Abstract
StartectR is a novel anthelmintic combination of derquantel and abamectin. It is hypothesized that derquantel and abamectin interact pharmacologically. We investigated the effects of derquantel, abamectin and their combination on somatic muscle nicotinic acetylcholine receptors and pharyngeal muscle glutamate gated chloride receptor channels of Ascaris suum. We used muscle-strips to test the effects of abamectin, derquantel, and abamectin + derquantel together on the contraction responses to different concentrations of acetylcholine. We found that abamectin reduced the response to acetylcholine, as did derquantel. In combination (abamectin + derquantel), inhibition of the higher acetylcholine concentration responses was statistically greater than the predicted additive effect. A two-micropipette current-clamp technique was used to study electrophysiological effects of the anthelmintics on: 1) acetylcholine responses in somatic muscle and; 2) on L-glutamate responses in pharyngeal preparations. On somatic muscle, derquantel (0.1 - 30 μM) produced a potent (IC50 0.22, CI 0.18-0.28 μM) reversible antagonism of acetylcholine depolarizations. Abamectin (0.3 μM) produced a slow onset inhibition of acetylcholine depolarizations. We compared effects of abamectin and derquantel on muscle preparations pretreated for 30 minutes with these drugs. The effect of the combination was significantly greater than the predicted additive effect of both drugs at higher acetylcholine concentrations. On the pharynx, application of derquantel produced no significant effect by itself or on responses to abamectin and L-glutamate. Abamectin increased the input conductance of the pharynx (EC50 0.42, CI 0.13-1.36 μM). Our study demonstrates that abamectin and derquantel interact at nicotinic acetylcholine receptors on the somatic muscle and suggested synergism can occur.
Keywords: abamectin, derquantel, combination, interaction, nAChRs, GluCls
1. Introduction
Nematode parasites cause severe problems for humans and animals. Globally, >1 billion people are infected with ascariasis, hook worms and whipworms; soil transmitted gastro-intestinal (GI) nematodes. These infections are endemic in a majority of tropical countries [1,2]. Similar GI nematodes also infect most domestic animals. Nematode infections in both humans and livestock result in debility, reduced productivity, severe economic losses and contribute to poverty [3,4].
In the absence of effective vaccines and sanitation, anthelmintic drugs are used for treatment and prophylaxis. Unfortunately, the regular use of anthelmintics has resulted in the emergence of anthelmintic resistance in domestic animals with similar concerns developing for humans. In Australia [6-8], anthelmintic resistance threatens the economics of the entire sheep industry. In humans, helminth isolates (hookworm and schistomiasis) have been have been described which are resistant to anthelmintic treatment [8-11]. The limited number of anthelmintics available for therapy, coupled with the development of resistance in parasites poses a serious threat to livestock and are a concern for human health [4,12].
A majority of anthelmintics exert their effect selectively on membrane ion-channels of nematode parasites. Nicotinic acetylcholine receptors (nAChRs) are present on nematode somatic muscles and nerves. Anthelmintics that act as selective agonists at muscle nAChRs produce spastic paralysis, while selective antagonists produce flaccid paralysis. The nAChR agonists include: the imidazothiazoles (levamisole); the tetrahydropyrimidines (pyrantel & oxantel) [13] and; the amino-acetonitrile derivatives (monepantel) [14]. The nAChR antagonists include the spiroindoles (derquantel, Fig. 1) [15]. Inhibitory glutamate gated chloride channels (GluCls) are present on nematode pharyngeal muscle [16] and are also widely distributed on nematode neurones. The avermectins (ivermectin, abamectin Fig. 1) and milbemycins (moxidectin, milbemycin) increase opening of GluCls [16,17], inhibiting movement, and also pharyngeal pumping and feeding [18].
Fig 1.
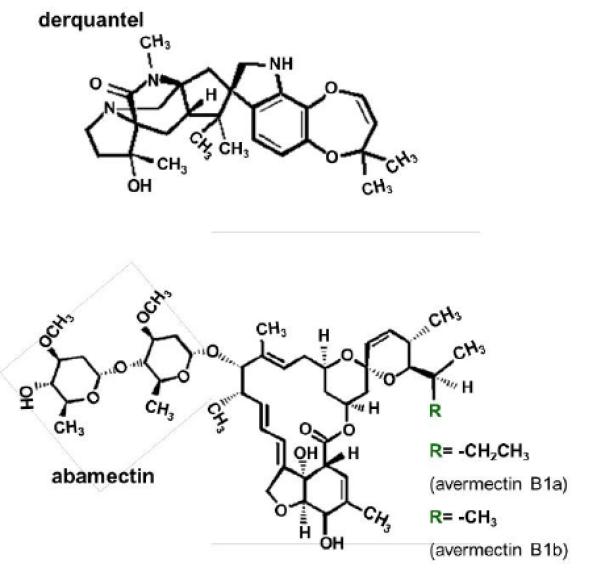
Structures of derquantel and abamectin. (A) Structure of derquantel. (B) Structure of abamectin. Abamectin is a mixture containing more than 80 % avermectin B1a and less than 20 % avermectin B1b. Avermectin B1a differs from avermectin B1b by a functional group at the ‘R’ position.
If control of parasitic nematodes relies on only a single class of anthelmintic drug, the selection pressure for resistance is strong [19]. However, if a combination of anthelmintic drugs is used from different drug classes, the development of resistance is predicted to be slower because simultaneous development of resistance for two classes of anthelmintic is required [20-24]. The combination of two or more anthelmintics also has the potential of producing additive and synergistic effects, increasing the efficacy of the combination.
In this study, we used isolated tissues from A. suum to study effects of derquantel and abamectin. We used somatic muscle flaps for contraction assays. We used somatic muscle flaps and pharyngeal muscles for electrophysiological assays. We studied the effects of derquantel alone, abamectin alone and both in combination; we found that the effects of derquantel and abamectin combined and found that synergism can occur on the nAChRs of the muscle. Derquantel had no effect on the pharynx.
2. Materials and methods
Adult A .suum were collected weekly from the JBS packing plant at Marshalltown, Iowa. Worms were maintained in Locke's solution [composition (mM): NaCl 155, KCl 5, CaCl2 2, NaHCO3 1.5 and glucose 5, at a temperature of 32 °C. The Locke's solution was changed twice daily and each batch of worms was used within 4 days of collection.
2.1.1 Muscle-flap for contraction
We prepared 1 cm muscle body flaps by dissecting the anterior part of the worm, 2–3 cm caudal to the head. Each flap was monitored isometrically by attaching a force transducer in an experimental bath maintained at 37oC containing 10 ml Ascaris Perienteric Fluid Ringer/APF Ringer (mM): NaCl, 23; Na-acetate, 110; KCl, 24; CaCl2, 6; MgCl25; glucose, 11; HEPES, 5; pH 7.6 with NaOH and 0.1% DMSO and bubbled with nitrogen. After dissection, the preparations were allowed to equilibrate for 15 min under an initial tension of 0.5 g. Different concentrations of acetylcholine were then added to the preparation and the maximum contraction observed before washing and subsequent application of the next concentration of acetylcholine. The responses for each concentration were expressed as a % of the maximum tension produced by each individual flap preparation. The effects of abamectin, and derquantel on control acetylcholine dose-response plots were determined. Contraction was monitored on a PC using a MacLab interface. The system allows for recording, displaying and analysis of experimental data. Sigmoid dose-response curves for each individual flap preparation at each concentration of antagonist were described by the Hill equation. The contraction responses were normalized by dividing each response by the mean of all of the responses of the control 100 μM acetylcholine responses (n=15 preparations).
2.1.2 Muscle flap for current-clamp recording
We also prepared the 1 cm muscle body flaps for electrophysiology by dissecting the anterior part of the worm, 2–3 cm caudal to the head which were then pinned onto Sylgard™ contained in a lined double jacketed bath chamber maintained at 35 °C by an inner circulation of warm water (Fisher scientific Isotemp 3016H, PA, USA). The preparation was continuously perfused, with APF-Ringer, composition (mM): NaCl 23, Na-acetate 110, KCl 24, CaCl2 6, MgCl2 5, glucose 11, and HEPES 5; 0.1 % DMSO; NaOH or acetic acid was used to adjust the pH to 7.6. The incoming perfusate was pre-warmed to 35 °C with an in-line heating system (SH 27B Warner instruments, CT, USA) before application. The rate of perfusion was 3.5–4 ml min−1 through a 20 gauge needle placed directly above the muscle bag recorded from. Test compounds were dissolved in APF-Ringer and applied as described in the results. A two-microelectrode current-clamp technique was employed to examine the electrophysiological effects in the bag region of somatic muscle. We used 3 M potassium acetate in the micropipettes which had resistances of 20–30 MΩ. The recordings were made by impaling the bag region of somatic muscle with two microelectrodes, namely current-injecting (I) and voltage-recording electrodes (V). The step current was −40 nA was injected for 500 ms at 0.3 Hz. All experiments were performed using an Axoclamp 2A amplifier, a 1320A Digidata interface and Clampex 9 software (Molecular Devices, CA, USA). All data were displayed and analyzed on a PC based desktop computer. Our somatic muscle preparations had resting membrane potentials greater than −25 mV and the resting input conductances less than 4 μS.
2.1.3 The pharyngeal muscle preparation
The pharynx of Ascaris is a large muscular tube amenable to electrophysiological study. The cuticle and the muscle in the head region were dissected out to expose the pharynx. The beginning of the intestine was pinned to the muscle and the cuticle to secure the pharynx for recording. We increased the stability of the preparation by using calcium-free APF Ringer to limit contraction and by lowering the temperature of the incoming perfusate to 28°C. A microperfusion needle with a flow rate of 3.5–4 ml min was used for perfusion of the pharynx. The recordings were made by impaling at the posterior region of pharynx with two microelectrodes, namely current-injecting (I) and voltage-recording electrodes (V). The step current of −1000 nA was injected for 500 ms at 0.3 Hz. Our pharyngeal muscle preparations had resting membrane potentials greater than −15 mV and the resting conductances less than 250 μS. Test compounds were dissolved in the calcium free APF-Ringer composition (mM): NaCl 23, Na-acetate 110, KCl 24, MgCl25, glucose 11, and HEPES 5; 0.1 % DMSO; NaOH or acetic acid was used to adjust the pH to 7.6.
2.1.4 Drugs
Derquantel (Der) and abamectin (Aba) were provided by Pfizer Animal Health (Pharmacia and Upjohn Co., Kalamazoo, MI). Acetylcholine and the L-glutamic acid monosodium salt hydrate was obtained from Sigma-Aldrich (MO, USA). GABA was obtained from Calbiochem (EMD Serono, Inc, Rockland, MA, USA). Derquantel and abamectin were dissolved in the APF-Ringer at their final concentration in 0.1% DMSO. 0.1% DMSO was tested and found to have no effect on acetylcholine or GABA responses.
2.1.5 Analysis
In the contraction and electrophysiology studies, sigmoid concentration response and additive effect curves were described by the equation:
| equation 1, |
where EC50 is the concentration of agonist (Xa) producing 50% of the maximum response and nH is the Hill coefficient (slope). Prism 5.0 (GraphPad Software, San Diego, CA.) was used to estimate the constants EC50 and nH in equation 1, by non-linear regression for each preparation. For the contraction studies nH was constrained to the value 1.0 since all unconstrained estimates were close to this value. In the electrophysiology, we used parameters which were consistent and measurable across different batches of worms in order to describe and study the responses to the drugs. In somatic muscle preparations we determined changes in resting membrane potential and in pharyngeal preparations we determined changes in the resting conductance. Our experiments were spread across different batches of worms to control for the batch variations.
We calculated the Bliss additive effect dose-response relationship for acetylcholine-contractions and acetylcholine-depolarizations to determine the interaction (antagonistic or synergistic) of derquantel + abamectin on the somatic muscle nAChRs [25]. We determined: mean contraction and depolarization responses to each concentration of acetylcholine (control acetylcholine responses: CR[Ach]); mean responses to each of the concentrations of acetylcholine in the presence of derquantel (derquantel effect) and; mean responses to each concentration of acetylcholine in presence of abamectin (abamectin effect).
We determined the additive effect from the fractional inhibition produced by derquantel alone (mean reduction in response /CR[Ach]) and abamectin alone at each concentration of acetylcholine. The fractional inhibition produced by derquantel alone was denoted Fd and the fractional inhibition produced by abamectin alone was denoted Fa. The fractional inhibition produced by abamectin when derquantel is already present is Fa(1-Fd).
The calculated fractional additive inhibition produced by the mixture of the two drugs is:
The calculated additive response is then:
| equation 2. |
Equation-2 calculates the size of the response when the two drugs are behaving additively [25]. We tested significance of the difference between the additive effect (equation-2) and the observed response for the combination of derquantel and abamectin using the t-test. Statistical analysis were done using Graph Pad Prism software.
Mean ± S.E. values are quoted throughout. Paired t-tests were used to test that control recordings were followed by test recordings from the same cell. Unpaired t-tests were used to compare control and test responses recorded from separate cells or preparations. Confidence levels are expressed as the predicted ± 95% range.
3. Results
3.1.1. Contraction: Inhibitory effects of derquantel and abamectin
Fig 2A shows a representative trace of the effects of adding increasing concentrations of acetylcholine and washing on isometric contractions of an Ascaris muscle flap preparation. When 1 μM derquantel was added there was little or no change in the resting contraction but the responses to the concentrations of acetylcholine were inhibited. The effect of derquantel reached equilibrium within 5 minutes, retesting did not produce further change. When 0.3 μM abamectin was added in addition to derquantel, the responses to acetylcholine were further inhibited. Washing reversed the inhibition but did not completely return the contractions to the control levels even after 10 minutes. We also tested 0.3 μM abamectin by itself and found, Fig. 2B, that it also inhibited the acetylcholine contractions by itself. Fig 2B shows normalized concentration-response plots (mean ± S.E.) for these experiments. The control EC50 for acetylcholine was 7 μM with a Rmax of 102% and the inhibitory effect of 1 μM derquantel was to increase the EC50 to 52 μM with little change in the Rmax to 97%. When 0.3 μM abamectin by itself there was an increase in the EC50 to 18 μM with little change in the Rmax. However, the combination produced greater inhibition with an increase in EC50 to 68 μM and a clear reduction in Rmax to 67%. Washing the preparation only partially reversed the inhibition. Fig 2B also shows the calculated additive effect of derquantel and abamectin (dashed line) and that that the observed inhibition of the combination appears to be greater. The inhibition of the combination was significantly greater than the predicted additive effect at concentrations greater than 10 μM (p<0.05 at 10 μM, p<0.03 at 30 μM & p<0.0001 at 100 μM, n =11). The difference was not significant at lower acetylcholine concentrations. These observations suggest that derquantel and abamectin effects are greater than additive at higher acetylcholine concentrations.
Fig 2.
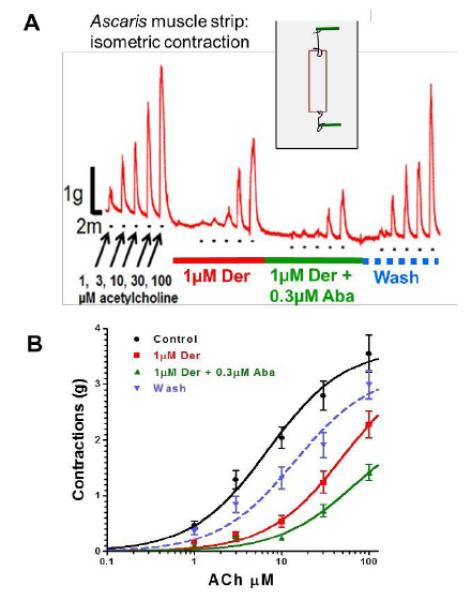
(A) Isometric contraction of Ascaris suum muscle strips produced by application of increasing concentrations of acetylcholine and antagonism by 1μM derquantel (red bar), 1μM derquantel+0.3μM abamectin (green bar) and wash (blue bar). Note that derquantel decreases the responses to acetylcholine and that the addition of abamectin increases the inhibition. (B) The concentration-depolarizing-response plots of acetylcholine showing mean ± S.E. bars. Control ((n=11, black); in the presence of 1μM derquantel (n=11, red); 0.3μM abamectin (n=4,blue); 1μM derquantel+0.3μM abamectin (n=11, green). The predicted additive effect is shown by the dashed line. The derquantel abamectin combination is statistically (p <0.5) more inhibitory than additive at concentrations > 10 μM ACh.
3.1.2.1 Electrophysiology: derquantel inhibits muscle nAChRs
Fig 3A illustrates the experimental arrangement. Derquantel (0.1 - 30 μM) by itself produced little or no significant change in the muscle membrane potential or conductance. However, derquantel had a rapid (within 4 min) reversible inhibitory effect on acetylcholine depolarizations under current-clamp. Fig.3B shows a representative trace of the inhibition produced by short application of 0.1 μM derquantel: the mean depolarizations were decreased from 7.2 ± 1.0 mV to 4.6 ± 0.5 mV (n=9) by derquantel, recovering to 7.0 ± 1.1 mV on wash. The bar chart in Fig. 3C summarizes results from 9 separate preparations: derquantel produced a significant inhibition (p = 0.001) which was reversible on washing (p = 0.004). Fig 3D illustrates the concentration-dependent inhibition of these acetylcholine depolarizations by derquantel and shows that the IC50, was 200 nM (n = 18).
Fig 3.
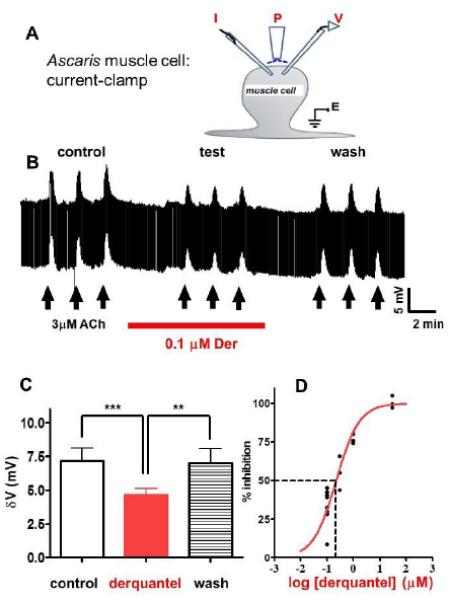
Current-clamp setup for making recordings from the somatic muscle of A .suum. (A) A diagram the position of the positioning of I; current injecting electrode and V; voltage recording electrode for making current clamp recording from the bag region of A .suum muscle cell. P; perfusion needle over the muscle bag for a localized perfusion of APF Ringer and or drugs, E; the earth electrode to complete the circuit. (B) Representative trace shows depolarizations to three control applications of 3 μM acetylcholine (15s) vertical arrows. The applications of acetylcholine were repeated in the presence 0.1 μM derquantel after exposure (4 min) of the preparations to derquantel. (C) Bar chart summarizing the results show a significant reduction in amplitude of control acetylcholine depolarizations in the presence of derquantel (0.1 μM) (p < 0.001). Note that during the wash period the acetylcholine depolarizations recovered when compared to the test (p < 0.01, t-test). (D) Concentration-inhibition plot for derquantel to inhibit 3 μM acetylcholine induced depolarizations. Concentration-inhibition responses were fitted with non-linear regression to determine the IC50. The IC50 determined from this study was 0.2 μM (n = 18).
3.1.2.2 Effects of derquantel on the ACh concentration response
Fig 4A is a representative trace showing the concentration-dependent effects of acetylcholine (0.3 to 30 μM, each applied for 15s) in a control preparation. Fig 4B shows representative trace of responses to acetylcholine applications in the presence of derquantel (1 μM). The control EC50 was 4.5 μM and the maximal response (Rmax) was 21 mV (n = 11), Fig 5. With derquantel the EC50 was 10.2 μM and the Rmax of 14.5 mV (n = 8). The major effect of derquantel was to produce a shift to the right of the EC50, as predicted for a competitive antagonist.
Fig 4.
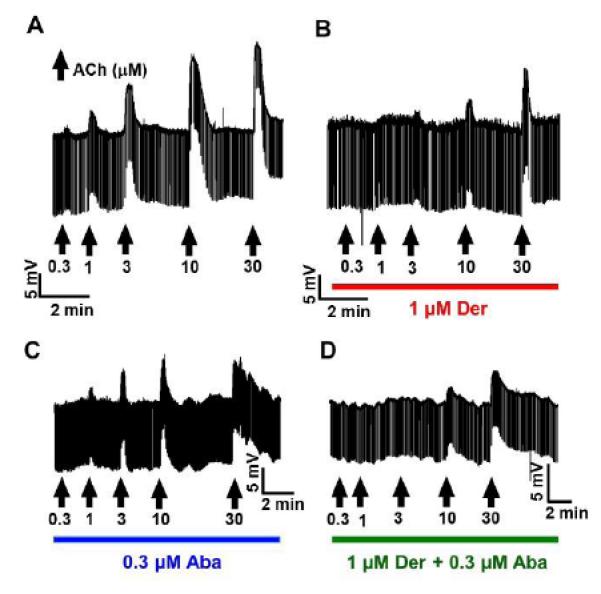
Representative traces showing acetylcholine concentration responses of controls and in the presence of 1 μM derquantel, 0.3 μM abamectin and the combination. Increasing concentrations of acetylcholine (0.3 - 30 μM) shown in black arrows were applied for 15s in controls. In test, the acetylcholine applications followed 30 min pretreatment of the preparations with the drugs. (A) Representative trace, one from 11 such experiments, shows a concentration dependent increase in the depolarizations to the acetylcholine applications in the controls. (B) Representative, one from 8 such experiments trace shows, a reduction in acetylcholine induced depolarizations in the presence of derquantel. (C) Representative trace, one from 9 such experiments shows a reduction in the acetylcholine induced depolarization in the presence of abamectin. (D) Representative trace, one from 7 such experiments shows a reduction in acetylcholine induced depolarizations in the presence of the combination.
Fig 5.
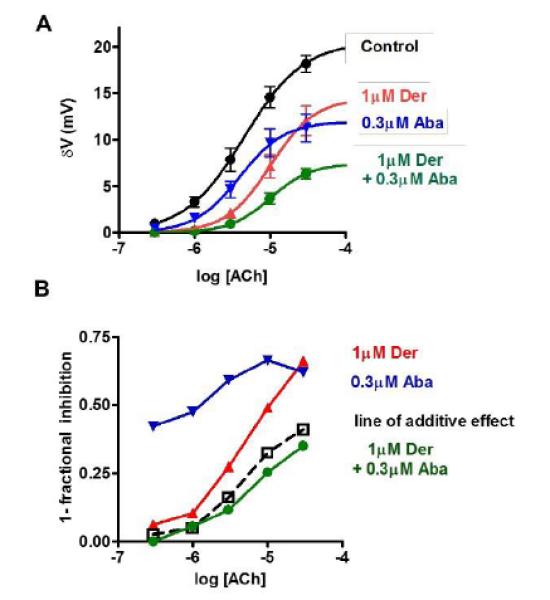
Acetylcholine concentration-depolarization and additive effect response plots in the presence of derquantel, abamectin and the combination. Concentration-depolarization responses for acetylcholine (0.3 - 30 μM, 15 s) in the controls fitted with non-linear regression ●. The test consists of increasing concentrations of acetylcholine were applied after a 30 min pretreatment of the preparations with 1 μM derquantel  , 0.3 μM abamectin
, 0.3 μM abamectin  and the combination (1 μM derquantel + 0.3 μM abamectin)
and the combination (1 μM derquantel + 0.3 μM abamectin)  . The EC50 and Rmax of acetylcholine in the controls was 4.5 μM and 20.6 mV (n = 11). The EC50 and Rmax of acetylcholine in the presence of derquantel was 10.2 μM and 14.5 ± 3.3 mV (n = 8). The EC50 and Rmax of acetylcholine in the presence of abamectin was 4 μM and 12 ± 1.7 mV (n = 9). The EC50 and Rmax of acetylcholine in the presence of the combination was 10.1 μM and 7.5 ± 1.2 mV (n = 7). The additive effect was calculated as described in the methods. The additive Effect showed less inhibition than the observed effect of the combination and reached statistically significance at 3 μM and 30 μM ACh.
. The EC50 and Rmax of acetylcholine in the controls was 4.5 μM and 20.6 mV (n = 11). The EC50 and Rmax of acetylcholine in the presence of derquantel was 10.2 μM and 14.5 ± 3.3 mV (n = 8). The EC50 and Rmax of acetylcholine in the presence of abamectin was 4 μM and 12 ± 1.7 mV (n = 9). The EC50 and Rmax of acetylcholine in the presence of the combination was 10.1 μM and 7.5 ± 1.2 mV (n = 7). The additive effect was calculated as described in the methods. The additive Effect showed less inhibition than the observed effect of the combination and reached statistically significance at 3 μM and 30 μM ACh.
3.1.2.3 Effects of abamectin on the ACh concentration response
Abamectin application by itself produced no significant change in membrane potential (p > 0.05). Fig 4C representative trace shows the effects of abamectin (0.3 μM) on the ACh concentration responses. In the presence of abamectin, the EC50 was 4 μM and the Rmax of 12 mV (n = 9). Comparison with the control, Fig. 5, shows that the major effect of abamectin was to produce a reduction in the Rmax without changing the EC50 in the manner of a non-competitive antagonist (negative allosteric modulator).
3.1.2.4 Combination can produce greater than additive inhibition
The inhibitory effects of 0.3 μM abamectin plus 1 μM derquantel on the acetylcholine responses were greater than those produced by either drug when administered alone, Fig. 4D & Fig. 5. The EC50 was 10.1 μM and the Rmax was 7.5 mV in the presence of the combinations (n = 7). The effect of the combination was to produce both a shift to the right of the EC50 and a reduction in Rmax.
We calculated the additive effect responses (equation 2) and the acetylcholine concentration response relationship, Fig 5 dashed line. The combination was more than additive at 3 μM and 30 μM (p≤0.05). A greater than additive effect is predicted if the two drugs act at separate sites on the acetylcholine receptor: derquantel acting mostly as a competitive antagonist and abamectin acting as a non-competitive antagonist.
3.2. No interaction at GABA receptors on somatic muscle
GABA is an inhibitory neurotransmitter that opens GABA gated chloride channels on the somatic muscle resulting in hyperpolarization. We tested the effects of derquantel, abamectin and the combination, applied for 4 min, on the hyperpolarizing response to GABA (10 μM, applied for 15s). We did not observe a significant (p > 0.05, paired t-test) effect of derquantel (1 μM, n = 4), abamectin (0.3 μM, n = 5) or the combination (1 μM derquantel+ 0.3 μM abamectin, n = 4) on the GABA responses. For example, the test response to GABA was 6.5 ± 1.5 mV and after 4 min in the presence of derquantel and abamectin the GABA response was 5.5 mV ± 1.5 mV, p>0.05 (data not shown). Our study suggests that the combination does not interact on GABA gated chloride receptor channels on the somatic muscle of Ascaris.
3.3.1 Abamectin activates GluCls of the pharynx
The Ascaris pharynx contains inhibitory glutamate-activated chloride channels (GluCls, [16]). Abamectin activated these channels when we applied abamectin for 2 min in cumulative doses in concentrations ranging from 0.01 to 1 μM. It produced hyperpolarization and a concentration-dependent increase in membrane conductance of the pharyngeal muscle, Fig 6B &D. The hyperpolarization varied between preparations and depended on the initial resting membrane potential. The resting input conductance of these preparations was 186.5 ± 18 μC and the change in conductance at the end of 1 μM abamectin (the highest concentration) was 282.6 ± 62.1 μS (n = 4). The EC50 for the concentration-conductance response for abamectin was 0.4 μM.
Fig 6.
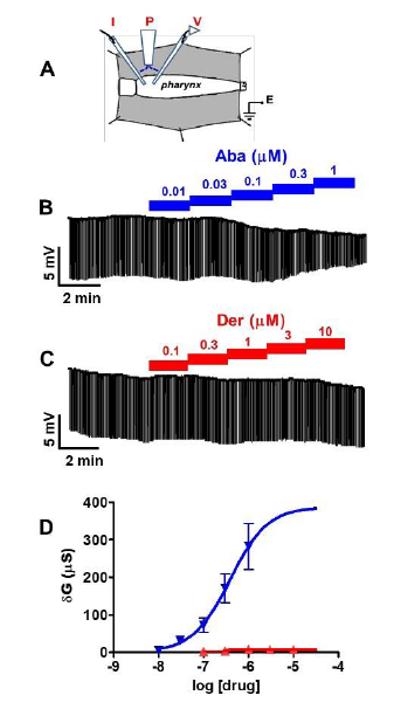
Recording technique used for the pharynx to record the conductance changes produced in response to cumulative applications of abamectin and derquantel in pharyngeal preparations. (A) Diagram of the technique. Positioning of: I, current injecting electrode and; V, voltage recording electrode in the terminal bulb of pharynx. P, perfusion needle above the pharynx for a localized perfusion of calcium free APF Ringer and or drugs E, the earth electrode to complete the circuit. The cumulative applications of abamectin (0.01 to 1 μM) and derquantel (0.1 to 10 μM). (B) Representative trace showing the conductance changes produced in response to cumulative applications of abamectin (C) Representative trace showing the conductance changes produced by the cumulative applications of derquantel. (D) The concentration conductance plots fitted with non-linear regression for abamectin  and derquantel
and derquantel  . The EC50 for abamectin was 400 nM (n = 4). It was not possible to fit non-linear regression for derquantel due to a little or no conductance change produced by derquantel (one sample t-test, n = 4).
. The EC50 for abamectin was 400 nM (n = 4). It was not possible to fit non-linear regression for derquantel due to a little or no conductance change produced by derquantel (one sample t-test, n = 4).
3.3.2. Derquantel does not affect GluCls of the pharynx
Derquantel was applied for 2 min in cumulative doses starting at a concentration of 0.1 and increasing up to10 μM . Fig 6C & D shows that derquantel produces no significant effect on the membrane potential or input conductance of the pharyngeal muscle (n = 4). We also tested the effects of cumulative-concentration application of abamectin in the presence of 0.1 and 1 μM derquantel in each set of 4 preparations. In the presence of 0.1 μM, the EC50 of abamectin was 0.3 μM; in the presence of 1 μM derquantel the EC50, was also 0.3 μM Derquantel did not change Log EC50 values for abamectin (p > 0.05, F-test) suggesting that derquantel does not interact with abamectin at the GluCls of the pharynx.
We tested the effect of derquantel on the conductance changes produced by applications of 10 μM and 100 μM L-glutamate for 15s. Fig 7 shows a representative trace of the L-glutamate control responses and L-glutamate responses in the presence of 1 μM derquantel. The input conductance response to 10 μM L-glutamate was 32 ± 8 μS (n = 4) and to 100 μM L-glutamate it was 126 ± 43 μS ( (n = 4). In the presence of 1 μM derquantel, the input conductance response to 10 μM L-glutamate was 51 ± 18 μS (n = 4) and the response to 100 μM L-glutamate was 116 ± 37 μS ( (n = 4). The presence of derquantel did not significantly change the L-glutamate (10 or 100 μM) responses (p > 0.1, paired t-test). These observations suggest that derquantel does not interact with L-glutamate at the GluCls of the pharynx.
Fig 7.
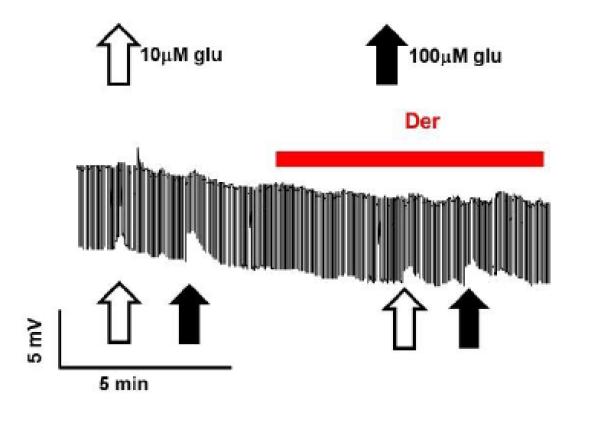
L-glutamate responses in the presence of derquantel in pharynx. Unfilled and filled arrows represents 15s applications of 10 μM L-glutamate and 100 μM L-glutamate respectively. The applications of L-glutamate followed after 4 min of 1 μM derquantel treatment. Representative trace shows control L-glutamate responses and those in the presence of derquantel (1 μM). Note that there is no effect of derquantel on the L-glutamate responses.
4. Discussion
4.1. Derquantel is a potent competitive nematode nAChR antagonist
Robertson et al. [15] used an Ascaris muscle strip contraction assay to describe the antagonistic effects of derquantel against a panel of cholinergic anthelmintics. Derquantel behaved like a competitive antagonist. The pA2 values for nicotinic agonists and cholinergic anthelmintics [26,27] were: methyridine (5.3), oxantel (5.4), thenium (5.5), levamisole (5.7), pyrantel (5.9), nicotine (6.3) and bephenium (6.5). Analyses of these observations lead to the conclusion that there are 3 subtypes of nAChR present in Ascaris muscle strips. There is the N-subtype preferentially activated by nicotine and oxantel; there is the L-subtype preferentially activated by levamisole and; the B-subtype preferentially activated by bephenium. In our present contraction studies with acetylcholine, we found that 1 μM derquantel shifted the acetylcholine EC50 from 7 μM to 52 μM giving a dose-ratio of 7.4 and pA2 of 6.8 (Schild equation). Interestingly, the pA2 is closest to bephenium, suggesting that acetylcholine, a quaternary ammonium like bephenium, may stimulate the B-subtype of nAChR during the contraction studies [27]. The potent inhibitory effects of derquantel in our Ascaris (Clade III nematode) contraction assays, is comparable to the potent inhibitory effects of derquantel seen in Clade V nematode parasites. For example, in T. colubriformis motility is inhibited by 100 nM derquantel [28] and in H. contortus, the EC50 for inhibition in motility assays is 200 nM [29].
In our electrophysiological studies we found, like Zinser et al [28], that derquantel has a rapid onset of action and no effect on membrane potential or conductance of the Ascaris somatic muscle, and that derquantel is a potent acetylcholine antagonist. In the electrophysiological studies, 1 μM derquantel shifted the acetylcholine concentration-depolarization plots to the right: the EC50 values were shifted from 4.5 μM to 10.2 μM giving a calculated pA2 of 6.1. The pA2 value of 6.1 is less than we saw in the contraction studies and may be explained if the mixture of subtypes of nAChR associated with contraction contains more B-subtypes than the nAChRs on the bag region of the Ascaris muscle which contain more L-subtypes [30]. The speed of action of derquantel and competitive mode of action is consistent with a site of action in the extracellular aqueous phase of the nAChR ion-channel.
In contrast to the effects on the somatic muscle, we found that derquantel had little or no effect on the GABA induced hyperpolarizations of somatic muscle or on effects of glutamate or abamectin on the pharynx. The potent effects on muscle nAChRs and the lack of effect on GABA receptor and GluCls provide further evidence that the somatic muscle nAChRs are the major target site for the therapeutic action of derquantel.
4.2. Effects of abamectin on the somatic muscle
Application of abamectin produced a slowly developing non-competitive antagonism of acetylcholine depolarizations and muscle contraction; the effect was to produce a reduction in the maximum response without a shift in the EC50. This inhibitory effect of abamectin, initially, was unexpected since a potentiating (positive allosteric effect) of Ivermectin is usually thought of as being selective for vertebrate α7 nAChRs [31,32]. This action of ivermectin, a macrocyclic lactone like abamectin, as positive allosteric modulator on nAChRs involves the transmembrane domains (TM1, TM2 and TM3) of the ion channel [33] as it does for the GluCls [34]. Interestingly however, the positive allosteric effect of ivermectin on α7 nAChRs can be converted to an antagonist by one of three mutations (S222M, M253L, and S276V located in TM1, TM2, and TM3 regions) [33]. With these mutations, ivermectin behaves like a non-competitive antagonist of the nAChRs that has no effect on the EC50 but reduced the maximum response in a manner similar to that we recorded for abamectin. Ivermectin also behaves as an inhibitor of C. elegans ACR-16 nAChRs expressed in Xenopus oocytes [34]. Thus abamectin may act like ivermectin in the outer lipid phase of the membrane to combine with a lipophilic region of the ion-channel [35,36] and depending on the nAChR subtype, act as a non-competitive antagonist (negative allosteric modulator). A site of action on the nAChR within the lipid phase of the membrane is consistent with a slow onset action. Further studies are required to identify which of the Ascaris somatic nAChR subunits are sensitive to abamectin
4.3. Effects of the combination on the somatic muscle
We selected 0.3 μM abamectin for testing based on the reported concentration inside H. contortus following a therapeutic dose of abamectin [37] which was 0.2 μM at 12 hours. We selected 1 μM derquantel for testing based on reports of intestinal (extra-worm) concentration of 13 μM at 12 hours [38] and the likely decrease in concentration of the drug within the worm [40]. We have seen that the effects of derquantel have a rapid onset and the drug behaves in a competitive manner with acetylcholine, suggesting a site of action on the extracellular surface of the nAChR. Abamectin is slower in onset, non-competitive and may have a site of action within the lipid phase of in the transmembrane region of the nAChR. When the two inhibitors are applied together the action at the two different putative sites of action on the nAChR combine and the effects are greater than additive at high (> 10 μM) concentrations of acetylcholine. At vertebrate neuro-muscular junctions, the released acetylcholine concentration can reach as high as 5 mM [40]. Hence, the inhibition of acetylcholine response at the somatic muscle of the worm produced by the combination may be higher than we have observed here. The inhibitory effects of the combination on the somatic muscle may translate into a greater therapeutic effect than is achievable using either of the individual drugs alone.
4.3. Effects of abamectin on the pharynx
The Ascaris nematode pharynx contains glutamate gated chloride channels (GluCls) which are the targeted by avermectin anthelmintics [16]. In C. elegans, glutamate mediates the hyperpolarizing actions of the inhibitory motor neuron M3 on the pharyngeal muscle [41]. GluCl activation is implicated in reducing locomotion mediated via command interneurons [17]. Single channel studies from the pharyngeal muscle vesicles of A. suum also show that the GluCl channels are activated by glutamate and ivermectin [42]. Previous studies on the GluCls show that they are sensitive low concentrations (nanomolar) of avermectin anthelmintics. The EC50 values for ivermectin in C .elegans GluCls were 0.1 - 0.2 μM [43] and H .contortus GluCls expressed in Xenopus oocytes had EC50 in the 0.1 - 1nM range [44]. In our pharyngeal preparations, abamectin produced hyperpolarization and a concentration dependent increase in the input conductance we attribute to the opening of GluCls of the pharynx. Abamectin had an EC50 of 0.4 μM showing that pharynx is sensitive to low concentrations of abamectin. The potency of avermectin anthelmintics on nematode worms is a combined effect of endogenous glutamate complimented by exogenous avermectins. The result is a massive increase in the chloride conductance of the pharyngeal muscle through irreversible opening of GluCls [17,45,46].
4.5. Effects of derquantel on the pharynx
Derquantel had no effect on the resting membrane conductance or on the conductance change produced by glutamate or abamectin. These observations suggest that derquantel has no effect on their GluCls and that GluCls are not involved in the mode of action of derquantel.
4.6. Anthelmintic resistance and the use of combination drugs in therapy
Anthelmintic resistance is a concern in parasitic nematodes of both humans and livestock [4,12,47]. Resistance limits the efficacy of current anthelmintics [48]. Electrophysiological studies in A. suum somatic muscle suggest that derquantel preferentially antagonizes the B-subtype of nAChRs [15]. Our studies show that abamectin can potentiate the effects of derquantel in the somatic muscle of A .suum.
Studies in Australia show that, resistance to both broad- and narrow-spectrum anthelmintics is widespread [49]. Suitable anthelmintic combinations favor elimination of those parasitic nematodes that carry resistant genes to only one of the anthelmintics. Despite the limitations of anthelmintic combinations being expensive, it is becoming clearer that the combinations should be used before resistance levels climb too high [50]. The use of combination therapy is also favored when the two anthelmintics have additive or synergistic effects. The combination of anthelmintics is less likely to be as successful if the compounds have an inhibitory effect on each other or if resistance to one of the combination were already present.
4.7 Conclusion
We have studied the interaction of derquantel and abamectin a novel anthelmintic combination. Our study focused on two important anthelmintic drug target sites in the worm namely the somatic muscle nAChRs and the pharyngeal GluCls. Our experiments on worm somatic muscle demonstrate that abamectin acts non-competitively at the nAChRs of somatic muscle and potentiates the competitive antagonism produced by derquantel. It is anticipated that the combination will allow a slower rate of development of resistance than either of the two drugs alone. The introduction of anthelmintic drugs with new mechanisms or new combinations will help move ahead the battle against the evolving resistance appearing in nematode parasites.
Acknowledgements
The authors acknowledge the support of NIH NIAID RO1 AI947194 to R.J.M, NIH NIAID 1R21AI092185-01A1 to APR and funding from Pfizer Animal Health to R.J.M and A.P.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
References
- 1.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 2.Savioli L, Albonico M. Soil-transmitted helminthiasis. Nat Rev Microbiol. 2004;2:618–619. doi: 10.1038/nrmicro962. [DOI] [PubMed] [Google Scholar]
- 3.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JR, Wroth R, de Chaneet GC, Besier RB, Karlsson J, Morcombe PW, Dalton-Morgan G, Roberts D. Survey of anthelmintic resistance in Western Australian sheep flocks. 1. Prevalence. Aust Vet J. 1986a;63:135–138. doi: 10.1111/j.1751-0813.1986.tb02950.x. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JR, Wroth R, de Chaneet GC, Besier RB, Karlsson J, Morcombe PW, Dalton-Morgan G, Roberts D. Survey of anthelmintic resistance in Western Australian sheep flocks. 2. Relationship with sheep management and parasite control practices. Aust Vet J. 1986b;63:139–144. doi: 10.1111/j.1751-0813.1986.tb02951.x. [DOI] [PubMed] [Google Scholar]
- 7.Jackson F, Coop RL. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120(Suppl):S95–107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J. Failure of mebendazole in treatment of human hookworm infections in the southern region of Mali. Am J Trop Med Hyg. 1997;57:25–30. doi: 10.4269/ajtmh.1997.57.25. [DOI] [PubMed] [Google Scholar]
- 9.Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 10.Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 11.Reynoldson JA, Behnke JM, Pallant LJ, Macnish MG, Gilbert F, Giles S, Spargo RJ, Thompson RC. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta Trop. 1997;68:301–312. doi: 10.1016/s0001-706x(97)00106-x. [DOI] [PubMed] [Google Scholar]
- 12.Geary TG. Are new anthelmintics needed to eliminated human helminthiases? Current Opin Infect Dis. 2012;25:709–717. doi: 10.1097/QCO.0b013e328359f04a. [DOI] [PubMed] [Google Scholar]
- 13.Martin RJ, Robertson AP. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. doi: 10.1017/S0031182007000029. [DOI] [PubMed] [Google Scholar]
- 14.Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, Weber SS, Wenger A, Wieland-Berghausen S, Goebel T, Gauvry N, Pautrat F, Skripsky T, Froelich O, Komoin-Oka C, Westlund B, Sluder A, Maser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- 15.Robertson AP, Clark CL, Burns TA, Thompson DP, Geary TG, Trailovic SM, Martin RJ. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J Pharmacol Exp Ther. 2002;302:853–860. doi: 10.1124/jpet.102.034272. [DOI] [PubMed] [Google Scholar]
- 16.Martin RJ. An electrophysiological preparation of Ascaris suum pharyngeal muscle reveals a glutamate-gated chloride channel sensitive to the avermectin analogue, milbemycin D. Parasitology. 1996;112:247–252. doi: 10.1017/s0031182000084833. Pt 2. [DOI] [PubMed] [Google Scholar]
- 17.Wolstenholme AJ, Rogers AT. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85–95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- 18.Sheriff JC, Kotze AC, Sangster NC, Martin RJ. Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology. 2002;125:477–484. doi: 10.1017/s0031182002002251. [DOI] [PubMed] [Google Scholar]
- 19.Sangster N. A practical approach to anthelmintic resistance. Equine Vet J. 2003;35:218–219. doi: 10.2746/042516403776148174. [DOI] [PubMed] [Google Scholar]
- 20.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes EH, Dobson RJ, Barger IA. Worm control and anthelmintic resistance: adventures with a model. Parasitol Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 22.Coles GC. Anthelmintic resistance--looking to the future: a UK perspective. Res Vet Sci. 2005;78:99–108. doi: 10.1016/j.rvsc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Smith G. A mathematical model for the evolutions of anthelmintic resistance in a direct life cycle nematode parasite. Int J Parasitol. 1990;20:913–921. doi: 10.1016/0020-7519(90)90030-q. [DOI] [PubMed] [Google Scholar]
- 24.Stepek G, Buttle DJ, Duce IR, Behnke JM. Human gastrointestinal nematode infections: are new control methods required? Int J Exp Pathol. 2006;87:325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greco WR, Faessel H, Levasseur L. The search for cytotoxic synergy between anticancer agents: a case of Dorothy and the ruby slippers? J Natl Cancer Inst. 1996;88:699–700. doi: 10.1093/jnci/88.11.699. [DOI] [PubMed] [Google Scholar]
- 26.Martin RJ, Bai G, Clark CL, Robertson AP. Methyridine (2-[2-methoxyethyl]-pyridine]) and levamisole activate different ACh receptor subtypes in nematode parasites: a new lead for levamisole-resistance. Br J Pharmacol. 2003;140:1068–1076. doi: 10.1038/sj.bjp.0705528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin RJ, Clark CL, Trailovic SM, Robertson AP. Oxantel is an N-type (methyridine and nicotine) agonist not an L-type (levamisole and pyrantel) agonist: classification of cholinergic anthelmintics in Ascaris. Int J Parasitol. 2004;34:1083–1090. doi: 10.1016/j.ijpara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Zinser EW, Wolf ML, Alexander-Bowman SJ, Thomas EM, Davis JP, Groppi VE, Lee BH, Thompson DP, Geary TG. Anthelmintic paraherquamides are cholinergic antagonists in gastrointestinal nematodes and mammals. J Vet Pharmacol Ther. 2002;25:241–250. doi: 10.1046/j.1365-2885.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson SS, Coscarelli EM, Davis JP, Zaya RM, Day JS, Barsuhn CL, Martin RA, Vidmar TJ, Lee BH, Conder GA, Geary TG, Ho NF, Thompson DP. Interrelationships among physicochemical properties, absorption and anthelmintic activities of 2-desoxoparaherquamide and selected analogs. J Vet Pharmacol Ther. 2004;27:169–181. doi: 10.1111/j.1365-2885.2004.00577.x. [DOI] [PubMed] [Google Scholar]
- 30.Qian H, Martin RJ, Robertson AP. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006;20:2606–2608. doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 31.Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- 33.Collins T, Millar NS. Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Mol Pharmacol. 2010;78:198–204. doi: 10.1124/mol.110.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond V, Mongan NP, Sattelle DB. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken α7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000;101:785–791. doi: 10.1016/s0306-4522(00)00279-7. [DOI] [PubMed] [Google Scholar]
- 35.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RJ, Kusel JR. On the distribution of a fluorescent ivermectin probe (4" 5,7 dimethyl-bodipy proprionylivermectin) in Ascaris membranes. Parasitology. 1992;104:549–555. doi: 10.1017/s0031182000063812. Pt 3. [DOI] [PubMed] [Google Scholar]
- 37.Lloberas M, Alvarez L, Entrocasso C, Virkel G, Ballent M, Mate L, Lanusse C, Lifschitz A. Comparative tissue pharmacokinetics and efficacy of moxidectin, abamectin and ivermectin in lambs infected with resistant nematodes: Impact of drug treatments on parasite P-glycoprotein expression Parasitology. Drugs & Drug Resist. 2013;3:20–27. doi: 10.1016/j.ijpddr.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedlander LS, Le Bizec B, Swan GI. Derquantel. 2012 ftp://ftp.fao.org/ag/agn/jecfa/vetdrug/12-2012-derquantel.pdf.
- 39.Ruiz-Lancheros E, Viau C, Walter TN, Francis A, Geary TG. Activity of novel nicotinic anthelmintics in the cut preparation of Caenorhabditis elegans. International Journal of Parasitology. 2011;41:455–461. doi: 10.1016/j.ijpara.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Jin T. Near-Infrared Fluorescence Detection of Acetylcholine in Aqueous Solution Using a Complex of Rhodamine 800 and p-Sulfonato-calix[8]arene. Sensors. 2010;10:2438–2449. doi: 10.3390/s100302438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avery L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J Exp Biol. 1993;175:283–297. doi: 10.1242/jeb.175.1.283. [DOI] [PubMed] [Google Scholar]
- 42.Adelsberger H, Scheuer T, Dudel J. A patch clamp study of a glutamatergic chloride channel on pharyngeal muscle of the nematode Ascaris suum. Neurosci Lett. 1997;230:183–186. doi: 10.1016/s0304-3940(97)00512-0. [DOI] [PubMed] [Google Scholar]
- 43.Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 44.McCavera S, Rogers AT, Yates DM, Woods DJ, Wolstenholme AJ. An ivermectin-sensitive glutamate-gated chloride channel from the parasitic nematode Haemonchus contortus. Mol Pharmacol. 2009;75:1347–1355. doi: 10.1124/mol.108.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hejmadi MV, Jagannathan S, Delany NS, Coles GC, Wolstenholme AJ. L-glutamate binding sites of parasitic nematodes: an association with ivermectin resistance? Parasitology. 2000;120:535–545. doi: 10.1017/s0031182099005843. Pt 5. [DOI] [PubMed] [Google Scholar]
- 46.Pemberton DJ, Franks CJ, Walker RJ, Holden-Dye L. Characterization of glutamate-gated chloride channels in the pharynx of wild-type and mutant Caenorhabditis elegans delineates the role of the subunit GluCl-alpha2 in the function of the native receptor. Mol Pharmacol. 2001;59:1037–1043. doi: 10.1124/mol.59.5.1037. [DOI] [PubMed] [Google Scholar]
- 47.Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James CE, Hudson AL, Davey MW. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol. 2009;25:328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Besier R, Love B. Anthelmintic resistance in sheep nematodes in Australia: the need for new approaches. Australian Journal of Experimental Agriculture. 2003;43:1383–1391. S, C, J. [Google Scholar]
- 50.Le Jambre LF, Martin P,J, Johnston A. Efficacy of combination anthelmintics against multiple resistant strains of sheep nematodes. Animal Production Science. 2010;50:946–952. [Google Scholar]


