Abstract
Catechol-O-methyltransferase (genetic locus, COMT) is a major enzyme involved in catecholamine metabolism and has been associated with numerous psychiatric phenotypes. We studied COMT SNPs and haplotypes in cocaine-induced paranoia (CIP) in African-American (AA) and European-American (EA) populations. We genotyped 17 SNPs across the COMT locus in 319 AA pedigrees (848 individuals) and 302 EA pedigrees (707 individuals). Family-controlled association analyses were conducted using FBAT. We found SNP rs737865 to be nominally significantly associated in the AA family population (p=0.05). In EAs, the best-known marker, rs4680 (Val158Met), was nominally significant in additive models (p=0.03). SNP rs174696 also showed nominal significance in additive models (p=0.02). We considered the 3 SNPs (rs737866-rs4680-rs174696) together in haplotype analysis in both family populations, using HBAT. The A-A-T haplotype was significantly associated with CIP in EAs (Z=2.845; p=0.0044, global p=0.020). We then studied COMT SNPs in an additional 738 AA and 404 EA unrelated cocaine dependent individuals with and without paranoia. The A-A-T haplotype was significantly associated to CIP in the AA unrelated population (p=0.0015). Two haplotypes, A-G-C and A-A-C, were significant in the EA unrelated population (p=0.001 and p=0.0003). We also identified rs4680 and three other SNPs, rs933271, rs5993883, and rs740603, as potentially functional variants, as predicted by a signature of positive selection in unrelated EAs and AAs. Based on our robust family-controlled and unrelated-affected analyses, we conclude that COMT is associated with CIP, possibly as a result of its role in the metabolism of dopamine and norepinephrine.
Keywords: COMT, cocaine-induced paranoia, family-based analysis, haplotype, SNP, positive selection
Introduction
Catechol-O-methyltransferase (COMT) plays a major role in brain catecholamine metabolism by catalyzing the transfer of a methyl group from S-adenosyl-methionine (SAM) to catecholamines. COMT maps to chromosome 22q11.21-q11.23, a region of particular interest because it is frequently included in the velocardiofacial syndrome (VCFS) deletion region (Grossman et al. 1992) Numerous genetic associations have been reported to several single nucleotide polymorphism (SNPs) or haplotypes at the COMT locus. These include VCFS-related traits (Bearden et al., 2005; Bearden et al.,2004; Gothelf et al., 2005; Gothelf et al.,2006; Lachman et al., 996), schizophrenia (Lachman et al., 1996; Shifman et al., 2002), anxiety-related personality traits (Stein et al., 2005), pain sensitivity (Diatchenko et al., 2005; Nackley et al., 2006), psychological stress response (Jabbi et al., 2007), and nicotine dependence (Beuten et al.,2006; Guo et al., 2007), among other traits. COMT haplotypes code for varying COMT enzymatic activity (Diatchenko et al., 2005), and associate with variation in mRNA structure and stability (Nackley et al., 2006).
Cocaine is an addictive drug that blocks the synaptic reuptake of catecholamines and serotonin. Its addictive properties are thought to result primarily from its dopamine transporter blocking activity, which leads to dopamine accumulation in the synaptic cleft contributing to the potent reinforcement produced by the drug. In addition to experiencing compulsive cocaine use, cocaine users often report psychiatric symptoms, among the most prominent of which is cocaine-induced paranoia (CIP). Paranoia has been estimated to occur in as many as 68-84% of cocaine dependent patients (Morton, 1999; Satel et al., 1991). In a study evaluating CIP risk in part of the present sample, Kalayasiri et al (Kalayasiri et al., 2006a) reported that 273 of 420 cocaine-dependent probands (65%) experienced paranoia related to cocaine use. CIP risk may be related in part to the higher levels of synaptic dopamine produced by cocaine. Previous studies have suggested that a haplotype in the gene encoding another enzyme involved in dopamine metabolism, dopamine β-hydroxylase (DBH), is associated with low plasma enzyme activity and CIP (Cubells et al., 2000). In addition, a major functional variant at DBH (-1021C→T) (Zabetian et al., 2001) is associated with self-reported paranoia during cocaine self-administration (Kalayasiri et al., 2006b).
Lohoff et al. (2008) reported an allelic and haplotypic association between COMT and cocaine dependence in individuals of African descent. The aim of our study was to examine potential functional variation at the COMT locus in CIP in both European-Americans (EAs) and African-Americans (AAs) by using SNP-haplotype analysis and identifying possible signatures of positive natural selection. These families were recruited for genetic linkage studies of cocaine or opioid dependence (Gelernter et al, 2005; Gelernter et al, 2006). (The first of these studies also identified a significant genetic linkage of CIP to markers on chromosome 9.) On the basis of COMT single SNP analysis, we found several nominally-significant associations. In addition, the rs737866-rs4680-rs174696 haplotype showed significant association in EA families and in both EA and AA unrelated populations. Haplotype A-A-T was associated with CIP in EA families and in unrelated AAs. The A-G-T haplotype frequency differed significantly only in male EA unrelateds based on the presence of CIP.
Materials and methods
DNA samples
319 AA pedigrees (848 persons, counting ungenotyped progenitors) and 302 EA pedigrees (707 persons, also counting ungenotyped progenitors) were recruited from 4 sites: Yale University School of Medicine (Yale; APT Foundation; New Haven, CT), University of Connecticut Health Center (UConn; Farmington, CT), McLean Hospital (Harvard Medical School; Belmont, MA), and Medical University of South Carolina (MUSC; Charleston, SC). These families were recruited on the basis of containing at least one affected sibling pair for cocaine or opioid dependence; additional relatives were recruited where possible (Gelernter et al., 2005; Gelernter et al., 2006). Probands with a clinical diagnosis of a major psychotic illness (schizophrenia or schizoaffective disorder) were excluded. Most subjects had comorbidity with other substances such as tobacco, opiates, or alcohol (Table 1). Nicotine dependence was the most common comorbid substance dependence. Additional unrelated self-identified AA (n=738; male 58% and female 41%) and EA (n=404; male 57% and female 43%) subjects were recruited at Yale, UConn, MUSC and UPenn (University of Pennsylvania School of Medicine). Only significant haplotypes derived from family-based analysis were selected for replication in unrelated subjects.
Table 1.
General information of the African-American and European-American family-based and unrelated samples.
| Family samples | ||
|---|---|---|
|
| ||
| African-American | European-American | |
| Number of individuals | 848 | 707 |
| Number of pedigrees | 319 | 302 |
| Males | 346 | 364 |
| Females | 502 | 343 |
| Cocaine-dependent individuals | 693 (81.7%) | 516 (73.0%) |
| CIP-affected individuals | 440 (51.9%) | 377 (53.3%) |
| Co-morbid substance dependencies | (% from total) | |
| -Co-morbid CIP with tobacco | 32.6% | |
| -Co-morbid CIP with alcohol | 24.9% | |
| -Co-morbid CIP with opiates | 15.2% | |
| -Co-morbid CIP with more than 2 substances | 30.0% | |
|
| ||
| Unrelated samples | ||
|
| ||
| African-American | European-American | |
|
| ||
| Number of individuals | 738 | 404 |
| Cocaine with CIP individuals | 514 (69.7 %) | 266 (65.8%) |
| Males | 314 | 161 |
| Females | 200 | 105 |
| Cocaine without CIP individuals | 224 (30.4%) | 138 (34.2%) |
| Males | 117 | 68 |
| Females | 107 | 70 |
| Co-morbid substance dependencies | (% from total) | |
| - Co-morbid CIP with tobacco | 51.9% | |
| - Co-morbid CIP with alcohol | 43.1 % | |
| - Co-morbid CIP with opiates | 26.7% | |
| - Co-morbid CIP with more than 2 substances | 70.0% | |
All subjects were interviewed using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA) (Gelernter et al., 2005; Pierucci-Lagha et al., 2005). A diagnosis of cocaine dependence was based on DSM-IV criteria, and CIP was defined by an affirmative answer to the question “Have you ever had a paranoid experience when you were using cocaine?” CIP is defined only in the context of cocaine use and most CIP subjects meet criteria for cocaine dependence as well. The overall reliability of this assessment of CIP is excellent (κ = 0.87) (Gelernter et al., 1994; Gelernter et al, 2005; Kalayasiri et al., 2006a). More information on the study sample is reported in Table 1.
For the study of selection, unrelated self-identified AA and EA subjects, screened by SSADDA to exclude psychiatric illness, were recruited at Yale University School of Medicine, University of Connecticut Health Center, and Medical University of South Carolina. All subjects provided informed consent as approved by the appropriate institutional review boards.
SNP genotyping
Most of the family DNA samples were obtained from immortalized cell lines, but some samples were directly extracted from blood or saliva. A total of 17 SNPs across the COMT gene were genotyped in all samples in the family-based study (Table 2). Locations of all SNPs are presented in Figure 1. Fifteen of these SNPs (all except rs737865 and rs165599) were genotyped in the set of unrelated individuals. In the samples obtained from families, we genotyped 3 SNPs, rs737865, rs4680 and rs165599, by TaqMan 5′ nuclease assay available through Applied-Biosystem's Assay-On-Demand service (Applied Biosystems, Foster City, CA). PCR amplifications were conducted using 2 ng of DNA, 1× TaqMan universal PCR master mix (Applied Biosystems, Foster city, CA), and 0.5× SNP genotyping assay mix (Applied Biosystems, Foster city, CA). PCR conditions were as follows: denaturation step of 95°C for 10 min, followed by 65 cycles of at 95°C for 15 sec and 60°C for 1 min. Amplification was performed on PTC-200 cyclers (MJ Research, Hercules, CA) and data were analyzed using the ABI Prism 7900HT Sequence Detector System and software version 2.1 (Applied Biosystems, Foster City, CA). All samples were run in duplicate for quality control purposes; discordant genotypes were discarded. In family and unrelated samples, 15 SNPs (including rs4680, genotyped by both methods) were genotyped by means of a customized Golden Gate SNP array (Illumina, San Diego, CA). For general quality control for this array, 11.5% of all samples were genotyped in duplicate, with no mismatches observed between the two assessments. Based on comparison of the duplicate runs (TaqMan 5′ nuclease and Illumina array genotyping) in 1586 samples, the mismatch rate for rs4680 was 0.19%.
Table 2.
Allele frequencies and additive model p-values of family-based associations between single SNP and cocaine-induced paranoia in African-American (AA) and European-American (EA).
| SNP ID | Allele | Allele frequency | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| AA | EA | AA | EA | |||
| 1 | rs737866 | A/G | 0.83/0.17 | 0.68/0.32 | 0.06 | 0.15 |
| 2 | rs737865 | T/C | 0.80/0.20 | 0.69/0.31 | 0.05 | 0.30 |
| 3 | rs933271 | T/C | 0.59/0.41 | 0.68/0.32 | 0.63 | 0.59 |
| *4 | rs5993883 | T/G | 0.51/0.49 | 0.43/0.57 | 0.32 | 0.92 |
| 5 | rs739368 | C/T | 0.76/0.24 | 0.98/0.02 | 0.16 | - |
| 6 | rs740603 | G/A | 0.50/0.50 | 0.56/0.44 | 0.66 | 0.61 |
| 7 | rs11569715 | C/G | 0.97/0.03 | 0.997/0.003 | - | - |
| 8 | rs11569720 | G/A | 1.00/0.00 | 0.997/0.003 | - | - |
| 9 | rs3218737 | C/T | 1.00/0.01 | 1.00/0.00 | - | - |
| 10 | rs2239393 | A/G | 0.58/0.42 | 0.57/0.43 | 0.12 | 0.43 |
| 11 | rs5031015 | G/A | 0.99/0.01 | 1.00/0.00 | - | - |
| 12 | rs4680 | G/A | 0.72/0.28 | 0.52/0.48 | 0.39 | 0.03 |
| 13 | rs4646316 | C/T | 0.78/0.22 | 0.75/0.25 | 0.75 | 0.33 |
| *14 | rs174696 | C/T | 0.56/0.44 | 0.25/0.75 | 0.53 | 0.02 |
| 15 | rs174697 | G/A | 0.82/0.18 | 0.93/0.07 | 0.82 | 0.09 |
| 16 | rs9332377 | C/T | 0.63/0.37 | 0.81/0.19 | 0.23 | 0.14 |
| *17 | rs165599 | A/G | 0.68/0.32 | 0.35/0.65 | 0.60 | 0.67 |
Bold and italic represent p-value ≤0.05.
Asterisks represent SNPs with different minor allele frequency (MAF) between EAs and AAs.
Figure 1.
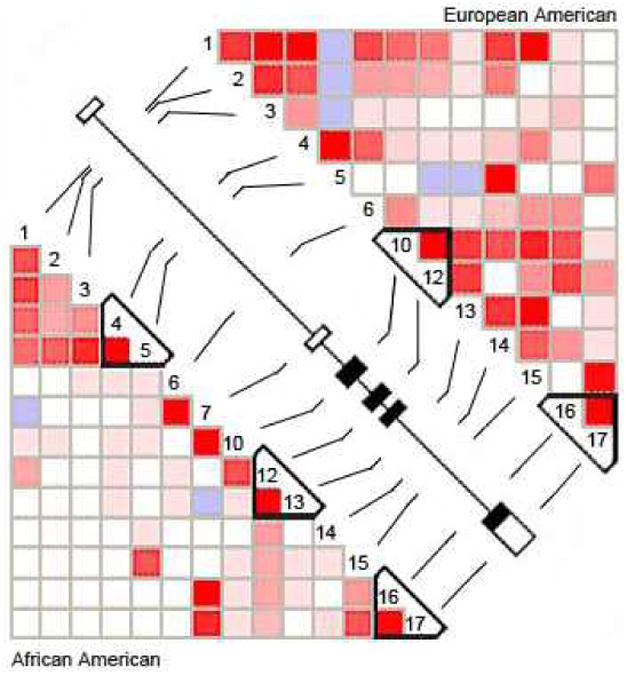
Pairwise LD of COMT gene in African-American (AA) and European-American (EA) families. AAs showed three short blocks and EAs showed two short blocks. Numbers 1 to 17 represent SNPs correlating with rs number in Table II across COMT gene. All COMT exons (the dark box represents coding region and the clear box represent the noncoding region; 3′ and 5′ UTR) shown in the center of picture include the position of the 17 SNPs genotyped in this study. From haploview, the bright red box represents D′ = 1 and LOD = 2, shades of pink/red represent D′ < 1 and LOD = 2, blue represents D′ = 1 and LOD < 2, and white represents D′ < 1 and LOD < 2.
Statistical analysis
CIP study
LD patterns at the COMT locus in EA and AA individuals from the family-based set of subjects were analyzed by HAPLOVIEW version 3.2 (Barrett et al., 2005). Tests of single SNP association with CIP were performed using FBAT (Family-Based Association Tests) (Horvath et al., 2001). HBAT (Haplotype-Based Association Tests) (Horvath et al., 2004) was used for haplotype analysis by focusing on SNPs that yielded a p-value ≤0.05 in single-SNP analyses in each population. We ran SNPSpD using all family members and the approach of Li and Ji (Li and Ji, 2005) to ensure an experiment-wide significance threshold of 5% to control type I error.
The chi-square test was used to compare allele, genotype and haplotype frequencies between groups of unrelated cocaine dependent individuals with and without paranoia. PHASE version 2.1 (Stephens and Scheet, 2005; Stephens et al., 2001), was also used to reconstruct haplotypes for each group.
Selection study
Haplotypes were reconstructed from genotypes using PHASE. A region spanning SNPs 2 through 5 (rs933271, rs5993883, rs739368, and rs740603) of the 15-SNP haplotype was chosen as a core from which to evaluate patterns in homozygosity. This was chosen as a core because the most common 15-SNP haplotype in EAs contains the derived allele state at rs933271, rs5993883, and rs740603. The web-based Extended Haplotype Homozygosity Calculator and Plotter (Mueller and Andreoli, 2004) was used to examine the decay of homozygosity for haplotypes of the core. Sweep software (Sabeti et al., 2002) was used to calculate Relative Extended Haplotype Homozygosity (REHH).
Results
COMT LD patterns in African-American and European-American families
Allele frequencies for the 17 SNPs are presented in Table 2. Low frequency SNPs (minor allele frequency <10%), including rs11569715 (marker 7), rs11569720 (marker 8), rs3218737 (marker 9) and rs5031015 (marker 11)m were excluded from family-based analyses in both AAs and EAs and rs739368 (marker 5) was excluded only from the EA family-based analysis. The LD structure of the COMT gene reconstructed by family-based SNP data set using HAPLOVIEW program (Barrett et al., 2005) is presented in Figure 2. According to the criteria of Gabriel et al. (2002), there were three and two small blocks of LD (less than or equal to 1 kb) observed in AA and EA family populations, respectively. The first block is found only in AAs, and it spans rs5993883 (marker 4) and rs739368 (marker 5). The second LD block spans rs4680 (marker 12) and rs4646316 (marker 13) in AAs, but rs2239393 (marker 10) and rs4680 (marker 12) in EAs. The third LD block, observed in both populations, spans rs9332377 (marker 16) and 165599 (marker 17).
Figure 2.
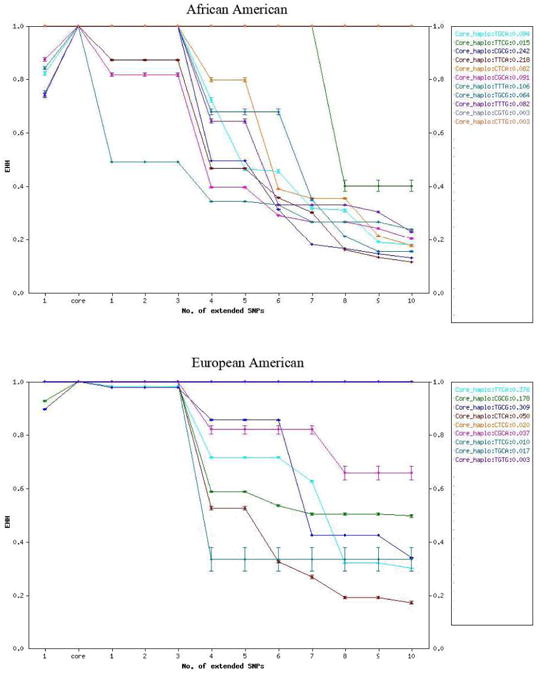
A core region of SNPs 2–5 within the 15-SNP haplotype includes rs933271, rs5993883, rs739368, and rs740603. The two populations display opposite patterns of homozygosity and frequency of haplotypes. The haplotype in which alleles are derived for rs933271, rs5993883, and rs740603 (T–T–C–A) is the most common in EAs and also has higher homozygosity than the three ancestral allele haplotype (C–G–C–G). The three derived allele haplotype is less common than the three ancestral allele haplotype in AAs and also has lower homozygosity. Graphics produced using the web-based Extended Haplotype Homozygosity Calculator and Plotter (http://ihg.gsf.de/cgi-bin/mueller/webehh.pl).
Family-based single SNP association
With the exclusion of rs11569715 (marker 7), rs11569720 (marker 8), rs3218737 (marker 9), and rs5031015 (marker 11) in AA family individuals, thirteen SNPs were included in the association analyses. Twelve SNPs were included in the EA families due to the exclusion of these and one additional SNP, rs739368 (marker 5). As shown in Table 2, rs737865 (marker 2) was nominally significantly associated with CIP only in the AA families (p=0.05). In the EA families, rs4680 (marker 12) and rs174696 (marker 14) showed nominal significance (p=0.03 and p=0.02, respectively). Rs737866 (marker 1) was nearly nominally significant only in AAs (p=0.06) and was nominally significant (p=0.02) in the dominant and recessive models in EAs (data not shown).
Family-based haplotype association
In AA families, only rs737865 was nominally significant; however, three SNPs gave nominally significant (or nearly so) results in EA families: rs737866, rs4680 and rs174696. Therefore, we applied haplotype analysis to the combination of those three SNPs in both family populations. Bonferroni correction for a total of 8 haplotypes yields a significance threshold of p<0.00625. The A-A-T haplotype in the EA family population was significant in the additive model (Z=2.845; p=0.0044; Table 3). In the AA family population, the G-G-T haplotype was significant in the additive (Z=-3.188; p=0.0014) (Table 3). However, the global p-value was significant only in EA families (p=0.020).
Table 3.
Haplotype frequencies and p-value from Haplotype-Based Association Test (HBAT) in cocaine-induced paranoia in African-American and European-American families.
| Haplotype | African- American (global p-value=0.187) | European-American (global p-value=0.020) | ||
|---|---|---|---|---|
| freq. | additive | freq. | additive | |
| rs737866-rs4680-rs174696 | ||||
| A-A-T | 0.203 | 0.6280 0.5299 |
0.347 | 2.8450 0.0044 |
| G-G-T | 0.062 | -3.1880 0.0014 |
0.260 | -1.0410 0.2979 |
| A-G-C | 0.455 | 0.2870 0.7744 |
0.105 | -1.8210 0.0685 |
| A-G-T | 0.147 | 0.7140 0.4754 |
0.138 | 0.5110 0.6095 |
| G-G-C | 0.070 | -0.0470 0.9625 |
0.007 | * |
| A-A-C | 0.053 | 0.3190 0.7496 |
0.107 | -0.6020 0.5472 |
| G-A-T | 0.008 | * | 0.029 | * |
| G-A-C | 0.002 | * | 0.007 | * |
Asterisks (*) represent data excluded from this analysis (for haplotype frequency less than 0.05). After Bonferroni correction for 8 haplotypes in the rs737866-rs4680-rs174696 haplotype analysis, significances at p< 0.00625 (0.05/8) level are marked with bold and underlined.
Association in unrelated individuals
We then performed haplotype analysis in additional unrelated EA and AA cocaine-dependent individuals, comparing subjects with and without CIP. Both populations showed global significance (p=0.017 in AAs and p=0.0001 in EAs). The frequency distribution for haplotype A-A-T differed significantly between CIP+ and CIP- unrelated AA subjects (p=0.0015 in all subjects, p=0.0004 in males only). Haplotypes A-G-C and A-A-C were significantly different by CIP status in both sexes in EA unrelateds (p=0.0010 and p=0.0003, respectively). The frequency distribution of the A-G-T haplotype was significantly different by CIP status after Bonferroni correction only in male EA unrelateds (p=0.0022) (Table 4 and Supplementary material).
Table 4.
Haplotype frequencies and chi-square p-value in unrelated cocaine dependent individuals with and without paranoia in African-American and European-American populations.
| Haplotype | African-American (global p-value=0.017) | European-American (global p-value=0.0001) | ||
|---|---|---|---|---|
| freq. (with/without) | p-value | freq.(with/without) | p-value | |
| rs737866-rs4680-rs174696 | ||||
| 1. A-G-T | 0.123/0.150 | 0.3056 | 0.188/0.196 | 0.6946 |
| 2. A-G-C | 0.462/0.410 | 0.0144 | 0.082/0.102 | 0.0010 |
| 3. A-A-T | 0.196/0.249 | 0.0015 | 0.293/0.343 | 0.0494 |
| 4. A-A-C | 0.077/0.062 | 0.3163 | 0.120/0.083 | 0.0003 |
| 5. G-G-T | 0.050/0.043 | 0.9959 | 0.227/0.243 | 0.7671 |
| 6. G-G-C | 0.083/0.069 | 0.3381 | 0.008/0.011 | 0.6397 |
| 7. G-A-T | 0.001/0.004 | 0.1717 | 0.030/0.004 | 0.0122 |
| 8. G-A-C | - | - | 0.004/0.004 | 0.9665 |
After Bonferroni correction for total 8 haplotypes in rs737866-rs4680-rs174696 haplotype analysis, significance at 0.00625 (0.05/8) level are marked in italic bold.
Selection Study
The most common reconstructed haplotype extended across all 15 SNPs in EAs (haplotype frequency 15%) and the second most common in AAs (haplotype frequency 4%) includes the derived rs4680 Met allele but also the derived alleles at three other SNPs (rs933271, rs5993883, and rs740603). The most common 15-SNP reconstructed haplotype in AAs (haplotype frequency 6%) contains the four ancestral alleles at the same loci. In EAs, this haplotype (i.e., the most common AA haplotype) has a frequency of 3%.
Although we did not find high linkage disequilibrium in this region overall, for a core region spanning these three additional markers in EAs we found higher Relative Extended Haplotype Homozygosity (high LD extending from a high frequency haplotype – a departure from neutrality) for the three-derived-allele haplotype than for the three-ancestral-allele haplotype. In AAs, we found the opposite effect.
Discussion
We found an association between COMT haplotypes and CIP. Numerous previous studies of psychiatric disorders and related phenotypes have demonstrated associations with this gene, most commonly with the nonsynonymous Val108/158Met variant (rs4680). This common functional variant was reported to associate with neurocognitive phenotypes hypothesized to be regulated by dopamine in the prefrontal lobe during working memory in schizophrenic patients and controls (Egan et al., 2001; Meyer-Lindenberg et al., 2005).
Shifman et al. (2002) reported a highly significant association between a COMT haplotype (rs737865-rs4680-rs165599) and schizophrenia. Bray et al. (2003) demonstrated that this haplotype is associated with lower expression of COMT mRNA in postmortem human brain tissue; much of this effect was from SNP rs165599. Chen et al (2004) found a small effect of rs209603, a SNP at the P2 promoter region of MB-COMT, on COMT enzyme activity. A two-SNP haplotype (rs2097603-rs4680) and a 3-SNP combination (rs2097603-rs4680-rs165599) were reported to have a strong impact on prefrontal memory response (Meyer-Lindenberg et al., 2006). In addition, other haplotypes (rs740603-rs4680-rs174699 and rs933271-rs4680-rs174699) showed sex-specific effects for nicotine dependence in AA and EA populations (Beuten et al., 2006). Another study (Lohoff et al, 2008) reported association between haplotype rs737865-rs4680 and cocaine dependence in a primarily African-ancestry population (Lohoff et al.,2008). These SNPs map close to our significant haplotype; rs4680 forms part of the haplotype for which we observed the most interesting results also. Our observations in the EA population were consistent with the study by Stein et al. (Stein et al., 2005) who found an association of COMT variants with the personality traits of neuroticism and extraversion. However, there is no haplotype that has been consistently studied and shown to be associated to these phenotypes. It is clearly the case that Val108/158Met is not the only important functional variant at this locus.
When we considered reconstructed haplotypes composed of 3 SNPs (rs737866-rs4680-rs174696), we found significant haplotypic association with CIP in EA families, and in EA and AA unrelated populations. Different haplotype frequencies in these two populations could explain the distinct association results. However, the A-A-T haplotype associated across EA families and the AA-case-control sample. Our EA family and EA and AA unrelated groups in this study contain variable proportions of affected individuals (53% and 52% in the EA and AA families, respectively, and 68% and 70% in the EA and AA unrelated populations, respectively). Occult population stratification is another possible concern in the unrelated samples. Nonetheless, the sample sizes are reasonably large, providing good statistical power, and the findings are generally consistent across samples and population groups. Additional replication is, however, desirable.
Additionally, we searched for potential functional variants in COMT by identifying possible signatures of positive natural selection. The three derived alleles in the most common EA15-SNP haplotype, in addition to rs4680 (Val108/158Met), may be functional variants, and are candidate risk loci for behavioral phenotypes where the Val108/158Met polymorphism has been implicated but does not fully explain phenotypic variation. We studied the decay over distance of haplotype homozygosity from a core haplotype of these three SNPs within the larger 15-SNP haplotypes. Under neutral conditions it is expected that haplotype frequency is a function of haplotype age; older haplotypes will be higher in frequency than those that have arisen recently. It is also expected that recombination, over time, will result in decreased homozygosity with increasing distance from a core haplotype. Natural selection may explain the departure from neutrality seen in a high frequency core haplotype for which homozygosity has been maintained rather than broken down by recombination relative to other core haplotypes. The SNPs we identified in COMT for which the derived alleles make up a high-frequency haplotype with relatively greater homozygosity than other EA haplotypes are good candidates to contain functional SNPs, although they did not show significance in single SNP association analysis in this study. As in the haplotype association analysis in this study, patterns seen were population-specific.
It is a reasonable conjecture that dopamine metabolism may be related to CIP. A VNTR polymorphism in the dopamine transporter gene (SLC6A3) and variants in the dopamine β-hydroxylase gene (DBH) have previously been shown to be associated with CIP (Cubells et al., 2000; Gelernter et al., 1994; Kalayasiri et al., 2006b). The dopamine transporter protein directly regulates the concentration of dopamine in the synaptic cleft by reuptake to the presynaptic neuron. The enzyme dopamine β-hydroxylase converts dopamine to norepinephrine. The COMT enzyme plays a role in the same pathways by metabolizing dopamine and norepinephrine. Therefore, the association of COMT with CIP is mechanistically consistent with earlier reports by virtue of having an impact on dopaminergic and noradrenergic neurotransmission. This study identifies a third important enzyme in catecholamine metabolism that may be related to CIP, and this should aid in our understanding of the pathophysiology of this trait.
Supplementary Material
Figure 3.
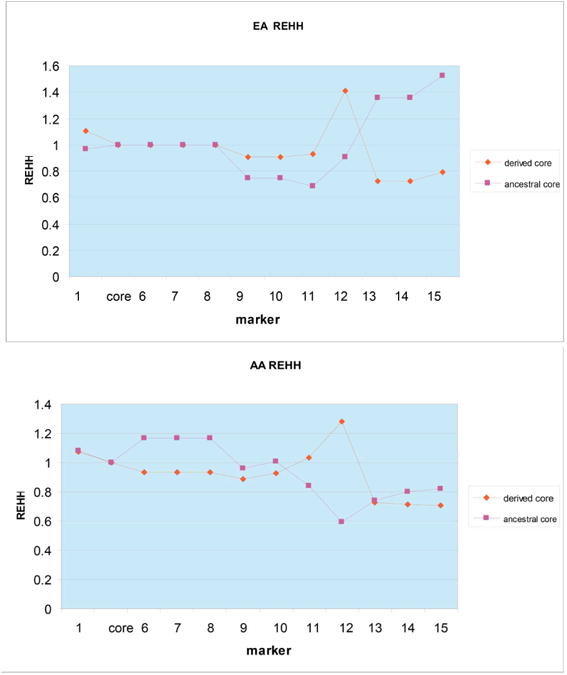
Relative extended haplotype homozygosity (REHH) calculated with Sweep software (http://www.broad.mit.edu/mpg/sweep/index.html) for EA and AA samples. REHH measures the comparative decay of homozygosity for one haplotype among haplotypes of a given core. The 15-SNP haplotypes compared have, for a core region of SNPs 2–5, either derived or ancestral alleles for the core SNPs.
Acknowledgments
Supported by the NIH (NIDA) grants R01 DA12690, R01 DA12849, R01 AA0175350, R01 AA11330, D43 TW06166, and K24 DA022288; GCRC M01 RR06192; and the US Department of Veterans Affairs (the VA Connecticut-Massachusetts Mental Illness Research, Education and Clinical Center (MIRECC)), and families who participated in this study. The authors are thankful for technical help from Ann Marie Lacobelle, Christa Robinson, and Greg Dalton-Key.
Footnotes
Disclosure/Conflict of Interest: Dr. Kranzler has been a paid consultant for Alkermes, GlaxoSmithKline and Gilead. He has received research support from Merck. He also reports associations with Eli Lilly, Janssen, Schering Plough, Lundbeck, Alkermes, GlaxoSmithKline, Abbott, and Johnson & Johnson, as these companies provide support to the ACNP Alcohol Clinical Trials Initiative (ACTIVE) and Dr. Kranzler receives support from ACTIVE
References
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Monterossso JR, Sokol S, McDonald-McGinn DM, Saitta SC, Harris SE, Moss E, Wang PP, et al. Effects of COMT genotype on behavioral symptomatology in the 22q11.2 Deletion Syndrome. Child Neuropsychol. 2005;11(1):109–17. doi: 10.1080/09297040590911239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM, Saitta SC, Harris SE, Moss E, Wang PP, et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry. 2004;161(9):1700–2. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2006;31(3):675–84. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O'Donovan MC. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73(1):152–61. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry. 2000;5(1):56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Satel SL, Rao PA. Genetic association between dopamine transporter protein alleles and cocaine-induced paranoia. Neuropsychopharmacology. 1994;11(3):195–200. doi: 10.1038/sj.npp.1380106. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits: significant linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet B Neuropsychiatr Genet. 2005;136(1):45–52. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, et al. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006;78(5):759–69. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8(11):1500–2. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M, Aviram-Goldring A, Frydman M, Yeshaya J, Shohat M, et al. Association of the low-activity COMT 158Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. Int J Neuropsychopharmacol. 2006:1–8. doi: 10.1017/S1461145706006699. [DOI] [PubMed] [Google Scholar]
- Grossman MH, Emanuel BS, Budarf ML. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1----q11.2. Genomics. 1992;12(4):822–5. doi: 10.1016/0888-7543(92)90316-k. [DOI] [PubMed] [Google Scholar]
- Guo S, Chen da F, Zhou DF, Sun HQ, Wu GY, Haile CN, Kosten TA, Kosten TR, Zhang XY. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology (Berl) 2007;190(4):449–56. doi: 10.1007/s00213-006-0628-4. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype--phenotype associations. Eur J Hum Genet. 2001;9(4):301–6. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol. 2004;26(1):61–9. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, Te Meerman GJ, Ormel J, den Boer JA. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genet. 2007;17(3):183–93. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Kranzler HR, Weiss R, Brady K, Gueorguieva R, Panhuysen C, Yang BZ, Farrer L, Gelernter J, Malison RT. Risk factors for cocaine-induced paranoia in cocaine-dependent sibling pairs. Drug Alcohol Depend. 2006a;84(1):77–84. doi: 10.1016/j.drugalcdep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. Dopamine beta-Hydroxylase Gene (DbetaH) -1021C-->T Influences Self-Reported Paranoia during Cocaine Self-Administration. Biol Psychiatry. 2006b doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95(3):221–7. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Weller AE, Bloch PJ, Nall AH, Ferraro TN, Kampman KM, Pettinati HM, Oslin DW, Dackis CA, O'Brien CP, et al. Association between the catechol-O-methyltransferase Val158Met polymorphism and cocaine dependence. Neuropsychopharmacology. 2008;33(13):3078–84. doi: 10.1038/npp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–6. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, Mattay VS, Egan M, Weinberger DR. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11(9):867–77. 797. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Morton WA. Cocaine and Psychiatric Symptoms. Prim Care Companion J Clin Psychiatry. 1999;1(4):109–113. doi: 10.4088/pcc.v01n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JC, Andreoli C. Plotting haplotype-specific linkage disequilibrium patterns by extended haplotype homozygosity. Bioinformatics. 2004;20(5):786–7. doi: 10.1093/bioinformatics/btg481. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314(5807):1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80(3):303–12. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sabeti PC, Reich DE, Higgins JM, Levine HZ, Richter DJ, Schaffner SF, Gabriel SB, Platko JV, Patterson NJ, McDonald GJ, et al. Detecting recent positive selection in the human genome from haplotype structure. Nature. 2002;419(6909):832–7. doi: 10.1038/nature01140. [DOI] [PubMed] [Google Scholar]
- Satel SL, Southwick SM, Gawin FH. Clinical features of cocaine-induced paranoia. Am J Psychiatry. 1991;148(4):495–8. doi: 10.1176/ajp.148.4.495. [DOI] [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71(6):1296–302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30(11):2092–102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005;76(3):449–62. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68(2):515–22. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


