Abstract
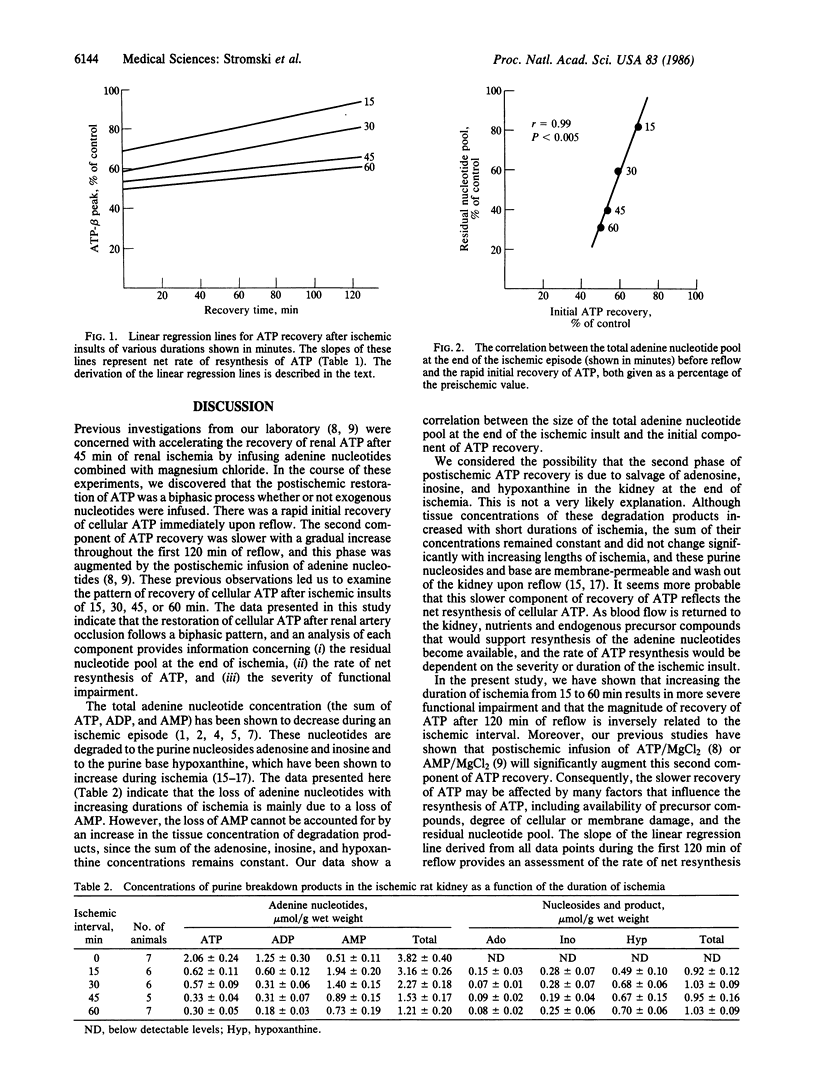
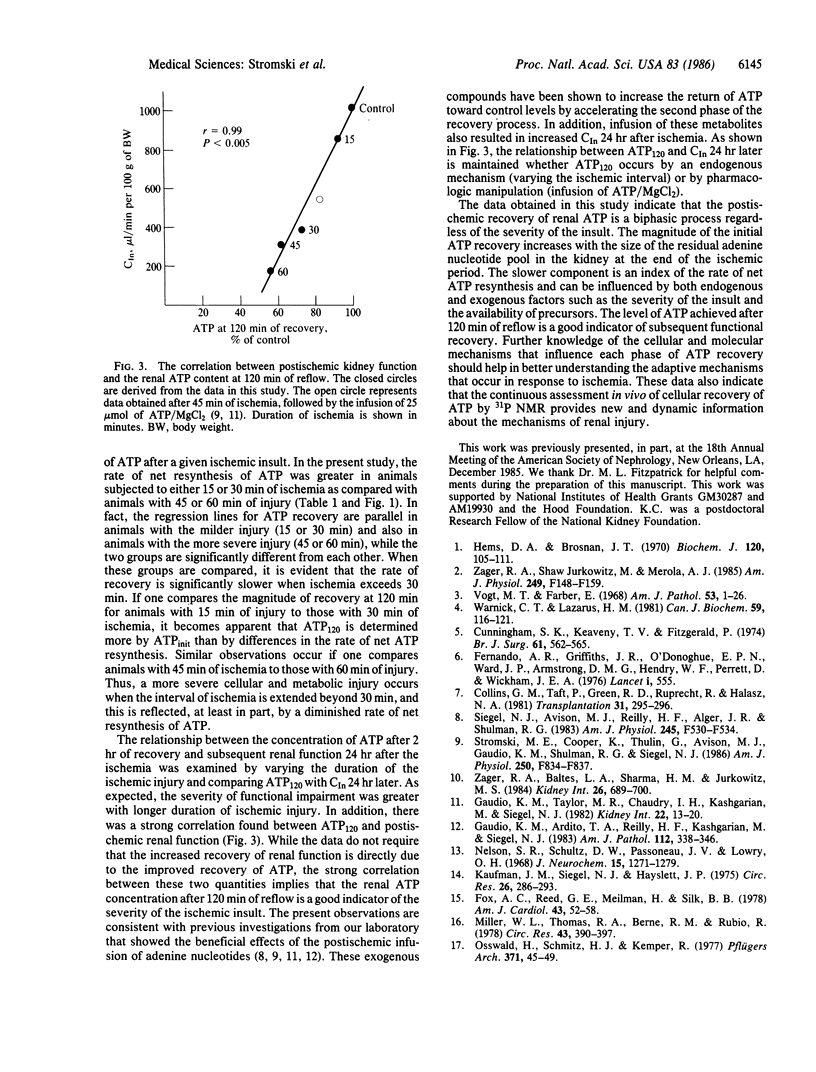
Renal energy metabolism was investigated before, during, and after ischemic insults of varying durations with in vivo 31P NMR spectroscopy. The postischemic recovery of renal ATP was found to be a biphasic process regardless of the length of the ischemia. This two-stage recovery consisted of a quick initial component immediately upon reflow followed by a slower, more gradual return toward preischemic levels. To characterize the source of each phase of the recovery, kidneys were extracted with perchloric acid at the end of the different periods of ischemia (before reflow). Concentrations of adenine nucleotides and breakdown products adenosine, inosine, and hypoxanthine were determined by 1H NMR spectroscopy. Excellent correlation was found between the residual nucleotide pool and the magnitude of the initial phase of ATP recovery. Additionally, the renal ATP content after 120 min of reflow was shown to have a strong correlation with subsequent functional recovery. These experiments show that in vivo 31P NMR can provide new and dynamic information concerning the biochemical recovery from ischemia. Furthermore, this data has the potential to predict the eventual functional recovery of the organ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins G. M., Green R. D., Carter J. N., Halasz N. A. Adenine nucleotide levels and recovery of function after renal ischemic injury. Transplantation. 1981 Apr;31(4):295–296. doi: 10.1097/00007890-198104000-00012. [DOI] [PubMed] [Google Scholar]
- Cunningham S. K., Keaveny T. V., Fitzgerald P. Effect of allopurinol on tissue ATP, ADP and AMP concentrations in renal ischaemia. Br J Surg. 1974 Jul;61(7):562–565. doi: 10.1002/bjs.1800610716. [DOI] [PubMed] [Google Scholar]
- Fernando A. R., Armstrong D. M., Griffiths J. R., Hendry W. F., O'Donoghue E. P., Perrett D., Ward J. P., Wickham J. E. Enhanced preservation of the ischaemic kidney with inosine. Lancet. 1976 Mar 13;1(7959):555–557. doi: 10.1016/s0140-6736(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Fox A. C., Reed G. E., Meilman H., Silk B. B. Release of nucleosides from canine and human hearts as an index of prior ischemia. Am J Cardiol. 1979 Jan;43(1):52–58. doi: 10.1016/0002-9149(79)90044-4. [DOI] [PubMed] [Google Scholar]
- Gaudio K. M., Ardito T. A., Reilly H. F., Kashgarian M., Siegel N. J. Accelerated cellular recovery after an ischemic renal injury. Am J Pathol. 1983 Sep;112(3):338–346. [PMC free article] [PubMed] [Google Scholar]
- Gaudio K. M., Taylor M. R., Chaudry I. H., Kashgarian M., Siegel N. J. Accelerated recovery of single nephron function by the postischemic infusion of ATP-MgCl2. Kidney Int. 1982 Jul;22(1):13–20. doi: 10.1038/ki.1982.126. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. M., Siegel N. J., Hayslett J. P. Functional and hemodynamic adaptation to progressive renal ablation. Circ Res. 1975 Feb;36(2):286–293. doi: 10.1161/01.res.36.2.286. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Thomas R. A., Berne R. M., Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978 Sep;43(3):390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- Nelson S. R., Schulz D. W., Passonneau J. V., Lowry O. H. Control of glycogen levels in brain. J Neurochem. 1968 Nov;15(11):1271–1279. doi: 10.1111/j.1471-4159.1968.tb05904.x. [DOI] [PubMed] [Google Scholar]
- Osswald H., Schmitz H. J., Kemper R. Tissue content of adenosine, inosine and hypoxanthine in the rat kidney after ischemia and postischemic recirculation. Pflugers Arch. 1977 Oct 19;371(1-2):45–49. doi: 10.1007/BF00580771. [DOI] [PubMed] [Google Scholar]
- Siegel N. J., Avison M. J., Reilly H. F., Alger J. R., Shulman R. G. Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol. 1983 Oct;245(4):F530–F534. doi: 10.1152/ajprenal.1983.245.4.F530. [DOI] [PubMed] [Google Scholar]
- Stromski M. E., Cooper K., Thulin G., Avison M. J., Gaudio K. M., Shulman R. G., Siegel N. J. Postischemic ATP-MgCl2 provides precursors for resynthesis of cellular ATP in rats. Am J Physiol. 1986 May;250(5 Pt 2):F834–F837. doi: 10.1152/ajprenal.1986.250.5.F834. [DOI] [PubMed] [Google Scholar]
- Vogt M. T., Farber E. On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol. 1968 Jul;53(1):1–26. [PMC free article] [PubMed] [Google Scholar]
- Warnick C. T., Lazarus H. M. Recovery of nucleotide levels after cell injury. Can J Biochem. 1981 Feb;59(2):116–121. doi: 10.1139/o81-017. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Baltes L. A., Sharma H. M., Jurkowitz M. S. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984 Nov;26(5):689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- Zager R. A., Jurkowitz M. S., Merola A. J. Responses of the normal rat kidney to sequential ischemic events. Am J Physiol. 1985 Jul;249(1 Pt 2):F148–F159. doi: 10.1152/ajprenal.1985.249.1.F148. [DOI] [PubMed] [Google Scholar]



