Abstract
The role of inflammation in inducing visceral hypersensitivity (VHS) in ulcerative colitis patients remains unknown. We tested the hypothesis that acute ulcerative colitis-like inflammation does not induce VHS. However, it sets up molecular conditions such that chronic stress following inflammation exaggerates single unit afferent discharges to colorectal distension. We used dextran sodium sulfate (DSS) to induce ulcerative colitis-like inflammation and a 9-day heterotypic chronic stress protocol in rats. DSS upregulated Nav1.8 mRNA in colon-responsive DRG neurons, TRPV1 in colonic muscularis externae (ME) and BDNF in spinal cord without affecting the spike frequency in spinal afferents or VMR to CRD. By contrast, chronic stress did not induce inflammation but it downregulated Kv1.1 and Kv1.4 mRNA in DRG neurons, and upregulated TRPA1 and NGF in ME, which mediated the increase of spike frequency and VMR to CRD. Chronic stress following inflammation exacerbated spike frequency in spinal afferent neurons. TRPA1 antagonist suppressed the sensitization of afferent neurons. DSS-inflammation did not affect the composition or excitation thresholds of low-threshold and high-threshold fibers. Chronic stress following inflammation increased the percent composition of high-threshold fibers and lowered the excitation threshold of both types of fibers. We conclude that not all types of inflammation induce VHS, whereas chronic stress induces VHS in the absence of inflammation.
Keywords: Colon inflammation, sensory neurons, inflammatory bowel disease, abdominal pain, chronic stress
INTRODUCTION
The sensitization of primary afferent neurons by peripheral inflammation and central facilitation of peripheral nociceptive signals underlie chronic pain (Woolf, 2011). Since profound colonic inflammation occurs in ulcerative colitis patients, the tacit assumption is that colonic inflammation in these patients sensitizes primary afferent neurons to cause visceral pain. However, clinical studies in ulcerative colitis patients did not consistently find visceral hypersensitivity (VHS) in response to colorectal distension (CRD); some reported visceral hypersensitivity (Rao et al., 1987), others found normosensitivity (Bernstein et al., 1996, Mayer et al., 2005) or hyposensitivity, (Chang et al., 2000), suggesting that the afferent nervous system may not be sensitized in all patients.
Clinical findings show that chronic stress exacerbates the symptoms of IBD patients, including abdominal pain (Levenstein et al., 2000b, Maunder and Levenstein, 2008). Likewise, animal studies show that the application of various chronic stress paradigms to rodents produces hypersensitivity to CRD by sensitizing colon primary afferent neurons (Bradesi et al., 2005, Winston and Sarna, 2013). Therefore, it is likely that the lack of consideration of concurrent chronic stress might be one of the reasons for the divergent findings of VHS in ulcerative colitis patients. We hypothesized that ulcerative colitis-like colonic inflammation in rats, by itself, does not sensitize the primary afferents or increase VMR to CRD. However, chronic stress following inflammation super sensitizes the primary afferents. We tested this hypothesis by applying a chronic stress protocol to rats following DSS-induced colonic inflammation that mimics the morphological, immunological, and histological features of ulcerative colitis (Elson et al., 1995). We found that acute ulcerative colitis-like inflammation alone did not induce visceral hypersensitivity, although it sensitized high threshold colonic pelvic nerve afferent fibers and up regulated the expression of pro-nociceptive genes, brain-derived neurotrophic factor (BDNF) and NaV1.8 in colon afferent neurons and TRPV1 in colonic muscularis externae (ME). However, chronic stress, following colonic inflammation, induced robust sensitization of afferent neurons by up regulating the expression of nerve growth factor (NGF) and transient receptor potential ankyrin repeat 1(TRPA1) in the ME, and down-regulating Kv1.1 and Kv1.4 in colon DRG neurons. Treatment with a TRPA1 antagonist significantly reduced visceral hypersensitivity and reduced sensitization of colonic pelvic nerve afferent fibers. In addition, DSS inflammation and chronic stress had differential effects on the recruitment of low-threshold (LT) and high-threshold (HT) fibers and their excitation thresholds.
EXPERIMENTAL METHODS
Animal models
We used 6–10 week old male Sprague-Dawley rats (180–280 g). The Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston, TX approved the procedures. Oral administration of 5% w/v DSS in drinking water for 5 days was used to induce colonic inflammation (Shi et al., 2011). Visceromotor response (VMR) to graded (10–80 mmHg) CRD or single fiber recordings from the sacral level S1 dorsal root as well as tissues for molecular experiments were obtained on day 6 after start of DSS treatment (Fig. 1A). A separate group of rats received psychological stress by a 9-day heterotypic intermittent chronic stress (HeICS) protocol comprised of randomly distributed daily application of water avoidance stress (WAS; 60 min), forced swimming stress (FSS; 20 min), or cold restraint stress (CRS; 45 min), as described previously (Winston et al., 2010) (figure 1A). The behavior, electrophysiological results, and tissues were obtained on day 10, one day after the end of HeICS (figure 1A). The third group of rats received 9-day HeICS starting on day 6 after start of DSS treatment (figure 1A); experiments were performed on day 16, one day after the end of HeICS. Age-matched control rats consumed regular drinking water.
Fig. 1.
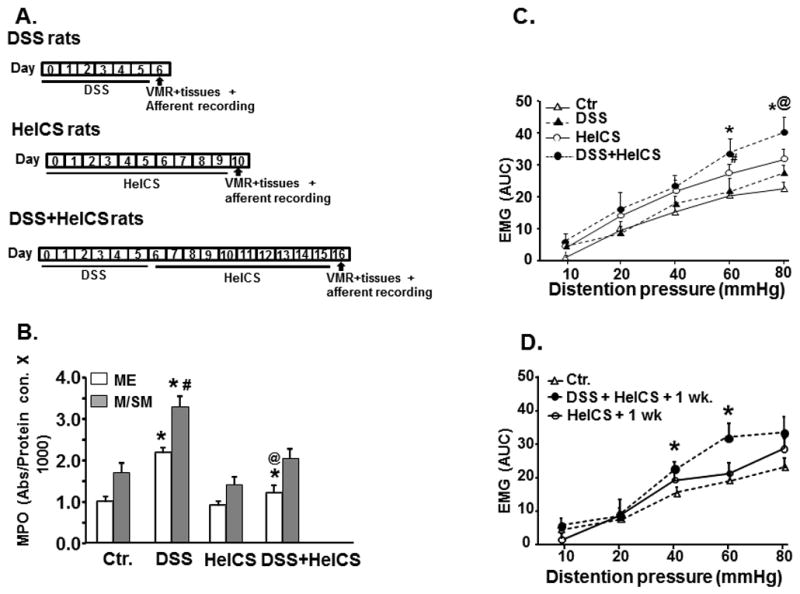
Study design, inflammation and the effect of DSS, HeICS and DSS+HeICS on VMR by CRD. (A) Inflammation and chronic stress protocols in DSS-, HeICS- and DSS+HeICS-rats. (B) MPO in ME and M/SM increased significantly on day 6 in DSS-rats vs. saline-treated controls (*p<0.05); MPO in M/SM was significantly greater than in ME (#p<0.05). HeICS did not affect MPO in ME or M/SM. In DSS+HeICS rats, MPO in ME, but not in M/SM, remained elevated on day 16 compared with controls (n=6 rats, *p<0.05). The MPO in DSS+HeICS rats on day 16 was significantly lower than in DSS rats on day 6 (@p<0.05). (C) Comparison of the VMR to graded CRD in control (Ctr.), DSS, HeICS-, and DSS+HeICS-rats. Electromyographic (EMG) activity is expressed as area under the curve (AUC) in units of volts ×seconds. DSS inflammation had no effect on VMR to CRD. HeICS significantly increased VMR to CRD (n=6 rats, #p<0.05 vs. Ctr.); HeICS+DSS further increased VMR vs. HeICS alone (n=6 rats, *p<0.05 vs. Ctr.; @p<0.05 vs. HeICS). (D) The VMR to CRD in DSS+HeICS rats remained significantly elevated one week after the last stressor (n=6 rats, *p<0.05 vs. Ctr.).
Measurement of VMR to Graded CRD
CRD was performed by rapidly inflating the balloon to constant pressures: 10, 20, 40, 60, and 80 mmHg, for 20 seconds followed by 2-minute rest, as described previously (Winston and Sarna, 2013). The area under the curve for the electrical signal, during each 20 seconds of distention, was calculated using Acknowledge software (Biopac Systems, Inc., Santa Barbara, CA). The net value for each distension response was calculated by subtracting the baseline value derived from the average area under the curve for the 20 seconds before and 20 seconds after the distention.
Electrophysiological recordings
Multiunit afferent discharges were recorded from the distal ends of S1 dorsal rootlets decentralized close to their entry into the spinal cord. A bundle of multiunit fibers was distinguished into 2–6 single units off-line using wave mark template matching in Spike 2 software that differentiates spikes by shape and amplitude. Colonic afferent fibers were identified by their response to CRD.
In vivo single fiber recording of S1 DRG rootlets
Isoflurane, 2.5%, followed by 50 mg/kg, i.p. sodium pentobarbital induced general anesthesia that was maintained by infusing (3 ml/h) a mixture of 1ml pentobarbital sodium (50 mg) + 2 ml pancuronium bromide + 1.7 ml saline by intravenous infusion through the tail vein. Adequacy of anesthesia was confirmed by the absence of corneal and pupillary reflexes and stability of end-tidal CO2 level. A tracheotomy tube connected to a ventilator system provided a mixture of room air and oxygen (3.0 ml, 54 breaths/min). Expired CO2 was monitored and maintained at 3.5%. Body temperature was monitored and maintained at 37 °C by a servo-controlled heating blanket. A laminectomy from T12 to S2 exposed the spinal cord. The head was stabilized in a stereotaxic frame. The dura was gently opened and a warm mineral oil pool, contained by skin flaps, covered the exposed spinal cord and roots.
Data analysis
Excitatory responses (imp/s) to CRD were calculated by subtracting spontaneous activity from the mean of 30 s of the maximal activity during distension. Fibers were considered responsive when CRD increased the activity 30% greater than the baseline value. Mechanosensitive single units were classified into high-threshold (>20 mmHg) and low-threshold (≤20 mmHg) on the basis of their response threshold and profile during colon distensions. The CRD data were analyzed using ANOVA with repeated measures; CRD intensity was the repeated factor and experimental group as the group factor.
Western Blot
Western blots were performed as described earlier (Winston et al., 2010). NGF (sc-546; Santa Cruz Biotechnology, CA) and BDNF antibodies (Santa Cruz Biotechnology, CA) were diluted 1/100 and incubated with tissues overnight at 4°C in phosphate-buffered saline + 1.5% goat serum. Rabbit anti-TRPA1 was diluted at 1:1,000; and goat anti--actin at 1:5,000 (both from Santa Cruz Biotechnology, Santa Cruz, CA). Band density was determined via the Imaging Densitometer (Odessy, Li-COR Biosciences, Nebraska).
Laser capture microscopic (LCM) dissection
We injected CTB-488 (Invitrogen, Calsbad, CA), 4 mg/ml in PBS, into the colon wall (6 injections of 2 μl each/rat); DRG were collected 6 days later and frozen in OTC on dry ice. We identified CTB-488 labeled neuronal profiles and captured them with a Pixel IIe LCM microscope (Applied Biosytems, Foster City, CA). RNA was prepared with a Qiagen microRNA kit. SYBR green RT-PCR was performed with Applied Biosystems reagents and Step One Plus real-time PCR apparatus. We used β-III-TUB as a normalizer and compared fold change to control by using the DDCt procedure. Primers were designed using Primer Express Software (Applied Biosystems) and validated through control experiments: a single amplimer was observed by melting curve analysis; no amplimer was produced without reverse transcription or template; amplification efficiency was 100%.
Immunostaining
Ten micron frozen sections cut from formalin fixed LS spinal cords were incubated overnight with primary antibodies: chicken anti-BDNF (Promega, G164A, 1:250) and with biotinylated goat anti-chicken antibody (Vector 9010, 5μg/ml), followed by incubation with streptavidin conjugated to HRP(DAKO, LSAB+system-HRP), visualized with DAB chromogen. DAB stained sections were imaged on an Olympus BX51 light microscope.
Statistics
Data are expressed as mean ± SEM. Two-way or one-way ANOVA followed by Fisher post-hoc analysis and t-test were used for comparison of means. Chi-square analysis was used to compare the number of neurons in different categories. P<0.05 was considered statistically significant in all cases. We used SPSS (SPSS, Inc., Chicago, IL) for all analyses.
RESULTS
Interaction between inflammation and chronic stress in inducing VHS
We called the rats treated with DSS alone DSS-rats, those treated with HeICS alone HeICS-rats, and those treated with DSS followed by HeICS, DSS+HeICs-rats (Fig. 1A). DSS treatment significantly increased MPO expression in the colonic ME and mucosal/submucosal (ME/SM) tissues on day 6 post inflammation (Fig. 1B), but had no significant effect on the VMR to CRD (Fig. 1C). By contrast, HeICS applied to naïve rats had no significant effect on MPO in the ME or the M/SM (Fig. 1B); however, it significantly enhanced the VMR to CRD (Fig. 1C). The application of HeICS following DSS inflammation exacerbated the VMR to CRD to levels significantly greater than in HeICS-rats (Fig. 1C). Note that inflammation due to DSS treatment returned to baseline on day 16 post-inflammation in M/SM. The inflammation in ME significantly decreased (p<0.05), but it was still elevated modestly at this time vs. control rats (Fig. 1B). The increase of VMR in HeICS-rats returned to baseline by day 7 after the last stressor. However, the VMR to CRD in DSS + HeICS rats remained significantly elevated at this time (Fig. 1D), suggesting the exacerbation of VHS by concurrent inflammation and chronic stress.
Differential effects of inflammation and chronic stress on activation and sensitization of colon-responsive spinal afferent fibers
Activation of dorsal root fibers
We recorded afferent fiber activity in response to graded CRD to investigate whether DSS inflammation and chronic stress affected their activation differentially. We identified 226 dorsal root fibers responsive to CRD in 24 rats: 57 in 7 control rats, 56 in 6 DSS-rats, 52 in 5 HeICS-rats, and 61 in 6 DSS+HeICS-rats. The alterations in the responses of single unit fibers from control-, DSS-, and DSS+HeICS-rats were similar to those of VMR to CRD (Fig. 1C and Fig. 2). DSS treatment alone had no significant effect on the spike frequency; HeICS increased it significantly, while DSS+HeICS exacerbated the spike frequency to levels greater than in response to HeICS alone (Fig. 2).
Fig. 2.
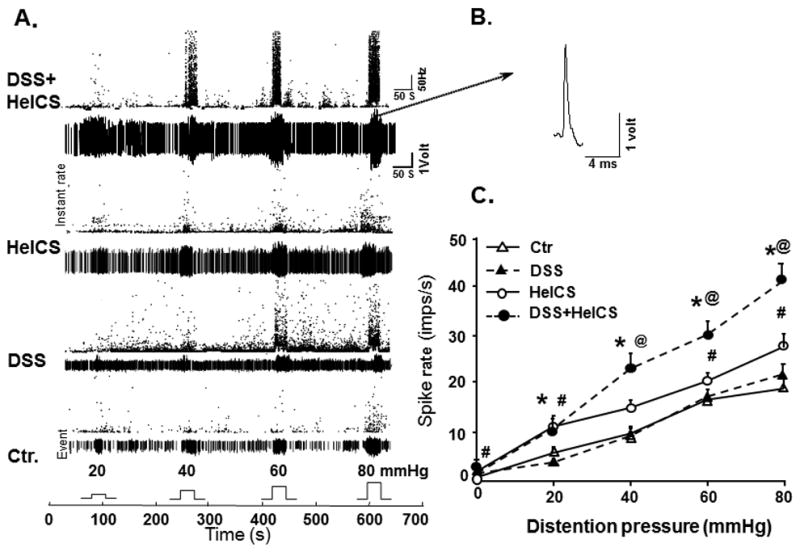
Spinal afferent single unit signals generated by graded CRD and the effect of DSS, HeICS and DSS+HeICS on primary afferent activation. (A) Representative responses of spinal LS afferent fiber to graded CRD (20, 40, 60 and 80 mmHg). The Waveform histograms showing the spike frequency (dot plots), background activities and responses of an individual afferent single unit to graded CRD in 4 group rat afferents recording to CRD. The bar pulses shown at the bottom indicate the duration of CRD. The numbers above the pulses show the pressure applied respectively (20, 40, 60 or 80 mmHg). (B) shows a single action potential from the template for a representative unit of DSS+HeICS afferent recording. (C) The effect of DSS, HeICS and DSS+HeICS on spinal dorsal root single unit afferent responses to CRD. HeICS enhanced afferent single unit signals to CRD significantly vs. DSS inflammation and control rats (n=6 rats in each group, 52–59 fibers in each group, *p<0.05 DSS+HeICS vs. DSS rats at the same distension pressure; #p<0.05 HeICS vs. Ctr.; @p<0.05 DSS+HeICS vs. HeICS).
We investigated the contribution of DSS and HeICS to the exacerbation of VMR to CRD in DSS+HeICS rats on day 16, at which time the inflammation in the M/ME had subsided. We found that the spike frequency in afferent fibers of DSS rats was significantly greater vs. the control rats on day 16 and HeICS elevated it significantly vs. that in DSS rats (Fig. 3A). The VMR in DSS rats during the post-inflammation period was also greater than in control rats. The increase following HeICS in DSS rats did not reach statistical significance vs. DSS treatment only, indicating a disconnect between the increase in the sensitivity of afferent fibers by HeICS and VMR to CRD during the post-inflammation period (Fig. 3B).
Fig. 3.
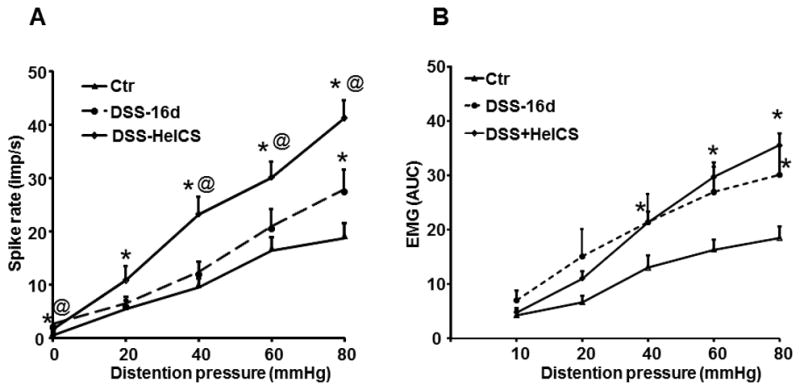
Contribution of DSS and HeICS to the increase of VMR to CRD during the post-inflammation period, day 16. (A) The frequency of single unit afferent discharges to graded CRD was significantly greater during the post-inflammation period on day 16. HeICS following DSS exacerbated the sensitivity of afferent fibers to CRD, 45–55 fibers from 6 rats in each group. (B) Although, VMR to CRD did not increase during the peak of DSS inflammation, it was greater than in control rats on day 16, when inflammation in the M/SM had subsided. The increase in VMR by HeICS following DSS did not reach significance. * p< 0.05 vs. Ctr. Rats, @ p<0.05 vs. DSS post inflammation, n=6 rats in each group.
Sensitization of LT and HT dorsal root fibers
We divided spinal afferent fibers into two subgroups: LT ≤ 20 mmHg) and HT (> 20 mmHg). The compositions of LT and HT fibers did not differ between control and DSS-rats (Fig. 4A and 4B). HeICS significantly decreased the percent of HT fibers vs. controls (Fig. 4A and 4C), while DSS+HeICS significantly enhanced the recruitment and proportion of HT fibers vs. control, DSS- and HeICS-rats (Fig. 4D).
Fig. 4.
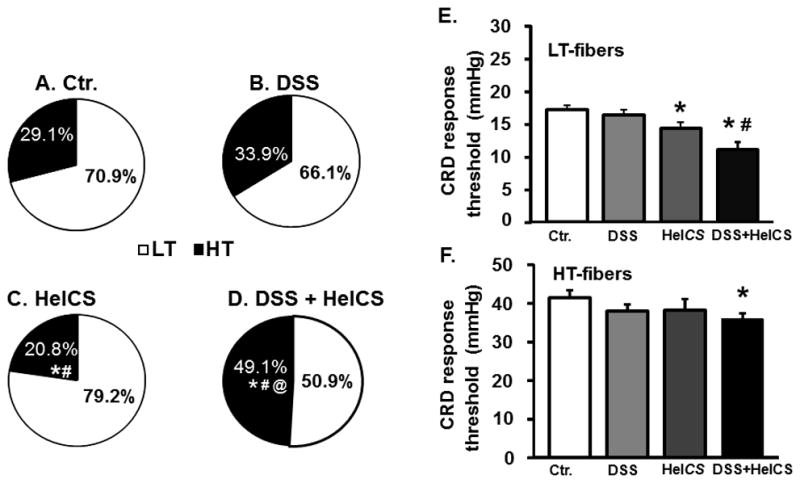
Effects of DSS inflammation, HeICS, and DSS+HeICS on the proportion and activation thresholds of low-threshold (LT) and (HT) fibers. (A-D) Pie graphs illustrate the percentage of LT and HT fibers detected in each treatment group. Chi-Square test: * indicates p<0.05 vs. Ctr.; # indicates p<0.05 vs. DSS group; @ indicates p<0.05 vs. HelCS. (E and F) Bar graphs depicting the average minimum pressure required to evoke responses from LT (E) or HT (F) fibers in each treatment group as determined by a ramp distention protocol. HeICS and DSS+HeICS significantly reduced the mean activation threshold of LT fibers. Only DSS+HeICS reduced the threshold of HT fibers (n=6 rats, *p<0.05 vs. Control; #p<0.05 vs. DSS).
Ramp and graded distension protocols showed that HeICS and DSS+HeICS, but not DSS, significantly reduced the mean activation threshold of LT fibers (Fig. 4E). By contrast, only DSS+HeICS reduced the threshold of HT fibers (Fig. 4F).
DSS inflammation alone had no significant effect on the average spike frequency in response to CRD in LT fibers vs. control rats (Fig. 5A), while HeICS significantly increased it (Fig. 5B). By contrast, each stressor significantly enhanced the average spike frequency in response to CRD in HT fibers (Fig. 5E and 5F). Both stressors together significantly enhanced the average spike frequency response in response to CRD in LT and HT fibers vs. either DSS treatment alone (Fig. 5C and 5G) or HeICS alone (Fig. 5D and 5H).
Fig. 5.
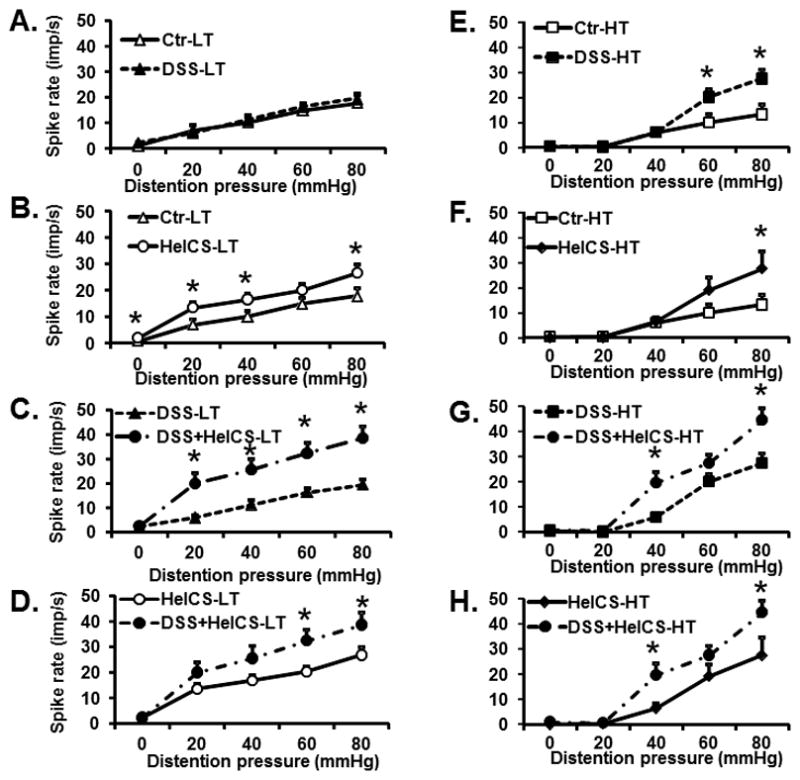
Effects of DSS, HeICS, and DSS+HeICS on LT and HT fiber activities in response to graded CRD. DSS inflammation significantly enhanced action potential frequency in response to CRD of high-threshold (HT) fibers (p<0.05), but not in low-threshold (LT) fibers (A and E). HeICS enhanced the response in both HT and LT afferent fibers (B and F). DSS+HeICS together significantly exacerbated the spike response frequency to CRD in both LT and HT fibers vs. DSS (C and G) and HeICS alone (D and H) (n=5–6 rats, * p<0.05).
Cellular regulation of VHS in response to DSS and HeICS
To identify potential targets for pharmacological interventions, we measured the expression of select neurotrophins, ion channels and TRP channels in colon-responsive S1 DRG neurons, colon ME and L6-S1spinal cord dorsal horn. DSS alone significantly increased BDNF mRNA in colon-projecting neurons; DSS+HeICS increased it to a lesser degree, while HeICS had no significant effect (Fig. 6A, top). DSS alone had no effect on Kv1.4 and Kv1.1 mRNA in these neurons, but HeICS and DSS+HeICS significantly suppressed their expression (Fig. 6A, bottom). By contrast, DSS treatment alone significantly increased Nav1.8 mRNA, while HeICS and DSS+HeICS had no effect (Fig. 6A, bottom). HeICS and DSS+HeICS treatments increased NGF and TRPA1 proteins in the colon ME, but DSS inflammation had no effect (Fig. 6B, top and middle). DSS treatment alone or in combination with HeICS significantly increased TRPV1 protein expression in colonic ME, but HeICS had no significant effect (Fig. 6B, bottom). Finally, DSS treatment alone or together with HeICS increased BDNF protein expression in the L6-S2 dorsal horn, but HeICS alone had no effect (Fig. 6C). Although all three treatments produced significant changes in pro-nociceptive gene expression, the increased VMR to CRD in HeICS and DSS+HeICS rats was associated with up-regulation of NGF and TRPA1 in the ME and the down-regulation of Kv1.4 and Kv1.1 in colon-responsive DRG neurons. BDNF up-regulation in DSS rats did not lead to VHS, but in combination with the changes produced by concurrent HeICS appeared to enhance VHS in DSS+HeICS rats. Therefore, we identified the increase of NGF and TRPA1 as potential molecules to target in suppressing VHS in DSS+HeICS rats.
Fig. 6.
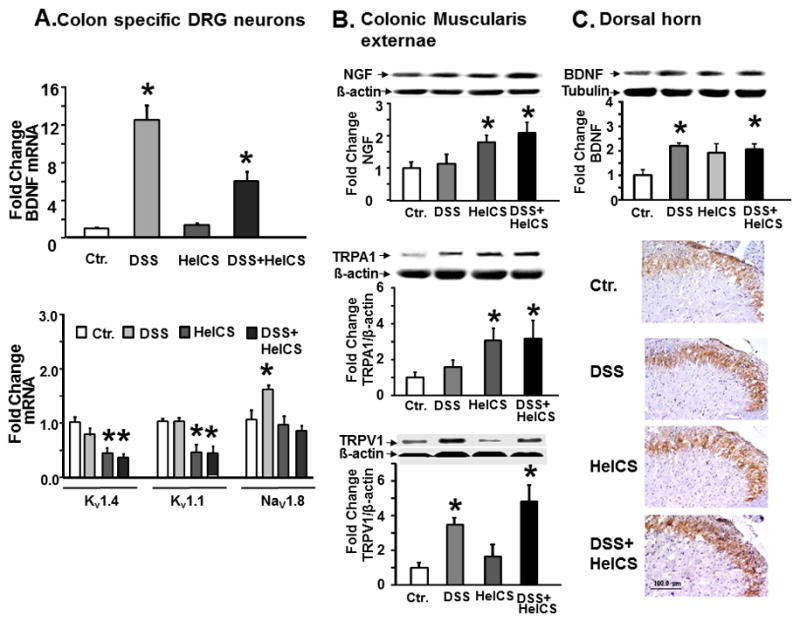
Changes in expression of nociceptive proteins and ion channels in colon-responsive DRG neurons, colon ME, and lumbar-sacral spinal cord. (A) Top panel: DSS inflammation increased BDNF mRNA expression in colon-responsive DRG neurons; the increase persisted on day-16 post-inflammation when HeICS followed DSS treatment. HeICS alone had no effect on BDNF expression. Bottom panel: DSS inflammation had no effect on Kv1.1, Kv1.4 mRNA, but it up-regulated Nav1.8 mRNA in colon responsive S1 DRG neurons. HeICS and HeICS+DSS down-regulated Kv1.1, Kv1.4 mRNA without affecting Nav1.8 mRNA. (B) Top and middle panels: western blots show that DSS had no effect on NGF and TRPA1 protein expression in colonic ME, but HeICS and DSS+HeICs significantly up-regulated both (n=5–6 rats; *P<0.05 vs. Ctr.; #P<0.05 vs. HelCS; @P<0.05 vs. DSS). Bottom panel: western blots show that DSS and DSS+HeICS up regulated the expression of TRPV1 in colonic ME, whereas, HeICS had no effect. (C) Top panel: Western blots show DSS and DSS+HeICS up-regulated BDNF protein in the spinal cord dorsal horn in L6-S2 segments (*P<0.05, n = 5–6). Bottom panel: Immunohistochemical staining for BDNF protein in transverse sections of sacral spinal cord dorsal horns show up-regulation of BDNF by DSS and DSS+HeICS.
Regulation of spinal afferent activity by TRPA1 and NGF
TRPA1 antagonist HC 030031 (50 mg/kg, i.p.) had no significant effect on the increase of spike frequency in response to graded CRD in naïve rats; but it significantly inhibited the increase in spike frequency by CRD in DSS- and DSS+HeICS- rats (Figs. 7A, B and C). Vehicle treatment had no significant effect (Fig. 7D). TRPA1 antagonist also inhibited the increase of VMR to CRD in DSS+HeICS rats, while the vehicle had no effect (Fig. 7E). TRPA1 antagonist had no significant effect on the spike frequency response to CRD in LT fibers in DSS rats (Fig. 7F), but it significantly inhibited the frequency response in HT fibers (Fig. 7G). In DSS+HeICS rats, TRPA1 antagonist inhibited the spike frequency response to a lesser degree in LT than in HT fibers (Fig. 7H and 7J). Treatment of DSS+HeICS rats daily with 16 μg/kg NGF neutralizing antibody i.p. for 5 days significantly suppressed the increase of TRPA1 in the colon ME (Fig. 8A) and VMR to CRD in DSS+HeICS rats (Fig. 8B).
Fig. 7.
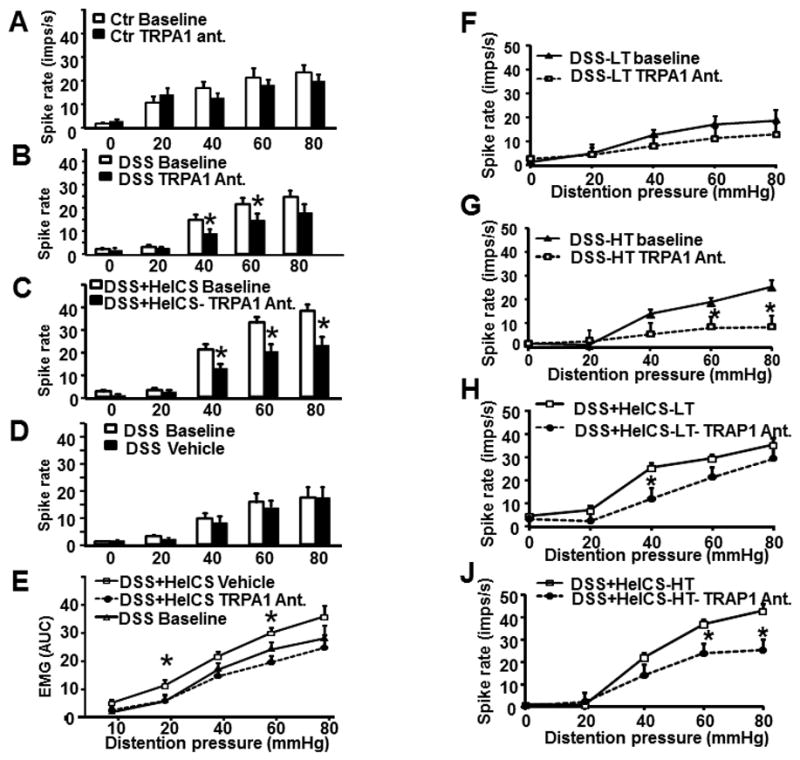
TRPA1 antagonist (HC 030031) attenuated VHS to CRD and colonic afferent activity in response to CRD. (A) HC 030031 significantly reduced the spike rate in naïve controls and (B) in DSS+HeICS rats (n=5–6 rats in each group, *p<0.05 vs. baseline). (C) Vehicle treatment had no effect. (D) HC 030031 significantly reduced VMR to CRD in DSS+HeICS rats. (E –F) HC 030031 suppressed the spike rate of HT fibers but not of LT fibers in DSS rats. (G–H) HC 030031 suppressed the spike rate in LT and HT fibers of DSS+HeICS rats (HT fibers n=15–32; *p<0.05 TRPA1 antagonist vs. each baseline).
Fig. 8.
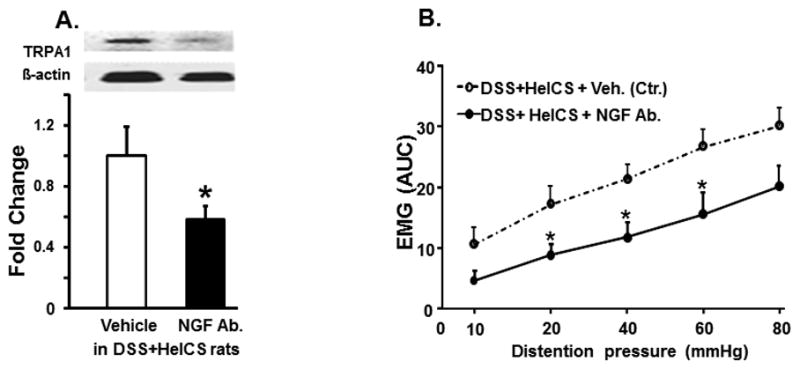
The role of NGF neutralizing antibody on VMR and TRPA1. (A) Systemic treatment of DSS+HeICS rats with NGF neutralizing antibody suppressed the increase of VMR to CRD in DSS+HeICS rats and (B) the expression of TRAP1 in colon ME. * indicates p<0.05 vs. vehicle control, n=6.
DISCUSSION
Our findings show that ulcerative colitis-like inflammation may not sensitize the spinal afferents enough to enhance VHS to CRD. The intensity of DSS inflammation with the dose we used is robust enough to impair smooth muscle function by suppressing its reactivity to acetylcholine (Shi et al., 2011), indicating that the biological effects of inflammation are cell type-specific. By contrast, other reports have shown that TNBS inflammation induces VHS to CRD (Gschossmann et al., 2002, Adam et al., 2006, Lamb et al., 2006, Zhou et al., 2008). This difference may be due to the different type of inflammation induced by DSS and TNBS treatments. DSS inflammation has a dominant component of oxidative stress with minor Th1-type cell recruitment, whereas TNBS inflammation shows a robust transmural increase in Th1-type cells as well as oxidative stress (Shi et al., 2011), suggesting that the pathological effects of inflammation may depend on the profile and intensities of the inflammatory mediators. MPO expression in tissues relates to the intensity of neutrophil granulocyte recruitment/activation. The increase of MPO in TNBS-inflammation is several-fold greater than in DSS-inflammation (Shi et al., 2011). Taken together, it seems that Th1-type inflammatory mediators are more potent than oxidative stress in inducing VHS to CRD in colonic inflammation.
Even though acute inflammation following DSS treatment did not induce VHS, increase of spike frequency and VMR to graded CRD were present when the inflammation had subsided. Chronic stress following DSS treatment further enhanced the spike frequency to graded CRD during the post-inflammation period; however, it did not significantly increase VMR to CRD. The mechanisms of increase of visceral sensitivity during the post-inflammation period following DSS treatment remain unknown.
Most studies on VHS monitored inflammation intensity by visual inspection of the mucosa, immunostaining the mucosa for immunocytes, or measuring MPO activity in full thickness of the colon wall (Larsson et al., 2006, Cattaruzza et al., 2010). However, accumulating evidence from clinical and in vitro electrophysiological studies shows that the peripheral nerve endings of the spinal nociceptive afferents responsive to stretch terminate in the ME/serosa Lembo, 1997 #1537; Brierley, 2004 #1785}. Changes in the immediate microenvironment of the nerve endings have the potential to affect their behavior, rather than changes at somewhat distant locations such as the M/SM tissues. The concentrations of inflammatory mediators and their time-courses differ between the ME and the mucosal/submucosal layers in colonic inflammation (Shi et al., 2011). Therefore, we assayed MPO expression separately in the ME and the M/SM, but correlated VHS with the MPO levels and changes in nociceptive proteins only in the ME. In support of this, we found alterations in the expression of nociceptive proteins in the ME correlate with the alterations in VMR to CRD, as discussed below.
Clinical studies show that chronic stress exacerbates the symptoms in IBD patients (Anton, 1999, Levenstein et al., 2000b). In spite of this understanding, the clinical studies evaluating visceral hyperalgesia in IBD patients did not factor co-existing chronic stress into their analysis, which may account for the variability of findings on VHS among different studies (Rao et al., 1987, Bernstein et al., 1996, Chang et al., 2000, Mayer et al., 2005). Our findings showed that chronic stress enhances the responses of primary afferents to CRD to a greater degree in the presence of underlying ulcerative colitis-like inflammation than in its absence. Chronic stress by itself induced VHS to CRD without causing inflammation in the colonic ME and it did not enhance inflammation induced by DSS.
The transduction of a mechanical event in the colon, such as a contraction or distension, to stimulate pseudoaffective behavior, such as VMR, involves at least two steps. 1) The generation of actions potentials in the peripheral endings of the afferent nerves at a frequency proportional to the amplitude of deformation of neurites and their conduction to the synaptic junctions in the dorsal horn (Brierley et al., 2011). 2) Neurotransmission of the signals generated in spinal cord neurons to the supraspinal structures. Our findings show a complex interplay between the above two factors in generating pseudoaffective responses during ulcerative colitis-like inflammation and chronic stress (Fig. 9). DSS inflammation up regulated the expression of Nav1.8 channels in colon-responsive L6-S1 DRG neurons, TRPV1 in the ME and BDNF in L6-S1 dorsal horns (Fig. 9A and 9B). However, as evidenced by the single fiber recordings from the dorsal roots, the noted increase of Nav1.8 and TRPV1 was unable to elevate the action potential frequency in the afferent neurons (Figs. 2 and 9B). The Nav1.8 channels contribute to the action potential kinetics and conduction (Renganathan et al., 2001, Beyak et al., 2004). Note that the pathological effects of nociceptive molecules depend on the intensity of changes in their expression in response to inflammation. TNBS inflammation increased the expression of Nav1.8 more than three-fold (King et al., 2009). The increase of Nav1.8 in DSS inflammation was less than one-fold, which may explain its inability to affect action potential frequency. The increase in the expression of BDNF in the spinal cord potentiates synaptic neurotransmission (Merighi et al., 2008, Numakawa et al., 2010). However, in the absence of a concurrent increase in action potential frequency in DSS inflammation, this potentiation could not affect the pseudoaffective response (VMR).
Fig. 9.
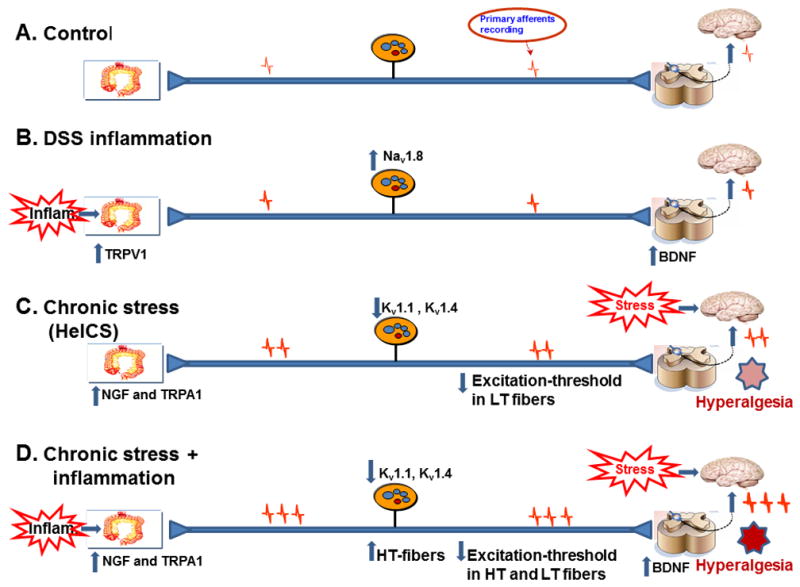
Cartoon showing the changes in expression of pro nociceptive genes in DSS, HeICS and DSS+HeICS vs. control rats to induce VHS. (A) The afferent pathway regulating visceral sensitivity to CRD. (B) DSS inflammation up regulated the expression of TRPV1 In colonic ME, Nav1.8 channels in colon specific spinal afferents and BDNF in the dorsal horn. However, these changes did not induce VHS. (C) HeICS up regulated NGF and TRPA1 in the colonic ME, down regulated Kv1.1 and Kv1.4 channels in colon-responsive afferent neurons and reduced the excitation threshold of LT fibers, which together induced VHS. (D) DSS+HeICS up regulated the expression of NGF and TRPA1 in the colonic ME, down regulated Kv1.1 and Kv1.4 channels in colon specific afferent neurons, recruited additional HT fibers, reduced the excitation threshold of LT and HT fibers and up regulated BDNF in the spinal cord to induce robust VHS that lasts for at least 7 days.
By contrast, chronic stress up regulated the expression of NGF and TRPA1 in the ME and suppressed the expression of Kv1.1 and Kv1.4 channels in colon-responsive DRG neurons (Fig. 9C). Together, these alterations increased the action potential frequency in spinal afferents and even in the absence of a significant increase of BDNF in the dorsal horn, chronic stress increased the VMR to CRD (Fig. 9C). Chronic stress elevates plasma catecholoamines that up regulate the expression of NGF (Winston et al., 2010, Ibeakanma et al., 2011) and NGF antibody blocks visceral hyperalgesia induced by chronic stress (Winston et al., 2010).
Chronic stress depolarizes the colon-responsive DRG neurons, decreases the rheobase, and induces the electrogenesis of action potentials (Winston et al., 2010). We believe the increase in expression of TRPA1 following chronic stress is critical in depolarizing the DRG neurons to make them hyperexcitable by CRD. These channels mediate long-lasting Ca2+ entry and resting calcium in neurons (Inoue et al., 2003). The sustained increase in the expression of TRPA1 channels following chronic stress suggests an increase in the pore density that would increase calcium entry to depolarize the neurons. The increase in expression of TRPA1 increases the intermediately-adapting mechanically-activated current (Brierley et al., 2011). The concurrent suppression of Kv1.1 and Kv1.4 by chronic stress would further increase membrane excitability by modulating both the resting membrane potential and firing rates of action potentials (Yoshimura and de Groat, 1999, Chi and Nicol, 2007). In support of this hypothesis, the down regulation of Kv1.1 by intrathecal administration of its siRNA in naive rats increased the VMR to CRD (Winston and Sarna, 2013). Kv1.1 channels carry the IK current, whereas Kv1.4 channels give rise to IA current. It is noteworthy that inflammatory insult to the stomach, the small intestine and pancreas also reduced the IA and IK currents in the small DRG neurons associated with each organ (Stewart et al., 2003, Dang et al., 2004, Xu et al., 2006). It appears, therefore, that some of the molecular changes inducing visceral hypersensitivity to CRD by inflammatory and non-inflammatory chronic stress may overlap. The molecular changes during concurrent chronic stress and DSS inflammation were a combination of those seen with each stressor alone, which may explain the exacerbation of afferent activity in the presence of both stressors (Fig. 9D).
Our findings suggest that TRPA1-expressing nociceptors in the ME are critical in mechanosensation in non-inflammatory hyperalgesia induced by chronic stress. TRPA1 antagonist significantly suppressed the action potential frequency in response to CRD in DSS+HeICS and naive rats. Other investigators found that deletion of Trpa1 in mice blocked the inflammatory hyperalgesia induced by TNBS (Dai et al., 2007, Brierley et al., 2009, Cattaruzza et al., 2010). However, the VMR to CRD or action potential frequency increase in response to muscular stretch did not differ between the non-inflamed Trpa1+/+ and Trpa1−/− mice, suggesting that TRPA1 channels may not function in naïve mice (Brierley et al., 2009). Our findings show that the increase of NGF in the colon ME following chronic stress up regulates TRPA1.
DSS inflammation and chronic stress differently affected the recruitment of LT and HT fibers and their threshold for excitation. DSS inflammation did not affect the composition of LT and HT fibers or the threshold of their excitation, another reason for the lack of induction of hyperalgesia by this type of inflammation. Chronic stress, on the other hand, increased the percent of LT fibers and reduced their threshold of excitation, which may contribute to the increase of VMR to CRD and increase of action potential frequency in response to CRD in LT fibers. The concurrent application of inflammation and chronic stress showed synergistic effects: a dramatic increase of HT fibers, decrease of excitation threshold of both LT and HT fibers, and elevation of action potential frequency in response to CRD in both LT and HT fibers. The increase in the composition of HT fibers under concurrent inflammation and chronic stress is likely due to the activation of silent nociceptors (Feng and Gebhart, 2011). These synergistic effects explain the exacerbation of visceral hyperalgesia during concurrent inflammation and chronic stress and suggest similar effects in ulcerative colitis patients (Levenstein et al., 2000a).
We conclude that chronic stress induces visceral hypersensitivity without inducing an inflammatory response in the colonic ME that contain the nociceptors. The down regulation of Kv1.1 and Kv1.4 channels in the colon-responsive DRG neurons and up regulation of TRPA1 channels in the colonic ME together sensitize the colon afferent neurons to increase afferent spike frequency and induce visceral hypersensitivity to CRD following chronic stress. By contrast, ulcerative colitis-like inflammation, comprised predominantly of oxidative stress and minor recruitment of Th1 proinflammatory cytokines, up regulates the expression of Nav1.8 channels in colon-responsive DRG, TRPV1 in the ME and BDNF in the spinal cord. However, these alterations are below the threshold to increase the spike frequency in afferent nerves. Even though the up regulation of BDNF in the spinal cord is expected to potentiate neurotransmission, it did not induce visceral hypersensitivity because the firing rates of spinal afferents did not increase. The increase in the recruitment of HT fibers and reduction in the excitation thresholds in HT and LT fibers both exacerbate visceral hypersensitivity during concurrent inflammation and chronic stress. Our findings also show that alterations in nociceptive proteins and ion channels in the muscularis externae correlate with changes in visceral sensitivity to CRD.
Highlights.
Novel findings on visceral hypersensitivity, inflammation and stress
Chronic stress exacerbates hypersensitivity in the presence of inflammation
Chronic stress downregulated select Kv channels in DRG neurons
Chronic stress upregulated TRPA1 and NGF in colon muscularis externae
TRPA1 antagonist suppressed the sensitization of afferent neurons.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Sarah Toombs Smith in proofing the manuscript.
Footnotes
COMPETING INTERESTS
None for all authors
Disclosures
This work was supported by NIDDK Grant 5R01DK032346.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–186. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Anton PA. Stress and mind-body impact on the course of inflammatory bowel diseases. Semin Gastrointest Dis. 1999;10:14–19. [PubMed] [Google Scholar]
- Bernstein CN, Niazi N, Robert M, Mertz H, Kodner A, Munakata J, Naliboff B, Mayer EA. Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain. 1996;66:151–161. doi: 10.1016/0304-3959(96)03062-x. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol. 2011;589:3575–3593. doi: 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O’Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. e2083. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G81–91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Munakata J, Mayer EA, Schmulson MJ, Johnson TD, Bernstein CN, Saba L, Naliboff B, Anton PA, Matin K. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi XX, Nicol GD. Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. J Neurophysiol. 2007;98:2683–2692. doi: 10.1152/jn.00437.2007. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol. 2004;286:G573–579. doi: 10.1152/ajpgi.00258.2003. [DOI] [PubMed] [Google Scholar]
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol. 2011;300:G170–180. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschossmann JM, Adam B, Liebregts T, Buenger L, Ruwe M, Gerken G, Mayer EA, Holtmann G. Effect of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Eur J Gastroenterol Hepatol. 2002;14:1067–1072. doi: 10.1097/00042737-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, Cattaruzza F, Hurlbut D, Vanner S, Bunnett N, Vergnolle N. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098–2108. e2095. doi: 10.1053/j.gastro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Inoue R, Hanano T, Shi J, Mori Y, Ito Y. Transient receptor potential protein as a novel non-voltage-gated Ca2+ entry channel involved in diverse pathophysiological functions. J Pharmacol Sci. 2003;91:271–276. doi: 10.1254/jphs.91.271. [DOI] [PubMed] [Google Scholar]
- King DE, Macleod RJ, Vanner SJ. Trinitrobenzenesulphonic acid colitis alters Na 1.8 channel expression in mouse dorsal root ganglia neurons. Neurogastroenterol Motil. 2009;21:880–e864. doi: 10.1111/j.1365-2982.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G451–457. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- Larsson MH, Rapp L, Lindstrom E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil. 2006;18:144–152. doi: 10.1111/j.1365-2982.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- Levenstein S, Prantera C, Varvo V, Scribano ML, Andreoli A, Luzi C, Arca M, Berto E, Milite G, Marcheggiano A. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000a;95:1213–1220. doi: 10.1111/j.1572-0241.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- Levenstein S, Prantera C, Varvo V, Scribano ML, Andreoli A, Luzi C, Arca M, Berto E, Milite G, Marcheggiano A. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000b;95:1213–1220. doi: 10.1111/j.1572-0241.2000.02012.x. [DOI] [PubMed] [Google Scholar]
- Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med. 2008;8:247–252. doi: 10.2174/156652408784533832. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Rao SS, Read NW, Davison PA, Bannister JJ, Holdsworth CD. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology. 1987;93:1270–1275. doi: 10.1016/0016-5085(87)90255-1. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Shi XZ, Winston JH, Sarna SK. Differential immune and genetic responses in rat models of Crohn’s colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G41–51. doi: 10.1152/ajpgi.00358.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol. 2003;552:797–807. doi: 10.1113/jphysiol.2003.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JH, Sarna SK. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology. 2013;144:570–579. e573. doi: 10.1053/j.gastro.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010;138:294–304. e293. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G424–431. doi: 10.1152/ajpgi.00560.2005. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Dig Dis Sci. 2008;53:429–435. doi: 10.1007/s10620-007-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


