reduced antinociceptive responses to morphine than those homologous for A112 allele (A/A mice) (Mague et al., 2009), similar to the results in humans. In addition, G/G mice showed reduced morphine-induced hyperactivity and locomotor sensitization (Mague et al., 2009). Moreover, female, not male, G/G mice failed to show a conditioned place preference to morphine-paired environments (Mague et al., 2009). Recently, Ramchandani et al. (2011) have also generated a mouse model of the OPRM1 A118G SNP by replacing the mouse exon 1 with the human exon 1 carrying the A118 or G118 allele. They found that homozygous G118 mice showed a 4-fold greater peak dopamine response to an alcohol challenge as homozygous A118 mice. Furthermore, Mahmoud, Thorsell, Sommer et al. (2011) reported that the potency and efficacy of morphine in modulating Ca2+ channels were reduced in sensory neurons of mice expressing the 118 GG gene compared with those expressing the 118AA version.
We have demonstrated that mice with oprm1 A112G displayed lower MOPR expression in some, but not all, brain regions (Wang, Huang, Ung et al., 2012). The effects of A112G on MOPR protein expression have sex differences in some brain regions (Wang et al., 2012). In the mouse brains A112G reduced N-glycosylation of the MOPR and in cultured cells A118G in the human MOPR decreased N-glycosylation and protein stability (Huang et al., 2012). The reduction in MOPR expression in brain regions by the A112G (Wang et al., 2012) is likely to be involved in attenuated morphine-induced antinociception, hyperactivity and locomotor sensitization (Mague et al., 2009). The observed behavioral changes in A112G mice may also be due to alterations in MOPR-G protein coupling, in addition to changes in MOPR level. In the present study, we investigated whether A112G SNP affected MOPR-mediated G protein activation among brain regions by quantitative in vitro autoradiography of DAMGO-stimulated [35S]GTPγS binding.
Materials and methods
Animals
A112G-MOPR knock-in mice were generated on C57BL/6 genetic background in Dr. Blendy’s laboratory. The animals (8–15 weeks, 18–30 g) were housed in the University of Pennsylvania animal facility and maintained on a 12 h light/dark cycle with ad libitum access to food and water. Animals were used in accordance with the methods approved by University of Pennsylvania Animal Care and Use Committee. Mice homozygous for the A112 or G112 allele are used for the study.
Materials
DAMGO, GDP and GTPγS were purchased from Sigma Chemical Co (St. Louis, MO). [35S]GTPγS (1250 Ci/mmole) and phosphor screens were obtained from Perkin Elmer (Boston, MA). [14C]microscales were purchased from GE healthcare, formerly Amersham Biosciences (Arlington Heights, IL). All other reagent-grade chemicals were obtained from Sigma Chemical Co (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
In vitro autoradiography of DAMGO-stimulated [35S]GTPγS binding
The experiments were conducted according to our published procedures, which were adapted from those of (Sim and Childers, 1997; Sim-Selley, Selley, Vogt et al., 2000). Coronal sections (20 µm) of regions of interest were cut on a cryostat (Leica CM3050 S) maintained at −20°C, thaw-mounted onto gelatin-subbed slides, and stored at −80°C until processed. Sections were incubated with 2 mM GDP in [35S]GTPγS binding buffer (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4) for 15 min at 25°C, and then incubated for 2 hr at 25°C in [35S]GTPγS binding buffer with 0.04 nM [35S]GTPγS and 2 mM GDP with or without the µ agonist DAMGO (10 µM). After incubation, sections were rinsed twice (2 minutes each) in cold 50 mM Tris-HCl buffer, pH 7.4, once (30 seconds) in deionized H2O, and then dried thoroughly and exposed to phosphor screens for 48 hr in cassettes along with [14C]microscales for densitometric analysis.
Autoradiography data analysis
Images captured on phosphor screens were visualized using a Cyclone Storage Phosphor Scanner (Packard Bioscience) and data were analyzed using the OptiQuant program associated with scanner. Images were quantified by densitometric analysis with [14C] standards. A brain paste standard assay was performed to determine the correction factor necessary to calculate nCi/g for [35S] from nCi/g for [14C], as previously described (Sim and Childers, 1997). Net agonist-stimulated [35S]GTPγS binding was calculated by subtracting basal binding (obtained in the absence of agonist) from agonist-stimulated binding. Data were reported as mean values ± S.E.M.
Statistical analysis
Two-way ANOVA was used to determine statistical significant differences between groups, with sex and genetic factor as main factors. For comparison of two groups within one factor, two-tailed Student’s t test was performed. *p<0.05, **p<0.01 was the level of significance in all statistical analyses.
Results
DAMGO-stimulated [35S]GTPγS binding in A/A and G/G mouse brains
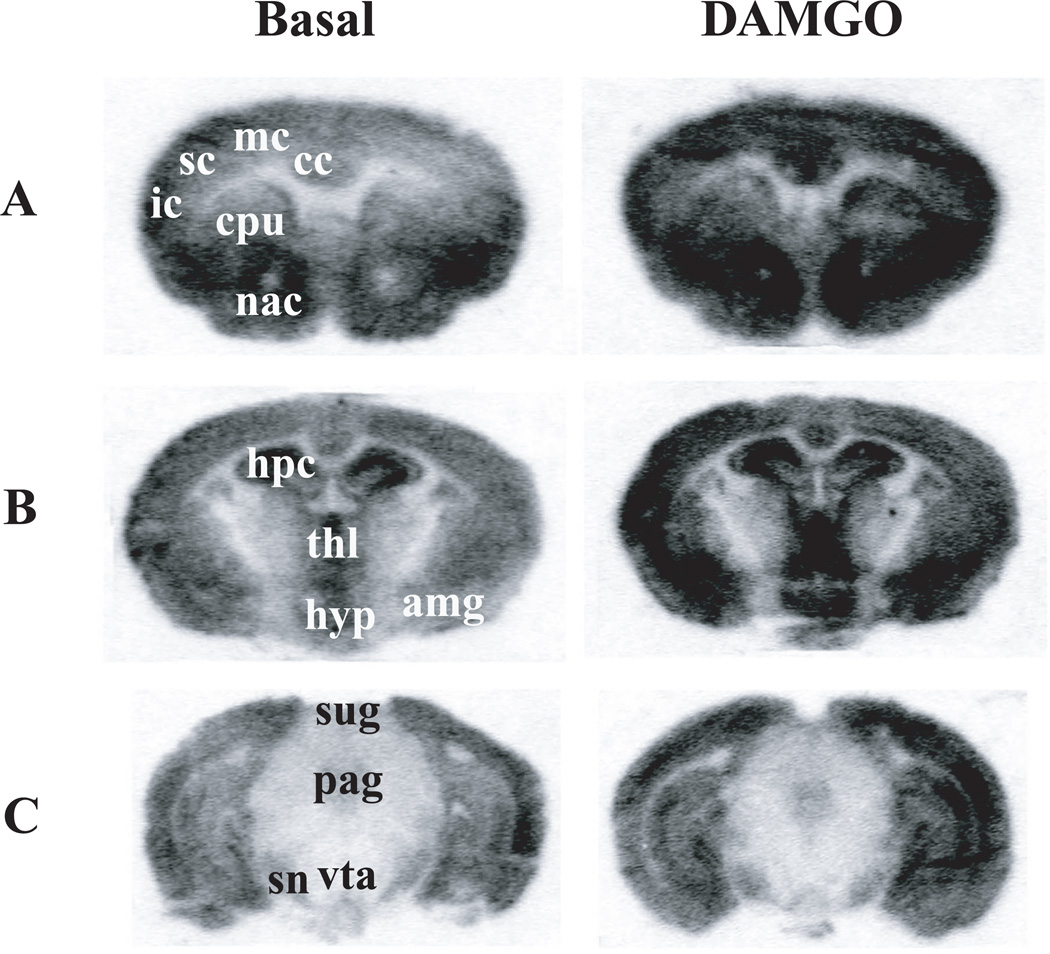
DAMGO-stimulated [35S]GTPγS binding, a measure of MOPR-mediated G protein activation (Traynor and Nahorski, 1995; Sim, Selley, and Childers, 1995), was performed on coronal sections of both the A/A and G/G mouse brains at 3 anatomical levels. There is no difference between A/A and G/G on the overall anatomical distribution of G protein activation; therefore, representative autoradiograms of DAMGO-stimulated [35S]GTPγS binding in male A/A mice are shown in Fig. 1. High levels of DAMGO-stimulated [35S]GTPγS binding were observed in the cortices, CPu, NAc in the telencephalon (Fig. 1A) and in the thalamus, hypothalamus and amygdala in the diencephalon (Fig. 1B). In the midbrain, DAMGO-stimulated [35S]GTPγS binding were found in the substantial nigra, PAG and VTA, albeit at lower levels (Fig. 1C).
Figure 1. Autoradiograms of DAMGO-stimulated [35S]GTPγS binding in selected coronal sections of male A/A mouse brains at the telencephalon (A), diencephalon (B) and midbrain (C) levels.
Sections were incubated with ~0.04 nM [35S]GTPγS and 2 mM GDP in the absence (basal) or presence of 10 µM DAMGO for 2 hr at 25°C, washed, dried, and exposed to phosphor screens for 48 hr. Abbreviation: cc, cingulate cortex; mc, motor cortex; sc, somatosensory cortex; ic, insular cortex; cpu, caudate putamen; acb, nucleus accumbens; hpc, hippocampus; hyp, hypothalamus; thl, thalamus; amg, amygdala; pag, periaqueductal gray; sn, substantial nigra; sug, superficial grey of superior colliculus; vta, ventral tegmental area.
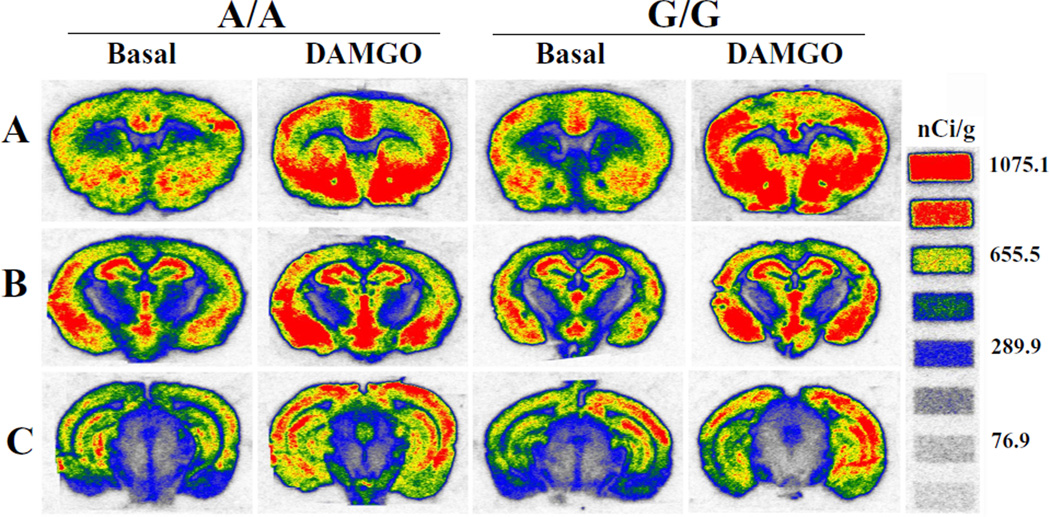
We compared the levels and distribution of the DAMGO-stimulated [35S]GTPγS binding between A/A and G/G mice brains. Representative pseudo-color autoradiograms of DAMGO-stimulated [35S]GTPγS binding in male A/A and G/G mice are shown in Fig. 2. A/A and G/G mice displayed similar regional distribution (Fig. 2). Quantitation of [35S]GTPγS binding in the presence and absence of DAMGO was performed by measuring the density of autoradiograms in brain regions. Net DAMGO-stimulated [35S]GTPγS binding was obtained by subtracting the basal binding from the binding in the presence of DAMGO. Density was converted to radioactivity using calibration curves generated from the [14C] standards. Table 1 shows the combined data of male and female mice of the same genotype and analyses of basal and net DAMGO-stimulated [35S]GTPγS binding. In the VTA, A/A mice displayed significantly lower basal [35S]GTPγS binding, but higher net DAMGO-stimulated [35S]GTPγS binding than G/G mice. In all the other brain regions, there are no significant differences between A/A and G/G mice in basal and net DAMGO-stimulated [35S]GTPγS binding, but G/G mice show a trend of higher basal [35S]GTPγS binding than A/A mice. Moreover, there are large regional variations in basal and net DAMGO-stimulated [35S]GTPγS binding.
Figure 2. Pseudo-color autoradiograms of DAMGO-stimulated [35S]GTPγS binding to the MOPR in selected coronal sections of A/A and G/G male mouse brains at 3 anatomical levels (A–C).
Experiments were carried out as described in Fig. 1 legend.
Table 1.
Quantitation of in vitro autoradiography of DAMGO-stimulated [35S]GTPγS binding in brains of A/A and G/G mice.
| Region | Basal [35S]GTPγS binding (pCi/mg) |
Net DAMGO-stimulated [35S]GTPγS binding (pCi/mg) |
||
|---|---|---|---|---|
| A/A | G/G | A/A | G/G | |
| Cingulate Cortex | 3644 ± 147 | 3933 ± 255 | 861 ± 134 | 888 ± 282 |
| Motor Cortex | 3206 ± 179 | 3562 ± 170 | 453 ± 100 | 569 ± 225 |
| Somatosensory Cortex | 3386 ± 211 | 3839 ± 105 | 328 ± 102 | 481 ± 251 |
| Insular Cortex | 3916 ± 206 | 4408 ± 158 | 599 ± 173 | 663 ± 187 |
| CPu | 2822 ± 179 | 2965 ± 80 | 1300 ± 121 | 1187 ± 184 |
| NAc | ||||
| Core | 4714 ± 259 | 4971 ± 237 | 1727 ± 160 | 1542 ± 254 |
| Shell | 4135 ± 245 | 4606 ± 150 | 1512 ± 150 | 1511 ± 217 |
| Hippocampus | 4108 ± 198 | 4713 ± 193 | 277 ± 89 | 308 ± 129 |
| Amygdala | 4016 ± 138 | 4192 ± 260 | 1090 ± 192 | 1177 ± 262 |
| Thalamus | 2959 ± 79 | 3073 ± 190 | 520 ± 101 | 519 ± 135 |
| Hypothalamus | 3514 ± 212 | 4332 ± 357 | 937 ± 130 | 996 ± 256 |
| PAG | 2432 ± 119 | 2278 ± 184 | 714 ± 93 | 600 ± 152 |
| Substantial nigra | 2730 ± 161 | 2892 ± 133 | 538 ± 88 | 445 ± 90 |
| VTA | 1127 ± 96 | 1419 ± 74* | 693 ± 161 | 339 ± 86* |
| Superifical layers of the superior colliculus | 5028 ± 249 | 6382 ± 592 | 1279 ± 272 | 706 ± 244 |
Quantitation was made from both left and right sides of each section. Data are expressed as mean ± S.E.M. of eleven to twelve animals, three to four sections from each brain.
p<0.05, different from A/A mice by the non-paired two-tailed Student’s t test
Sex Differences in net DAMGO-stimulated [35S]GTPγS binding
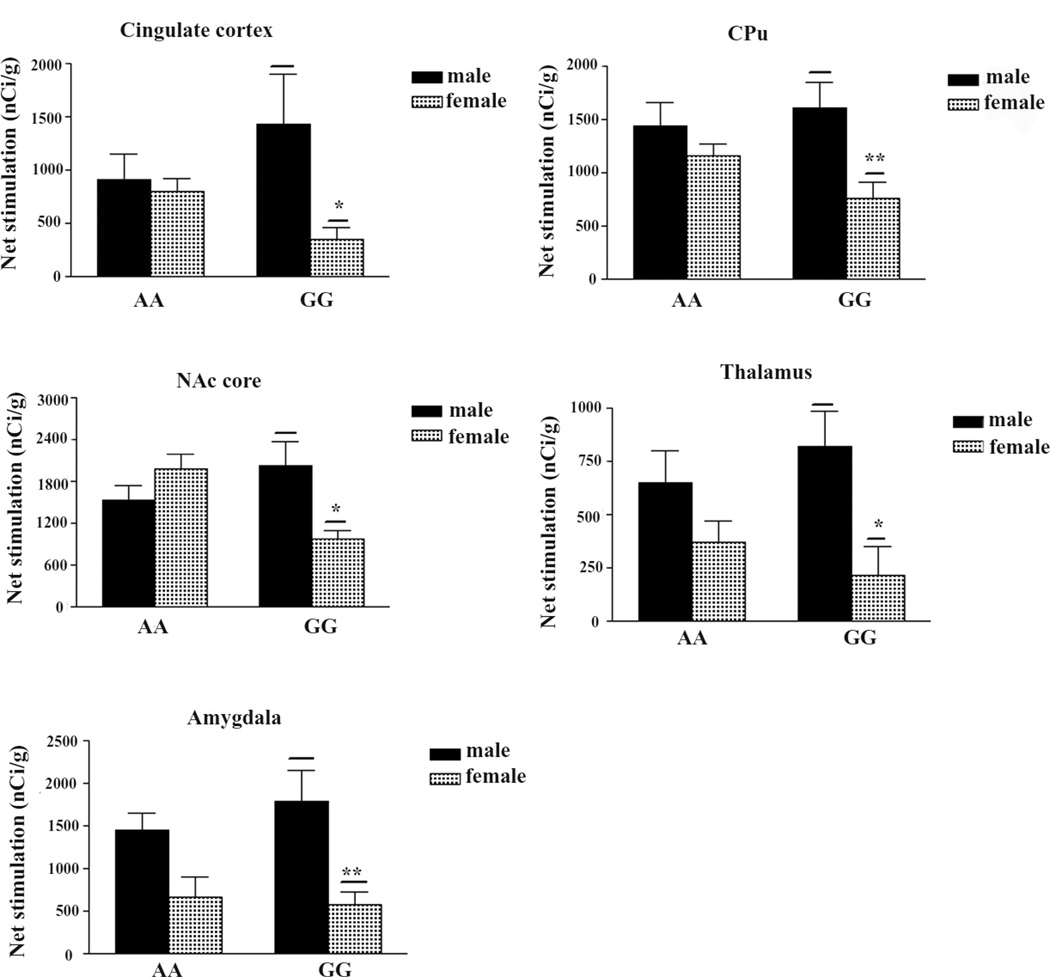
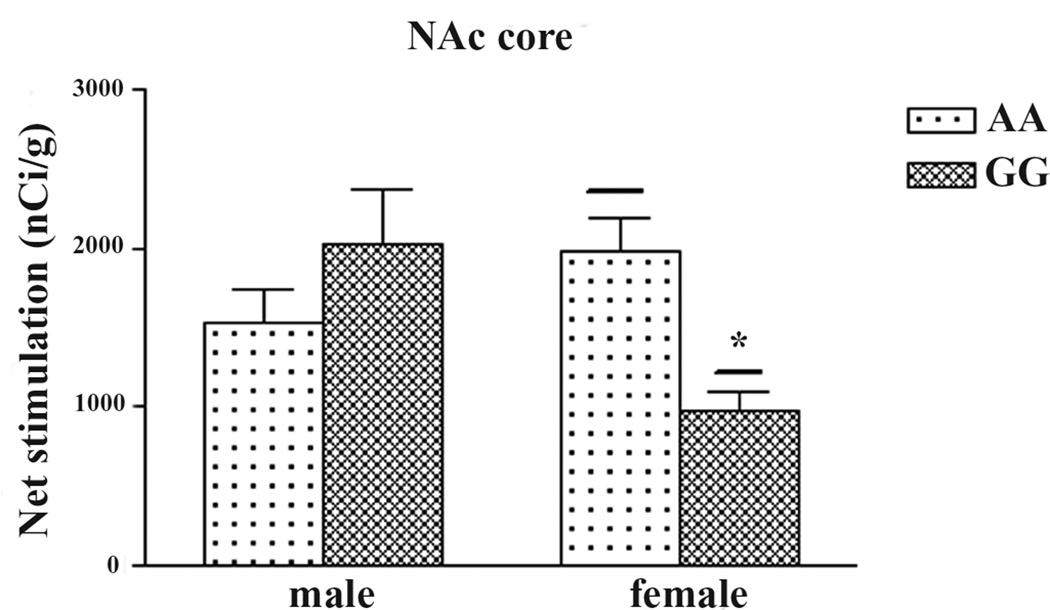
To explore if there are sex differences in basal or DAMGO-stimulated [35S]GTPγS binding in A/A and G/G mice, the data of males and females were analyzed separately. There were no sex differences in basal [35S]GTPγS binding in A/A or G/G mice in any of the brain regions. As shown in Fig. 3, males G/G mice showed significantly higher net DAMGO-stimulated [35S]GTPγS binding than female G/G mice in the cingulate cortex, CPu, NAc core, thalamus and amygdala. In contrast, there are no sex differences in A/A mice. In addition, in the NAc core, female A/A mice displayed significantly higher DAMGO-stimulated [35S]GTPγS binding than female G/G mice (Fig. 4).
Figure 3. Brain regions that show significant sex differences in DAMGO-stimulated [35S]GTPγS binding in G/G mice.
Data are expressed at mean ± S.E.M. of five to six animals, three or four sections from each brain. *p < 0.05 different from G/G male by the non-paired two-tailed Student’s t test.
Figure 4. In the nucleus accumbens core, there is significant genotype difference in DAMGO-stimulated [35S]GTPγS binding in female mice.
Data are expressed at mean ± S.E.M. of five to six animals, three or four sections from each brain. *p < 0.05, **p < 0.01 different from A/A female by the non-paired two-tailed Student’s t test.
Discussion
The current studies demonstrated that A112G SNP led to lower DAMGO-stimulated [35S]GTPγS binding in the VTA. In addition, A112G SNP resulted in reductions in DAMGO-stimulated [35S]GTPγS in the NAc core in females, but not in males. These biochemical changes resulting from A112G SNP may correlate with alterations in morphine-induced in vivo pharmacological effects, which are discussed below.
Our results are different from those of (Ramchandani et al., 2011) on the A118G mice they generated. They found that there were no differences between G/G and A/A mice in DAMGO-stimulated [35S]GTPγS binding in the dorsal striatum, NAc and VTA. The discrepancy may be due to different mouse models used. The mice used in the present study has A112G substitution in the mouse MOPR sequence, where those in the Ramchandani et al. (2011) study have the first exon of the human MOPR gene with A118 and G118 in A/A and G/G mice, respectively.
Reduced DAMGO-stimulated [35S]GTPγS binding by A112G SNP in the VTA may be related to decreased morphine-induced hyperactivity and locomotor sensitization
VTA plays an important role in morphine-induced hyperactivity and locomotor sensitization (Bunney, Massari, and Pert, 1984; Kalivas and Duffy, 1987). Our observations that A112G SNP led to reduced DAMGO-stimulated [35S]GTPγS binding in VTA are consistent with the findings that G/G mice showed decreased morphine-induced hyperactivity and locomotor sensitization (Mague et al., 2009). In addition, we have demonstrated that A112G SNP results in reduced MOPR expression in the VTA (Wang et al., 2012), which may play a role as well.
Reduced DAMGO-stimulated [35S]GTPγS binding in the NAc core of female G/G mice may contribute to impaired morphine-induced conditioned place preference
The NAc is a key neural substrate involved in rewarding properties of drugs of abuse, including morphine (Koob, Sanna, and Bloom, 1998). We found that in the NAc core female G/G mice displayed significantly lower DAMGO-stimulated [35S]GTPγS binding than female A/A mice; however, there is no difference between male A/A and male G/G mice. These differences are likely to contribute to the previous report that female G/G mice failed to show a morphine-induced conditioned place preference, in contrast to female A/A, male A/A and male G/G mice (Mague et al., 2009).
Effects of A112G SNP on DAMGO-stimulated [35S]GTPγS binding have sex differences
We found that among G/G mice males displayed higher DAMGO-stimulated [35S]GTPγS binding than females in the cingulate cortex, CPu, thalamus, NAc core and amygdala; however, no sex differences were observed among A/A mice. These results indicate that the sex differences are limited to the G/G mice in these regions. In future studies, it may be important to examine if sex differences are present in the G/G mice in behavioral endpoints mediated by the MOPR in these brain regions.
We have found that male A/A mice showed higher [3H]DAMGO binding than G/G mice in more brain regions than female A/A over G/G (Wang et al., 2012). DAMGO-stimulated [35S]GTPγS binding did not show a similar trend as [3H]DAMGO binding. There are several possible contributing factors for the discordance between DAMGO-stimulated [35S]GTPγS binding and [3H]DAMGO binding to the MOPR, as we previously discussed (Wang et al., 2012). N-linked glycosylation (Knezevic, Polasek, Gornik et al., 2009; Stanta, Saldova, Struwe et al., 2010; Huang et al., 2012), regional differences in MOPR-G protein coupling efficiency (Wang, Rasakham, Huang et al., 2011), hormonal state (Loyd, Wang, and Murphy, 2008), and epigenetic regulation [reviewed in (Jessen and Auger, 2011)] may influence sex-specific protein expression. It appears that these factors affect G/G mice, but not A/A mice, of which the underlying mechanisms remain to be investigated.
Lack of correlation between DAMGO-stimulated [35S]GTPγS binding and receptor levels
We found that A112G SNP led to reduced DAMGO-stimulated [35S]GTPγS binding only in the VTA when data of male and female mice were combined. In contrast, our previous studies revealed that A112G SNP resulted in lower MOPR expression in several brain regions, including VTA, cortices, NAc, hypothalamus, amygdala, PAG, and superficial layers of the superior colliculus (Wang et al., 2012). Our data indicate that changes in DAMGO-stimulated [35S]GTPγS binding levels do not correlate with alterations in MOPR levels. A similar lack of a close correlation has been observed between mu, kappa or cannabinoid receptor and G protein activation (Breivogel, Sim, and Childers, 1997; Vogt, Sim-Selley, Childers et al., 2001; Wang et al., 2011). The mechanisms underlying these discrepancies are not yet clear, but some factors may be involved. The ratios of receptor to G proteins may vary among brain regions. MOPRs have been shown to activate different types of G proteins (Chakrabarti, Prather, Yu et al., 1995), which may lead to different numbers of G proteins activated per receptor in different regions. It is also possible that some MOPRs may not be coupled functionally, due to their intracellular location (Wang, Van Bockstaele, and Liu-Chen, 2008).
Regional differences in basal [35S]GTPγS binding
In the VTA, G/G mice (males and females pooled) exhibited significantly higher basal [35S]GTPγS binding than A/A mice. In most of the other regions, G/G mice showed a trend of higher basal levels, but they did not reach statistical significance. Previously Mague et al. (2009) reported lower MOPR protein levels by immunoblotting and lower Bmax value of [3H]DAMGO binding to the MOPR in the brains of G/G mice, compared to A/A mice. We also found that A/A mice displayed higher MOPR expression than G/G mice in both males and females (Wang et al., 2012). Therefore, the higher basal [35S]GTPγS binding in G/G mice is not due to differences in the MOPR level. Basal [35S]GTPγS binding may represent constitutive (agonist-independent) activation of Gα subunits of trimeric G proteins and many other guanine nucleotide binding proteins present in the brain. Differences in levels and activities of these guanine nucleotide binding proteins may contribute to the differences in basal [35S]GTPγS binding between A/A and G/G mice. It has also been shown that [35S]GTPγS binding to NDPK accounts for part of the high basal binding (Zhang, Li, Chen et al., 1999).
In addition, there is a marked variation in basal [35S]GTPγS binding across different brain regions. For example, in the A/A mice brain, the basal [35S]GTPγS binding ranged from 1127 nCi/g in the VTA to 5028 nCi/g in the superficial layers of the superior colliculus. Our findings are consistent with those of Sim, Selley, Dworkin et al. (1996). GDP is required to reduce basal [35S]GTPγS binding (Traynor and Nahorski, 1995) and the concentration required varies among cells and tissues (Huang, Xu, Yoon et al., 2007). It is conceivable that brain regions may require different GDP concentrations to achieve similar basal [35S]GTPγS binding. However, we had to use one concentration of GDP (2 mM) in the experiment for all brain regions, which may contribute to varying levels of basal [35S]GTPγS binding. Thus, the net DAMGO-stimulated [35S]GTPγS binding as % of basal binding is consequently variable across brain regions and is quite low in some regions, which are consistent with the finding of (Wang et al., 2011) and Sim et al. (1996).
In summary, A112G affected DAMGO-stimulated [35S]GTPγS binding in region- and sex-specific manners. A112G SNP led to reduced DAMGO-stimulated [35S]GTPγS binding in the VTA, which may be associated with lower morphine-induced hyperactivity and locomotor sensitization. There are sex differences in the effects of A112G SNP in NAc core that may contribute to lack of morphine-induced conditioned place preference in female G/G mice. In G/G mice males showed significantly higher net DAMGO-stimulated [35S]GTPγS binding than females in some brain areas, of which the in vivo pharmacological consequences remain to be examined.
Acknowledgements
This work was supported by National Institutes of Health Grants R01 DA17302 and P30 DA13429 (to LYLC) and R21-DA-027066 (to JAB).
ABBREVIATIONS
- A/A
homozygous for A112 allele
- G/G
homozygous for G112 allele
- MOPR
mu opioid receptor
- OPRM1 and oprm1
human and mouse MOPR genes, respectively
- SNP
single nucleotide polymorphism
- CPu
caudate putamen
- NAc
nucleus accumbens
- PAG
periaqueductal gray
- VTA
ventral tegmental area
Footnotes
Author contribution
Participated in research design: Liu-Chen, Wang, Huang, Blendy
Conducted experiments: Wang and Huang
Performed data analysis: Wang and Huang
Wrote or contributed to the writing of the manuscript: Liu-Chen, Wang
References
- Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. Mu opioid receptor gene variants: lack of association with alcohol dependence. Mol. Psychiatry. 1997;2:490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- Bunney WC, Massari VJ, Pert A. Chronic morphine-induced hyperactivity in rats is altered by nucleus accumbens and ventral tegmental lesions. Psychopharmacology (Berl) 1984;82:318–321. doi: 10.1007/BF00427677. [DOI] [PubMed] [Google Scholar]
- Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin. Pharmacol. Ther. 2008;83:559–566. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Prather PL, Yu L, Law P-Y, Loh HH. Expression of the mu-opioid receptor in cho cells: ability of mu-opioid ligands to promote alpha-azidoanilido[32P]GTP labeling of multiple G protein alpha subunits. J. Neurochem. 1995;64:2534–2543. doi: 10.1046/j.1471-4159.1995.64062534.x. [DOI] [PubMed] [Google Scholar]
- Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105:334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- Chou WY, Yang LC, Lu HF, Ko JY, Wang CH, Lin SH, Lee TH, Concejero A, Hsu CJ. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol. Scand. 2006;50:787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- Hayashida M, Nagashima M, Satoh Y, Katoh R, Tagami M, Ide S, Kasai S, Nishizawa D, Ogai Y, Hasegawa J, Komatsu H, Sora I, Fukuda K, Koga H, Hanaoka K, Ikeda K. Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype. Pharmacogenomics. 2008;9:1605–1616. doi: 10.2217/14622416.9.11.1605. [DOI] [PubMed] [Google Scholar]
- Huang P, Chen C, Mague SD, Blendy JA, Liu-Chen LY. A common single nucleotide polymorphism A118G of the mu opioid receptor alters its N-glycosylation and protein stability. Biochem. J. 2012;441:379–386. doi: 10.1042/BJ20111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Xu W, Yoon SI, Chen C, Chong PL, Unterwald EM, Liu-Chen LY. Agonist treatment did not affect association of mu opioid receptors with lipid rafts and cholesterol reduction had opposite effects on the receptor-mediated signaling in rat brain and CHO cells. Brain Res. 2007;1184:46–56. doi: 10.1016/j.brainres.2007.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen HM, Auger AP. Sex differences in epigenetic mechanisms may underlie risk and resilience for mental health disorders. Epigenetics. 2011;6:857–861. doi: 10.4161/epi.6.7.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons. J. Pharmacol. Exp. Ther. 1987;241:204–212. [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Kim JG, Choi YS, Kim HO, Kim SY, Oslin DW. A mu opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Knezevic A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, Wright A, Kolcic I, O'Donoghue N, Bones J, Rudd PM, Lauc G. Variability, heritability and environmental determinants of human plasma N-glycome. J. Proteome. Res. 2009;8:694–701. doi: 10.1021/pr800737u. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol. Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics. J. 2004;4:184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J. Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Blendy JA. OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. 2010;108:172–182. doi: 10.1016/j.drugalcdep.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud S, Thorsell A, Sommer WH, Heilig M, Holgate JK, Bartlett SE, Ruiz-Velasco V. Pharmacological consequence of the A118G mu opioid receptor polymorphism on morphine- and fentanyl-mediated modulation of Ca(2) channels in humanized mouse sensory neurons. Anesthesiology. 2011;115:1054–1062. doi: 10.1097/ALN.0b013e318231fc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol. Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, Teo YY, Tan EC. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Childers SR. Anatomical distribution of mu, delta, and kappa opioid- and nociceptin/orphanin FQ-stimulated [35S]guanylyl-5'-O-(gamma-thio)-triphosphate binding in guinea pig brain. J. Comp. Neurol. 1997;386:562–572. doi: 10.1002/(sici)1096-9861(19971006)386:4<562::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5'-[gamma-[35S]thio]-triphosphate binding. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on µ opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J. Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J. Neurosci. 2000;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanta JL, Saldova R, Struwe WB, Byrne JC, Leweke FM, Rothermund M, Rahmoune H, Levin Y, Guest PC, Bahn S, Rudd PM. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J. Proteome. Res. 2010;9:4476–4489. doi: 10.1021/pr1002356. [DOI] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5'-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RG, Vogt BA. Colocalization of mu-opioid receptors and activated G-proteins in rat cingulate cortex. J. Pharmacol. Exp. Ther. 2001;299:840–848. [PubMed] [Google Scholar]
- Wang Y, Van Bockstaele EJ, Liu-Chen LY. In vivo trafficking of endogenous opioid receptors. Life Sci. 2008;83:693–699. doi: 10.1016/j.lfs.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY. Reduced expression of the mu opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience. 2012;205:178–184. doi: 10.1016/j.neuroscience.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY. Sex Difference in {kappa}-Opioid Receptor (KOPR)-Mediated Behaviors, Brain Region KOPR Level and KOPR-Mediated Guanosine 5'-O-(3-[35S]Thiotriphosphate) Binding in the Guinea Pig. J. Pharmacol. Exp. Ther. 2011;339:438–450. doi: 10.1124/jpet.111.183905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li JG, Chen C, Liu-Chen LY. Nucleoside diphosphate kinase associated with membranes modulates mu-opioid receptor-mediated [35S]GTPgammaS binding and agonist binding to mu-opioid receptor. Eur. J. Pharmacol. 1999;377:223–231. doi: 10.1016/s0014-2999(99)00387-8. [DOI] [PubMed] [Google Scholar]