Abstract
Propargyl alcohol (PA) is a high production volume chemical used in synthesis of many industrial chemicals and agricultural products. Despite the potential for prolonged or accidental exposure to PA in industrial settings, the toxicity potential of PA was not well characterized. To address the knowledge gaps relevant to the toxicity profile of PA, the National Toxicology Program (NTP) conducted 2-week, 14-week and 2-year studies in male and female F344/N rats and B6C3F1/N mice. For the 2-week inhalation study, the rats and mice were exposed to 0, 31.3, 62.5, 125, 250 or 500 ppm. Significant mortality was observed in both rats and mice exposed to ≥ 125 ppm of PA. The major target organ of toxicity in both mice and rats was the liver with exposure-related histopathological changes (250 and 500 ppm). Based on the decreased survival in the 2-week study, the rats and mice were exposed to 0, 4, 8, 16, 32 or 64 ppm of PA in the 14-week study. No treatment-related mortality was observed. Mean body weights of male (≥ 8 ppm) and female mice (32 and 64 ppm) were significantly decreased (7–16%). Histopathological changes were noted in the nasal cavity, and included suppurative inflammation, squamous metaplasia, hyaline droplet accumulation, olfactory epithelium atrophy, and necrosis. In the 2-year inhalation studies, the rats were exposed to 0, 16, 32 and 64 ppm of PA and the mice were exposed to 0, 8, 16 and 32 ppm of PA. Survival of male rats was significantly reduced (32 ppm and 64 ppm). Mean body weights of 64 ppm male rats were significantly decreased relative to the controls. Both mice and rats showed a spectrum of non-neoplastic changes in the nose. Increased neoplastic incidences of nasal respiratory/transitional epithelial adenoma were observed in both rats and mice. The incidence of mononuclear cell leukemia was significantly increased in male rats. In conclusion, the key findings from this study indicated that the nose was the primary target organ of toxicity for PA. Long term inhalation exposure to PA led to nonneoplastic changes in the nose, and increased incidences of respiratory/transitional epithelial adenomas in both mice and rats. Increased incidences of harderian gland adenoma may also have been related to exposure to PA in male mice.
1. Introduction
Propargyl alcohol (PA) is a moderately volatile, colorless acetylinic primary alcohol which is used as a chemical intermediate in manufacturing of pharmaceutical and agricultural products. In various industries, PA is used as a reactant in formulation of soil fumigants, corrosion inhibitors, solvent stabilizers, anti-scaling agents and polymer modifiers (Lewis 1993); Kuney, 1994; (Dow Chemical Company 1964; Lington 1994); (ACGIH) 2005). Other names for PA include 2-propyn-1-ol, propynyl alcohol and 1-hydroxy-2-propyne. Exposure to PA could primarily occur in occupational settings through inhalation of vapors. In addition, accidental exposure through dermal contact cannot be excluded (Lington 1994). PA is a high production volume (HPV) chemical with an annual production ranging between 1 to < 10 million pounds (EPA, 2006 IUR) and hence there is a concern regarding the lack of toxicological data on PA. PA is structurally similar to allyl alcohol, a known irritant with lung, liver and kidney as its primary target organs of toxicity in animals (Auerbach et al. 2008; Li et al. 2012). Occupational Health and Safety Administration (OSHA) has established maximum permissible exposure limit (PEL) as 1 ppm (2.3 mg/m3) over an 8-hour shift with a skin notation. Propargyl alcohol was nominated for testing by the National Toxicology Program by the National Cancer Institute based on its high production volume, lack of toxicity information, and potential human exposure in occupational settings.
There is limited pharmacokinetic information available in the literature which indicates that PA is readily absorbed into the blood stream after both oral and inhalation exposure in F344/N rats and B6C3F1/N mice. Dermal absorption of PA is low because of the volatility of the chemical (Dix et al. 2001). Absorption from inhalation exposure was found to be approximately 60% for the 1 and 10 ppm exposure concentrations, but only 20% to 30% of the 100 ppm concentration was absorbed. PA has been shown to accumulate primarily in the liver and kidney and is rapidly cleared within 24 hours. Studies of the metabolism of PA demonstrate that it is biotransformed by the enzymes catalase and CYP2E1 to a biologically reactive metabolite, propargylaldehyde, (DeMaster et al. 1994,{Moridani, 2001 #9). It is thought that propargylaldehyde mediates the hepatotoxicity of PA (Basu and Marnett 1984).
Very little information is available in the literature on the short-term and chronic toxicity profile of PA but it is known to be acutely toxic by the oral (Archer 1985), dermal (Rowe 1982), and inhalation (Vernot et al. 1977) routes of exposure. It has been identified as an eye, skin, and respiratory tract irritant, and the primary targets of toxicity of PA are reported to be mucous membranes, liver, and kidney. However, complete details of these studies are not publicly available, making them difficult to evaluate. A few short term and subchronic inhalation studies have primarily reported liver and kidney toxicity ((BUA) 1998). In a 2-week inhalation study, male and female Wistar rats were exposed to 0, 10, 50, or 200 ppm, 6 hours/day. One female in the high dose group died. At study termination, the 200 ppm rats showed elevated serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities. In the highest dose group (200 ppm), the relative liver and kidney weights were significantly increased. Histopathological examination revealed lesions in the liver (hepatocellular hypertrophy, cytoplasmic granulation, and parenchymal single-cell necrosis at 200 ppm) and nasal mucosa (metaplasia was seen at ≥50 ppm) ((BUA) 1998). In a 90-day inhalation study, with male and female Wistar rats exposed to 0, 1, 5, or 25 ppm, 6 hours/day for 90 days showed increased relative liver and kidney weights in the highest exposure concentration in both sexes. However, histopathological examination revealed no treatment-related findings
Genotoxicity studies conducted by the National Toxicology Program (NTP) demonstrated that PA did not induce a remarkable increase in mutagenic activity in Salmonella typhimurium (SA) strains. Of all the standard strains tested, PA induced mutations only in the TA 100 strain particularly in the absence of S9 enzymes. Also, no significant increases in the micronucleated erythrocytes were observed in the male B6C3F1/N mice exposed to PA. However, the female mice showed a positive trend, but no statistically significant increase in number of micronucleated erythrocytes over controls (NTP 2008). These results were fairly consistent with evidence in the literature. Previously, PA was reported to be nonmutagenic in standard tester strains of SA, with and without S9 activation enzymes (Basu and Marnett 1984); (Blakey et al. 1994). However, weak mutagenic activity was detected in the absence of S9 in strain 3052 (Basu and Marnett 1984). Also, in cultured Chinese hamster ovary cells exposed to PA in both the presence and the absence of S9 enzymes significant dose-related increases in chromosomal aberrations were detected (Blakey et al. 1994).
Despite being a high production volume chemical, PA has not been adequately tested for toxicity and carcinogenicity. Given the paucity of toxicity data following long term exposure to PA, the NTP conducted short-term and chronic toxicity studies to characterize the toxicity and carcinogenicity of PA following whole-body inhalation exposure in F344/N rats and B6C3F1/N mice. More details of the complete study can be found in the NTP Technical Report (NTP 2008).
2. Material and Methods
2.1. Chemical and Characterization
Propargyl Alcohol (CAS 107-19-7) was obtained from International Specialty Products (Texas City, TX) in one lot. The chemical, a colorless liquid, was identified as PA by infrared and proton nuclear magnetic resonance spectroscopy. The purity was determined by gas chromatography and based on the analyses the overall purity of the lot was determined to be ≥ 99.0 %. Routine stability testing was conducted throughout the duration of the study. No degradation of the bulk chemical was detected.
2.2. Inhalation Exposure System
PA was pumped onto glass beads in a heated glass column where it was vaporized. Heated nitrogen flowed through the column and carried the vapor to a short vapor distribution manifold, where concentration was controlled by the chemical pump and nitrogen flow rates. Individual Teflon® delivery lines carried the vapor from the manifold to the chamber inlets. PA vapor was diluted with conditioned chamber air from the exposure chamber inlet duct to achieve the desired exposure concentration. Uniform vapor concentrations were maintained throughout the chamber. Chamber and room concentrations of PA were monitored by an on-line gas chromatograph. Samples were drawn from each exposure chamber approximately every 20 (2-week and 14-week studies) or 24 (2-year studies) minutes during each 6-hour exposure period. Evaluations of chamber uniformity and persistence and monitoring for PA impurities were conducted periodically throughout the studies by gas chromatography. Chamber uniformity was maintained; no degradation was detected.
2.3. Animals and Animal Maintenance
The inhalation exposures were conducted at AAALAC–accredited facility of Battelle Toxicology Northwest Operations (BTNW; Richland, WA). Male and female F344/N rats and B6C3F1/N mice were obtained from Taconic Farms, Inc. (Germantown, NY). Animals were quarantined for 11 days before the beginning of the studies. Rats and mice were 5 to 6 weeks old at the beginning of the studies. Feed (NTP-2000) was available ad libitum except during exposure periods; water was always available. Rats and mice were housed individually. Animals were killed by carbon dioxide asphyxiation. Cages and racks were changed weekly and approved by the BTNW Institute Animal Care and Use Committee.
Animal use was in accordance with the U.S. Public Health Service policy on humane care and use of laboratory animals and the Guide for the Care and Use of Laboratory Animals. Animal handling and husbandry met all National Institutes of Health (NIH) guidelines. These studies were conducted in compliance with the Food and Drug Administration Good Laboratory Practice Regulations (21 CFR, Part 58).
2.4. 2-week Study Design
Groups of five male and five female rats and mice were exposed to PA at concentrations of 0, 31.3, 62.5, 125, 250, or 500 ppm, 6 hours plus time required to reach 90% of concentration in the exposure chamber (T90-12 minutes) per day, 5 days per week for 16 (rats) or 17 (mice) days. Exposure concentrations were selected after review of a 2-week study conducted by industry using 200 ppm as the highest exposure concentration (BUA, 1998). Animals were weighed prior to in-life phase of studies, on days 6 and 13, and at the study termination. Animals were observed twice daily for signs of morbidity and mortality. Clinical findings were recorded daily. Necropsies were performed on all rats and mice. The heart, right kidney, liver, lung, right testis, and thymus were weighed. Histopathologic examinations were performed on all control and 500 ppm rats and mice. In addition to gross lesions and tissue masses, histopathological lesions were examined in following tissues to the no-effect level; left kidney, liver, lung, and nose.
2.5. 14-week Study Design
Groups of 10 male and 10 female rats and mice were exposed to PA at concentrations of 0, 4, 8, 16, 32, or 64 ppm, 6 hours plus (T90- 12 minutes) per day, 5 days per week for 14 weeks. Additional groups of 10 male and female rats exposed to the same concentrations for 23 days for clinical pathology analysis. Animals were observed twice daily for signs of morbidity and mortality. Clinical findings were recorded weekly for rats and mice. The animals were weighed prior to in-life phase of studies, during every week, and at study termination. Animals were anesthetized with 70% carbon dioxide, and blood was collected from the retroorbital sinus of clinical pathology rats on days 3 and 23 and from the core study rats and mice at the end of the studies for hematology (rats and mice) and clinical chemistry (rats only). Core study rats were placed in metabolism cages for 16-hour urine collection during week 12. Additional details on the hematology and clinical chemistry endpoints measured are available in the NTP technical report (NTP 2008). Direct-acting enzyme inhibitory effect of the PA was assessed (at National Institute of Environmental Health Sciences (NIEHS/NTP laboratories, RTP, NC) by incubating 0, 0.1, 1 and 10 mM (final) concentrations of propargyl alcohol with normal rat serum at room temperature for various time periods (up to 2.5 hours), and analyzed for cholinesterase activity by two methods utilizing either propionylthiocholine (PTC) or butyrylthiocholine (BTC) as substrates by a Cobas Mira analyzer (Roche Diagnostics). The reagents and procedures for the assays were obtained from Sigma Diagnostics and uses as per the manufacturer's instruction (St Louis, MO; PTC, procedure number 422; BTC procedure number 421).
Complete necropsies were performed on all core study animals. The heart, right kidney, liver, lung, right testis, and thymus were weighed. Tissues for microscopic examination were fixed and preserved in 10% neutral buffered formalin, trimmed, processed, embedded in paraffin, sectioned to a thickness of 4 to 6 μm, and stained with hematoxylin and eosin. Complete histopathologic examinations were performed on target organs of rats and mice until a no-effect-level was observed. Additional details regarding the pathology data generation, quality assurance review and the NTP pathology working group are available in the NTP technical report (NTP 2008).
2.6. 2-Year Study Design
Groups of 50 male and 50 female rats and mice were exposed to PA by inhalation at concentrations of 0, 8 (mice only), 16, 32, or 64 (rats only) ppm, 6 hours plus T90 (14 minutes) per day, 5 days per week for 105 weeks. All animals were observed twice daily. Body weights were recorded prior to in-life phase of the study, weekly for the first 13 weeks, and every 4 weeks through week 93, every 2 weeks for the rest of the study, and at study termination. Animals were observed twice daily for signs of morbidity and mortality and clinical findings were recorded every 4 weeks through week 93, every 2 weeks through rest of the study, and at the study termination. Complete necropsies and microscopic examinations of all NTP protocol required tissues were performed on all rats and mice. A detailed list of the tissues examined can be found in the NTP technical report (NTP 2008). At necropsy, all organs and tissues were examined for grossly visible lesions, and all protocol-required tissues were fixed and preserved in 10% neutral buffered formalin, trimmed and processed, embedded in paraffin, sectioned to a thickness of 4–6 μm, and stained with hematoxylin and eosin (H&E) for microscopic examination. For all paired organs (e.g. adrenal gland, kidney, ovary), samples from each organ were examined. For the nose, three sections, referred to as Levels I, II, and III, were taken following the standard NTP protocol. Proceeding from anterior to posterior, Level I was taken immediately posterior to the upper incisor teeth; Level II was taken through the level of the incisive papilla anterior to the first palatal ridge; and Level III was taken through the middle of the second molar teeth (Boorman G 1990). Additional details regarding the pathology data generation, quality assurance review and the NTP pathology working group are available in the NTP technical report (NTP 2008).
2.7. Statistical Methods
The probability of survival was estimated by the product- limit procedure (Kaplan and Meier 1958). Animals found dead of other than natural causes or missing were censored from the survival analyses; animals dying from natural causes were not censored. Statistical analyses for possible exposure related effects on survival used Cox's (1972) method for testing two groups for equality and Tarone's (1975) life table test to identify dose-related trends. All reported P values for the survival analyses are two sided. The Poly-k test (Astuti and Yanagawa 2002; Bailer and Portier 1988; Portier and Bailer 1989) was used to assess neoplasm and nonneoplastic lesion prevalence. This test is a survival-adjusted quantal-response procedure that modifies the Cochran-Armitage linear trend test to take survival differences into account. Unless otherwise specified, a value of k=3 was used in the analysis of site specific lesions. Tests of significance included pairwise comparisons of each dosed group with controls and a test for an overall dose-related trend. Continuity-corrected Poly-3 tests were used in the analysis of lesion incidence, and reported P values are one sided (Bieler and Williams 1993); (Portier and Bailer 1989); (Bailer and Portier 1988)). Organ and body weight data were analyzed with the parametric multiple comparison procedures (Dunnett and Crisafio 1955). Hematology and, clinical chemistry, urinalysis, were analyzed using the modified (Dunn 1964; Williams 1986)and Shirley's nonparametric multiple comparison methods. We used Jonckheere's test to assess the significance of the dose-related trends and to determine whether a trend-sensitive test was more appropriate for pairwise comparisons than a test that does not assume a monotonic dose-related trend (Dunn 1964; Dunnett and Crisafio 1955).
3. RESULTS
3.1. 2-Week Studies in F344/N Rats and B6C3F1/N Mice
Significant mortality was observed in both rats and mice in the 2-week study (data not shown). All male rats and male and female mice exposed to ≥ 125 ppm and female rats exposed to ≥ 250 ppm died within the first week of exposure. Final mean body weights of the surviving top dose group of rats and mice (62.5 ppm or 125 ppm, respectively) were significantly decreased (8 % and 12 %, respectively). Common exposure-related clinical findings in rats and mice included lethargy, ataxia, abnormal breathing and nasal/eye discharge. Absolute weights of kidney were significantly increased at 62.5 ppm (11%) and 125 ppm (17%) in male and female rats and in male (11%) and female (14%) mice at 31.3 ppm. Compared with controls, relative kidney weights were significantly increased in both male and female rats at 31.3 ppm, 62.5 ppm and 125 ppm (11% to 28%) and in male and female mice at 31.3 ppm and 62.5 ppm (11% to 17%). Relative liver weights were increased (11% to 13%) in both sexes of rats (62.5 ppm) and mice (31.3 ppm and 62.5 ppm). In the top surviving dose group (125 ppm) of female rats, statistically significant increases in both absolute (35%) and relative (47%) liver weights were observed. Histopathologically, livers of all mice and rats exposed to 250 ppm or 500 ppm contained large areas of degeneration and necrosis of hepatocytes, accompanied by marked congestion of affected areas and erythrophagocytosis. In mice, these were also seen in the 125 ppm group with less severity. The pattern of necrosis was predominantly periportal in these dose groups, with some larger degenerated and necrotic areas covering the major portion of the affected lobules. Male mice exposed to 125 ppm also showed periportal necrosis, but the male rats and female mice exposed to 125 ppm exhibited centrilobular necrosis of hepatocytes and moderate congestion. Based on the decreased survival of mice and rats exposed to ≥125 ppm, the PA exposure concentrations selected for the following 14-week inhalation studies were 4, 8, 16, 32, and 64 ppm. The highest exposure concentration for the 14-week studies in rats and mice was limited by toxicity observed in the 2-week studies of PA. More details on the organ weight changes, clinical chemistry parameters and histopathological changes in the 2-week PA studies are in the NTP Technical report (NTP 2008).
3.2. Subchronic (14-week) and Chronic (2-year) Studies in Rats
3.2.1. 14-week in F344/N Rats
In the 14-week study, there were no exposure-related changes in mortality or toxicologically relevant changes in body weights, clinical observations, hematology, urine analysis data or gross lesions in the PA exposed rats. The most dramatic change was observed in the clinical chemistry results in the serum cholinesterase activity (Figure 1A and B). An exposure-concentration and time-related decrease in serum cholinesterase activity occurred in exposed male and female rats; females were more affected. The female rats in the top exposure (64 ppm) group demonstrated a 19%, 42%, and 50% decrease in cholinesterase activity on days 3 and 23 (data not shown) and at week 14, respectively. However, male rats in the top exposure group demonstrated only 15% and 27% decreases on day 23 and at week 14, respectively. For simplicity of presentation, serum cholinesterase levels at only 14-week time point are shown in Figure 1 A and B. The sex differences observed in basal rat serum cholinesterase levels were not surprising as it is well established that adult female rats have 3- to 5- fold greater basal levels of cholinesterase than males (Schmidt and Schmidt 1978). In vitro assessment by addition of PA directly to the rat serum did not result in any significant inhibition (data not shown) of cholinesterase activity, indicating that PA is not a direct inhibitor. There were no other exposure-related changes in clinical chemistry parameters.
Figure 1. Serum cholinesterase levels in F344/N rats following 14-weeks of inhalation exposure to PA.
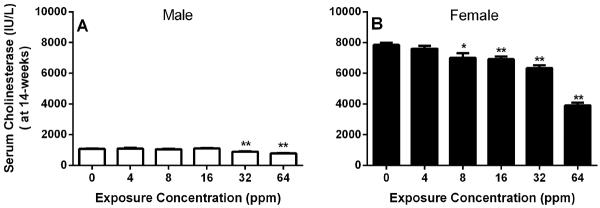
Each bar represents the average serum cholinesterase levels in F344/N rats exposed to varying concentrations of propargyl alcohol for 14-weeks. For simplification, the data from Day 4 and Day23 time-points was omitted. However at each time point there was an exposure-dependent significant decrease in serum cholinesterase levels (see text). Data expressed as Mean ± SEM. n=9–10. * Significantly different (p≤0.05) from the control group by Dunn's or Shirley's test. ** p<0.01. Ratios were calculated and statistical errors were performed on rounded data.
The exposure-related histopathology findings were limited to the nose (Table 1). Minimal to mild hyperplasia of respiratory epithelium of the nose was noted in all exposed groups and in two of the male controls; the incidences were significantly increased in all exposed groups except 8 ppm males and 4 ppm females. Respiratory epithelial hyperplasia was characterized by a taller mucosa, associated with increased numbers of columnar epithelial cells and gland-like spaces within the mucosal epithelium, and the presence of occasional dilated glands in the underlying lamina propria. Squamous metaplasia of the respiratory epithelium was noted in a few males and most females exposed to 64 ppm and was characterized by extensive involvement of septum, turbinates and lateral walls in nasal Level I. Necrosis of respiratory epithelium was also noted in two of the top dose females. These changes were noted in the septum, turbinates and lateral walls of Level I. In some animals, foci of transitional epithelial hyperplasia were noted on the lateral walls and the lateral sides of the nasoturbinates. Necrosis of olfactory epithelium was present in both males and females, exposed to 32 ppm and 64 ppm and was noted on the dorsal septum and adjacent tips of the turbinates of Level III. There was sloughed olfactory epithelium with pyknotic nuclei, and only a thin layer of flattened or vacuolated basal cells remained on the surfaces of affected areas. The transition from normal to necrotic olfactory epithelium was usually abrupt.
No significant changes in survival or body weight were observed in the 1 study, and the nasal lesions in the respiratory and olfactory epithelium were minimal to mild in severity and were not considered to be life threatening. Therefore, the PA exposure concentrations selected for the 2-year inhalation study in rats were 16, 32 and 64 ppm.
3.2.2. 2-Year Study in F344/N rats
Significant reduction in survival was observed in the 32 and 64 ppm exposure groups of male rats with survival rates of 30% and 32%, respectively, as compared to controls (55%). No exposure-related reduction in survival was observed in female rats. Mean body weights of males exposed to 64 ppm were less than those of the controls after week 24 of the study. Mean body weights of exposed females were similar to those of the controls.
In male rats, there was a positive trend in nasal respiratory/transitional adenomas with increased incidences observed in three rats in the 64 ppm exposure group (Table 2). One female rat in the 32 ppm exposure group also had a respiratory adenoma. These tumors presented as well-demarcated, sessile or pedunculated, polypoid proliferations of well- differentiated hyperplastic surface epithelium with transitional/respiratory features and without cytologic atypia (Figure 2A and B). All tumors arose in Level I from either the nasoturbinate, maxilloturbinate, or lateral wall.
Figure 2. Respiratory/transitional adenoma.
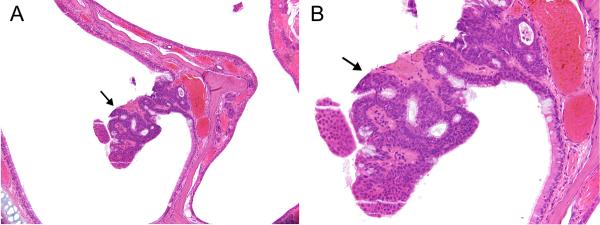
A) A small, polypoid (arrow) adenoma projects from the medial side of a nasoturbinate in Level I in a female rat exposed to 32ppm PA for 2-years. B) The adenoma is composed of epithelium having mixed transitional and respiratory features. Original objective magnification: A) 10×, B) 20×.
A spectrum of nonneoplastic changes was observed in the respiratory and olfactory epithelium at all levels of exposure (Table 2). All exposed groups had significantly increased incidences of respiratory epithelial hypertrophy and hyperplasia, and respiratory glandular hyperplasia, in Levels I and II. The respiratory epithelium lining the septum at Level I and the turbinates and septum at Level II was characterized by tall ciliated columnar cells interspersed with clusters of goblet cells, sometimes producing an irregular surface due to crowding of the cells. The transitional epithelium lining the lateral wall and tips of the turbinates in Level I was also thickened due to an increased number of cell layers.
Several exposure-related lesions were seen in the olfactory epithelium of levels II and III. Basal cell hyperplasia of a minimal to mild degree was present in many of the 16 ppm animals and in most of the 32 and 64 ppm animals. The lesion was seen mainly along the nasal septum and the rounded tips of the turbinates of Level III and consisted of crowding of the basal cells along the basement membrane, increased numbers of basal cell layers, and occasional formation of small rosettes compressing the olfactory epithelium. Olfactory epithelial hyperplasia was seen in a few males and one female exposed to 64 ppm and was characterized by increased thickness of the entire epithelium. Hyperplasia of the underlying olfactory epithelium glands (Bowman's glands) was noted in a few 64 ppm males and 16 ppm females.
Mild to moderate olfactory epithelial atrophy, consisting of a decrease in the number of olfactory cells lining the ethmoid turbinates and usually seen in the dorsal meatus of Level III, was present in the majority of 32 and 64 ppm males and females and in many of the 16 ppm males.
Respiratory metaplasia of the olfactory epithelium occurred in many of the males in all exposed groups and in many of the 64 ppm females; metaplasia consisted of replacement of the normal olfactory epithelium by ciliated respiratory epithelium in the dorsal meatus of Level II and especially in the turbinates of Level III.
Minimal to mild olfactory epithelial degeneration was diagnosed in a few 32 and 64 ppm males and females on the basis of either vacuolization of the epithelium or an increase in foci of mineralization. Olfactory epithelial necrosis, graded as minimal to mild on the basis of extent, was also noted in a few 32 and 64 ppm males and females. Minimal to mild olfactory epithelial hyaline droplet accumulation was diagnosed in a few males and females in all exposed groups.
Chronic active inflammation was present in many of the exposed animals, as well as in some of the controls. Lesion prevalence increased with exposure, although a concomitant increase in severity was not observed. The inflammation was characterized by infiltration of the lamina propria by lymphocytes, macrophages, and small numbers of neutrophils, and often by the presence of a suppurative exudate in the nasal cavity.
In male rats, the incidences of mononuclear cell leukemia (MCL) occurred with a positive trend (P<0.001), and the incidence in the 64 ppm group was significantly greater than in the control group. There was an exposure-dependent increase in incidences of MCL in females, but none of the exposed groups had significantly greater incidences than the controls (Table 3).
3.3. Subchronic (14-week) and Chronic (2-year) Studies in Mice
3.3.1. 14-week Study in B6C3F1/N mice
Due to decreased survival of male and female mice exposed to ≥125 ppm in 2-week studies, the propargyl alcohol exposure-concentrations selected for subchronic studies in mice were 4, 8, 16, 32 and 64 ppm. Survival, hematology, clinical chemistry parameters and organ weights of mice were not affected by exposure to propargyl alcohol and no exposure-related gross lesions were observed. The final mean body weights and body weight gains of males exposed to ≥8 ppm and females exposed to ≥64 ppm were significantly less (6% to 15%) than those of the control group.
Similar to the subchronic study in rats, histopathologic changes were noted primarily in the nasal cavity, involving both the respiratory and olfactory epithelium of all three levels. The changes were limited to males and females exposed to 16 ppm or greater (Table 4). Minimal to mild suppurative inflammation of the nasal cavity involving Level I and sometimes Level II occurred in many 64 ppm males, all 64 ppm females, and one 32 ppm female. Minimal to moderate squamous metaplasia of the respiratory epithelium was present in most 32 ppm and all 64 ppm animals and was characterized by extensive involvement of the septum, turbinates, and lateral walls in nasal Level I.
Minimal to mild hyaline accumulation was noted in the respiratory epithelium of nasal Level II in many of the 32 and 64 ppm males and females and manifested as brightly eosinophilic cytoplasmic globules in the epithelial cells lining the septum and dorsal meatus. Minimal to moderate olfactory epithelial atrophy involving Level II or III was present in most 32 and 64 ppm males and females and was characterized by decreased numbers of neuronal cells. In other areas, the olfactory epithelium was often replaced by respiratory metaplastic epithelium. Minimal to moderate hyperplasia and respiratory metaplasia of Bowman's glands in the olfactory region of Level II (dorsal meatus), and the dorsal meatus and septum in Level III, were found in nearly all 32 and 64 ppm males and females, in a few 16 ppm males, and in one 16 ppm female. The glands were dilated, sometimes contained inflammatory cells and proteinaceous cell debris, and were often located beneath an overlying respiratory metaplastic epithelium or an atrophic olfactory epithelium.
Minimal necrosis of olfactory epithelium was noted in nasal Level II of most 16 ppm females, a few 32 ppm females, and one 32 ppm male. The necrosis was minimal in all cases and was characterized by disruption and sloughing of olfactory epithelium at the junction of respiratory and olfactory epithelium on the septum or adjacent nasoturbinate.
Minimal to moderate turbinate atrophy was also present in all females and most males exposed to 32 or 64 ppm. The atrophy ranged from minimal to moderate and was characterized by loss of the hook shape of the nasoturbinates and shortening of the maxilloturbinates in Level I, and decrease in the length of the nasoturbinates in Level II. The change in shape and length of the turbinates was associated with, and largely due to, similar atrophic changes in the turbinate bone. In comparison to the rats, the nasal lesions in the mice were more severe and extensive. In particular, suppurative inflammation, turbinate atrophy, olfactory epithelial atrophy, and respiratory metaplasia of the olfactory epithelium with hyperplasia of Bowman's glands were noted only in the mice.
Based on the constant and significant reductions in the final mean body weights of males and females exposed to 64 ppm, PA exposure concentrations selected for the 2-year inhalation study in mice were 8, 16 and 32 ppm.
3.3.2. 2-Year Study in B6C3F1/N Mice
Survival of exposed groups of mice was similar to controls. At the end of study, mean body weights of 16 and 32 ppm females were less than those of the controls by 6 % and 19 %, respectively.
In both male and female mice, nasal respiratory/transitional epithelial adenomas occurred in a few animals in each exposed group; the incidences increased in an exposure concentration-related manner, and the incidence was significantly increased in the 32 ppm males and females (Table 5). No adenomas occurred in controls. The adenomas originated from the nasoturbinates and lateral walls of Levels I and II, forming sessile or pedunculated polypoid masses projecting into the nasal cavity. A few tumors arose from the tips of the maxilloturbinates. Microscopically, the adenomas were characterized by proliferation of well-differentiated, cuboidal to columnar transitional or respiratory epithelium. Many of the tumors were cystic or microcystic (Figure 3), while others were predominantly solid and cystic (Figure 4). Growth was primarily exophytic into the nasal cavity, and none of the tumors exhibited invasive patterns within the adenomas or within the underlying lamina propria. However, some tumors were large and a few virtually filled the nasal cavity unilaterally. One tumor was associated with perforation of the nasal septum, evidently by pressure necrosis, and extension into the nasal cavity on the opposite side (Figure 5). Scattered cytologic atypia was noted focally in one tumor, but the significance of these individual cell changes was uncertain.
Figure 3. Respiratory/transitional adenoma, cystic.
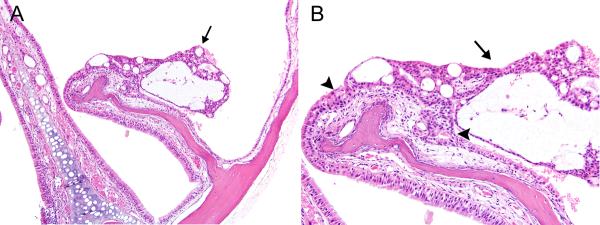
A) A small, polypoid, multicystic adenoma (arrow) projects from the lateral side of a nasoturbinate in Level I in a female mouse exposed to 16ppm PA for 2-years. B) The adenoma (arrow) exhibits predominantly transitional epithelial features, and is bordered by hyperplastic epithelium (arrowheads) on both sides. Original objective magnification: A) 10×, B) 20×.
Figure 4. Respiratory/transitional adenoma, solid and cystic.
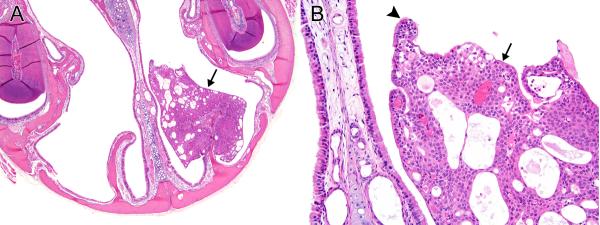
A) A large adenoma (arrow) surrounds the major portion of a nasoturbinate in Level I in a female mouse exposed to 16ppm PA for 2-years. The adenoma is solid in the basal portion, microcystic in the peripheral portions, and partially fills the dorsal meatus and the dorsal portion of the lateral meatus on this side of the nasal cavity. B) The adenoma exhibits both transitional (arrow) and respiratory, ciliated (arrowhead) epithelial features. Original objective magnification: A) 4×, B) 20×.
Figure 5. Respiratory/transitional adenoma, with expansion to opposite nasal cavity.
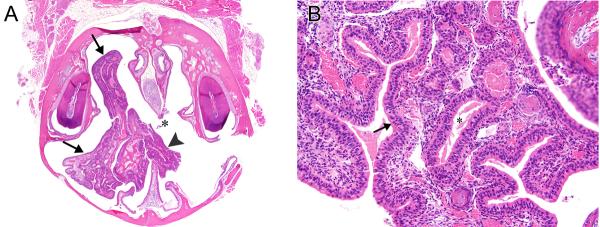
A) This large adenoma (arrows) surrounds a nasoturbinate in Level I, fills much of the nasal cavity on this side, and extends through a perforated nasal septum (asterisk) into the opposite nasal cavity (arrowhead). Female mouse exposed to 16ppm PA for 2-years. B) This adenoma is lined by a tall, columnar epithelium (arrow) that has more of a respiratory epithelial appearance. Glands (asterisk), or invaginations of the surface epithelium, are present in the stroma of the tumor, and are lined by epithelium similar to that on the surface of the tumor. Original objective magnification: A) 2×, B) 20×.
A spectrum of nonneoplastic changes was observed in the respiratory and olfactory epithelium at all exposure concentrations (Table 5). Transitional and respiratory epithelial hyperplasia, usually mild to moderate, occurred in most mice exposed to PA (Figures 6). The severity increased only minimally with exposure concentration. Transitional and respiratory hyperplasia involved the tips of the nasoturbinates and their lateral sides, the lateral walls, and the maxilloturbinates in nasal Level I. Hyperplasia was characterized by thickening of the transitional and respiratory epithelium by increased numbers of columnar and cuboidal lining cells. Foci of respiratory epithelial hypertrophy and hyperplasia were also seen in the nasal septum and medial side of the nasoturbinates in Level I. Respiratory glandular hyperplasia was also present in most 16 and 32 ppm mice and in many 8 ppm mice, with exposure concentration-related increases in incidence and severity. The glandular hyperplasia primarily involved the mucosa of the dorsal meatus of Levels I and II. Squamous metaplasia of the respiratory epithelium occurred in most 16 and 32 ppm males and females and several 8 ppm males, with exposure concentration-related increases in incidence and severity. The metaplasia was noted predominantly in Level I and, to a lesser extent, Level II. The extent of the metaplasia varied from a few foci on turbinate tips to nearly the entire nasal mucosa in Level I. Suppurative inflammation was often associated with the squamous metaplasia, presumably preceding it in response to injury of the nose. The inflammatory response consisted of a submucosal, mucosal, or luminal infiltration by neutrophils with occasional disruption of the surface epithelium and accumulation of inflammatory exudate and proteinaceous debris in the nasal airways.
Figure 6. Respiratory/transitional epithelial hyperplasia.
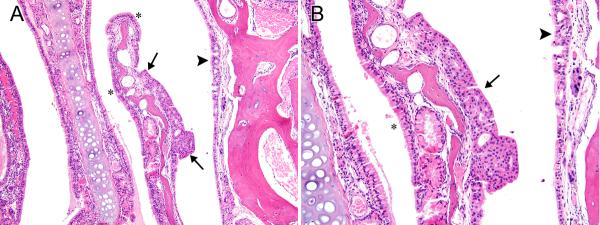
A) Epithelial hyperplasia, with transitional features (arrows) forms a plaque-like thickening of the epithelial lining on the lateral side of a nasoturbinate in Level II of the same female mouse with the adenoma in Level I (2-year studies) that is shown in Fig. 4. Respiratory hyperplasia is also noted on the lateral wall (arrowhead). Normal appearing respiratory epithelium is indicated by asterisks for comparison. B) The stratified, non-ciliated epithelium with transitional features (arrow), and the hyperplastic, ciliated epithelium with respiratory epithelial features (arrowhead) are better shown in this higher magnification image. The more normal columnar, ciliated respiratory epithelium is marked with an asterisk for comparison. Original objective magnification: A) 10×, B) 20×.
Turbinate atrophy with an exposure concentration related increase in severity was found in most exposed males and all exposed females. Atrophy was most apparent in Level I and the tips of the atrophic nasoturbinates frequently exhibited either hyperplasia or squamous metaplasia of the respiratory epithelium, as well as occasional respiratory adenomas. Incidences of olfactory epithelial atrophy were significantly increased in 16 and 32 ppm males and females. An exposure concentration-related increase in severity was noted. The designation of atrophy was restricted to lesions showing a decrease in the number of layers of olfactory cells and thus a decreased height of the olfactory epithelium; the lesion was usually present in the dorsal meatus of Level III. When the olfactory epithelium was replaced by ciliated, columnar respiratory epithelium, the alteration was designated respiratory metaplasia. This latter lesion was noted with significantly increased incidence in 16 ppm female mice and 32 ppm male and female mice. Increasing severity was represented by more extensive replacement of the olfactory epithelium by the respiratory metaplastic epithelium, which was frequently accompanied by underlying respiratory glandular metaplasia and atrophy of the olfactory nerve bundles.
The occurrence of Harderian gland adenoma was significantly increased in 8 and 32 ppm male mice (0 ppm, 3/50; 8 ppm, 10/50; 16 ppm, 6/50 and 32 ppm, 11/50). The adenomas were characterized by circumscribed, unencapsulated nodules composed of tall columnar cells with abundant cytoplasm and basal nuclei arranged in tubular and papillary patterns. In addition, chronic active inflammation of the cornea was observed with significantly increased incidence in 32 ppm males and females (Table 6). A few males and females exposed to lower concentrations also exhibited inflammation, but severities were similar across groups. Female mice in the top dose group (32 ppm) showed significant increase in incidences of cataract as compared to the controls, 6/48 and 1/49, respectively.
4. DISCUSSION AND CONCLUSIONS
Propargyl alcohol (PA) is a high production volume (HPV) chemical which is mainly used as an intermediate in production of a variety of chemical and pharmaceutical products. PA was one of chemicals included by the EPA for testing under the HPV challenge, which aims at assessing potential health effects and addressing the gap in basic hazard information for chemicals produced or imported in United States in quantities of ≥ 1 million pounds per year. Based on widespread pattern of use of PA, lack of adequate toxicological information and a concern about the carcinogenic potential and chronic toxicity, the NTP conducted genetic, short term and long term toxicity studies of PA in rats and mice following whole body inhalation exposure. The goal of this study was to analyze potential deleterious health effects of PA and generate hazard information on PA that would contribute to regulatory decision making process.
In the 2-week inhalation toxicity studies conducted by the NTP, both rats and mice showed a significant mortality following exposure to ≥125 ppm PA. The high rate of mortality was not expected as the exposure concentrations for the 2-week studies were selected based on published results from an acute study reported in the literature ((BUA) 1998). Also, previously reported ADME studies demonstrated that there is a decrease in systemic absorption of PA following respiratory exposure to higher (100 ppm) concentrations (Dix et al. 2001). The primary target organ of toxicity in both rats and mice was found to be the liver. A sharp dose response in hepatotoxicity was observed and a range of microscopic hepatic lesions including moderate to marked periportal necrosis, congestion, and hepatocellular erythrophagocytosis was observed in both rats and mice. A structurally similar chemical allyl alcohol has been previously shown to cause necrosis in periportal regions of the liver lobule in rodents (Badr 1991). Allyl alcohol-induced hepatotoxicity is known to be mediated by a reactive metabolite, acrolein, which is formed via catalytic oxidation of allyl alcohol by alcohol dehydrogenase (ADH). Similar to allyl alcohol, the toxicity of PA is hypothesized to be due to metabolism to the more reactive and mutagenic aldehyde, propargylaldehyde or propioaldehyde (Banijamali et al. 2003; Basu and Marnett 1984; Moridani et al. 2001). However, propargyl alcohol is a relatively poor substrate for ADH and is mainly metabolized by CYP2E1 (Moridiani MY et.al., 2001). Each molecule of propargylaldehyde can react with two molecules of glutathione, which potentially can lead to depletion of glutathione more rapidly than allyl alcohol. Also, numerous other metabolites of PA with unknown biological activity have been identified (Banijamali et al. 2003; Dix et al. 2001).
In contrast to the liver toxicity observed in the 2-week studies and the previously published subchronic inhalation studies (Dow Chemical Company 1964); (Lington 1994); (BUA) 1998), the results from 14-week studies did not demonstrate any significant signs of liver toxicity. This was surprising given the structural similarity of propargyl alcohol to allyl alcohol. This apparent discrepancy could be attributed to the lower exposure concentrations used in the 14-week study than the 2-week study. However, some signs of potential hepatic dysfunctions were noted in the 14-week studies. In both male and female rats, inhibition of total serum cholinesterase activity was observed in a time- and concentration-dependent manner. Variation in the serum cholinesterase activity could be due to changes in either acetyl- or butyryl-cholinesterase (BuChE) levels. Because BuChE is synthesized in the liver, reduced cholinesterase levels could be an indicator of hepatic insufficiency; however, in this study, there were no alterations in other markers of hepatic injury or in liver histopathology.
The decreased cholinesterase activity could be due to either selective inhibition or reduced production of the enzyme. To identify potential mechanisms for decreased serum cholinesterase activity the ability of PA to directly inhibit cholinesterase was tested in vitro. The results demonstrated that the parent compound, PA, had no direct inhibitory effect. Thus, if enzyme inhibition were the mechanism, it presumably would have been related to a metabolite of PA. It is also possible that a decrease in serum cholinesterase activity may be indicative of neurotoxicity. However, no clinical observations that could be related to decreased serum cholinesterase such as tremors, salivation and lacrimation, were observed in the 14-week study in rats.
The nose was the primary target organ of toxicity in the 14-week inhalation studies of PA. In both rats and mice, there were exposure –related increases in a variety of histopathological lesions. These lesions occurred predominantly in the two highest exposure concentration groups (32 and 64 ppm). The no-observed-effect level for the nose in mice was 8 ppm in the subchronic studies. A no-observed-effect level for the nose in rats was not identified.
In the 2-year rat study, survival of 32 and 64 ppm males was significantly reduced compared to controls. The decreased survival was mostly the result of moribund sacrifice of animals due to signs of excessive lethargy. Similar to the 14-week study, the nose was the primary target organ of toxicity in the 2-year inhalation studies of PA. Neoplastic lesions were observed primarily in the nose of both sexes of rats and mice with increased incidences of respiratory/transitional adenomas. Three of the male rats exposed to 64 ppm and one female rat exposed to 32 ppm developed nasal respiratory adenomas compared to none in the controls. The male rats and both male and female mice showed statistically significant trends in exposure-related increases in the incidences of respiratory/transitional epithelial adenoma. In male and female mice exposed to 32 ppm, there was a significant pair wise increase in incidences of nasal respiratory adenoma. Nasal respiratory neoplasms are extremely rare in control F344/N rats and B6C3F1/N mice in NTP studies. No nasal adenomas had been reported in NTP historical control mice at the time the report had been written, and adenomas had only been identified in 1/447 chamber controls in inhalation studies of rats. The occurrence of these rare nasal respiratory adenomas in both species and in both sexes of mice is indicative of the neoplastic potential of PA. . There is some indirect evidence that nasal adenomas may undergo malignant progression ((Brown et al. 1991); (Morgan and Harakema 1996; U. Mohr et al. 1992); however, there was no indication of malignancy in the current study.
A variety of nonneoplastic nasal lesions was also observed in the respiratory and olfactory epithelium at all exposure concentrations in the 2-year studies (≥16 ppm in rats, ≥ 8 ppm in mice) and severities of these lesions showed an exposure concentration-related increase. Rats developed a wider spectrum of nonneoplastic lesions of the olfactory epithelium as compared to the mice. These lesions included hyperplasia, basal cell hyperplasia, degeneration, necrosis, and hyaline droplet accumulation. Higher incidences and severities of nonneoplastic lesions were observed at lower exposure concentrations in mice than in rats. This apparent species difference in sensitivity may be potentially due to the more active production of toxic metabolites of PA. Previous studies have reported that CYP2E1 the primary P450 enzyme involved in metabolism of PA has higher activity in the lungs and liver of mice than in that in the rat (Green et al. 2001; Nakajima et al. 1993). Although, CYP2E1 is known to be present in the nasal mucosa of mice and rats, no published reports on comparisons of CYP2E1 activity levels in nasal mucosa of the mice and rats were found in the literature.
The correlation between the nonneoplastic proliferative lesions in the nasal cavity and the formation of nasal neoplasms is not clear. Hyperplasia of the transitional/respiratory epithelium was often noted in areas where the nasal adenomas also occurred, such as the nasoturbinates and the lateral wall, and was sometimes noted in the epithelium adjacent to adenomas. Hyperplasia may be a regenerative response to degeneration and necrosis. However, it is not always clear histologically whether hyperplasia is just a regenerative response or part of a morphological continuum to neoplasia (Boorman 1990; Ward et al. 1993). In the current study many animals developed nonneoplastic proliferative lesions in the nose but only a few developed nasal neoplasms.
The nasal lesions observed in the current studies were mainly found in the lateral walls of the anterior nasal cavity and may have been related to enzymatic metabolism of PA to an active metabolite. The transitional epithelium predominantly lines the lateral wall of the lateral meatus and the sides of the nasoturbinates and maxilloturbinates. Therefore the transitional epithelium receives a major portion of the nasal airflow in the rat (Morgan and Monticello 1990); (Morgan and Harakema 1996). Both the olfactory epithelium and the transitional epithelium of the anterior nasal cavity have been reported to be rich in xenobiotic metabolizing enzymes (Dahl and Hadley 1991; Dahl and Lewis 1993). Additionally, the water solubility of PA would be expected to enhance its absorption by the watery nasal secretions, favoring its uptake by the transitional epithelium.
In the male rats, there was an exposure concentration-related increase in the incidence of mononuclear cell leukemia and the incidence was significantly increased in the 64 ppm group compared to the controls. Although mononuclear cell leukemia is fairly common in F344/N rats control male rats (NTP historical range of 38% to 66% for inhalation studies), the incidences in this study exceeded the NTP historical control ranges for inhalation studies and for all study routes. In females, there was a positive trend in incidences with increasing exposure concentration; however, there were no statistical differences and the incidences were within the NTP historical control ranges. It has been reported that the odds ratios for developing lymphoid and hematopoietic cancers were increased in humans exposed to allyl alcohol (the structurally related unsaturated alcohol) (Ott et al. 1989a, b). However, the significance of this correlation was confounded by the exposure of workers to multiple chemicals along with allyl alcohol.
In addition, chronic (2-year) exposure to PA led to a statistically significant increased incidence of cataract and corneal inflammation in the top dose group of male and female mice. Incidences of Harderian gland adenoma were significantly increased in male mice exposed to 8 ppm or 32 ppm of PA. These incidences slightly exceeded the NTP historical control range for inhalation studies. Further analysis of individual animal data suggested that PA had a direct irritant effect on the cornea, which may have been exacerbated by the presence of Harderian gland neoplasms. Almost all mice with Harderian gland neoplasm appeared to also have keratitis or corneal inflammation. However, not all mice with keratitis developed Harderian gland neoplasms.
In summary, the primary PA exposure-related lesions were in the nose. Increased incidences of rare respiratory/transitional epithelial adenoma were indicative of neoplastic potential of PA in male rats and in male and female mice. An increased incidence of mononuclear cell leukemia was also found in male rats. Along with the neoplastic lesions, a variety of proliferative and inflammatory nonneoplastic lesions was observed in the nasal respiratory, transitional, and olfactory epithelium at all exposure concentrations in both sexes of mice and rats. The eyes of exposed mice were affected by exposure, most likely due to the irritant properties of PA, and the incidences of Harderian gland adenomas were also found to be increased in male mice.
Supplementary Material
Figure 7. Respiratory/transitional epithelial hyperplasia.
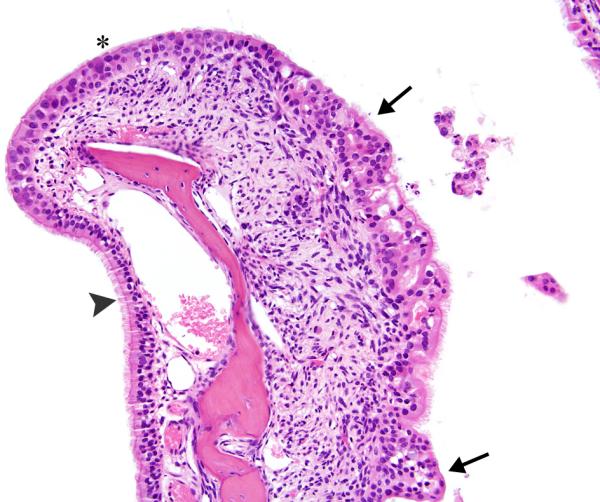
The epithelium lining this nasoturbinate in Level II exhibits variation from normal respiratory, ciliated epithelium (arrowhead) to hyperplastic transitional epithelium (asterisk) to hyperplastic, ciliated epithelium with mixed respiratory and transitional epithelial features (arrows). The subepithelial stroma of the nasoturbinate shows inflammation and fibroplasia. Male mouse exposed to 32ppm PA for 2-years. Original objective magnification: 20×.
Acknowledgement
We thank Dr. Mathew Stout (DNTP/NIEHS) and Dr. Deepa Rao (Contractor, DNTP/NIEHS) for their helpful review of the manuscript.
Footnotes
Disclaimer This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare that there are no conflicts of interest.
REFERENCES
- American Conference of Governmental Industrial Hygienists (ACGIH) 2005 TLVs and BEIs. Threshold Limit Values of Chemical Substances & Physical Agents and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Inc; Cinncinnati, Ohio. 2005. p. 48. [Google Scholar]
- Beratergremium fur Altstoffe (BUA) Propargyl Alcohol. In: BUA, editor. 213 BUA-Report: Propargyl Alcohol CAS-No. 107-19-7, GDCh-Advisory Committee on Existing Chemicals of Environmental Relevance. Stuttgart; Germany: 1998. [accessed on 7 Aug. 2013]. Available at http://www.hirzel.de/titel/51876.html. [Google Scholar]
- Archer TE. Acute oral toxicity as LD50 (mg/kg) of propargyl alcohol to male and female rats. J Environ Sci Health B. 1985;20:593–596. doi: 10.1080/03601238509372497. [DOI] [PubMed] [Google Scholar]
- Astuti ET, Yanagawa T. Testing trend for count data with extra-Poisson variability. Biometrics. 2002;58:398–402. doi: 10.1111/j.0006-341x.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- Auerbach SS, Mahler J, Travlos GS, Irwin RD. A comparative 90-day toxicity study of allyl acetate, allyl alcohol and acrolein. Toxicology. 2008;253:79–88. doi: 10.1016/j.tox.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr MZ. Periportal hepatotoxicity due to allyl alcohol: a myriad of proposed mechanisms. J Biochem Toxicol. 1991;6:1–5. doi: 10.1002/jbt.2570060102. [DOI] [PubMed] [Google Scholar]
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. [PubMed] [Google Scholar]
- Banijamali AR, DeMatteo V, Sumner SJ. A mechanism for the formation of bisglutathione conjugates of propargyl alcohol. Pest Manag Sci. 2003;59:331–338. doi: 10.1002/ps.641. [DOI] [PubMed] [Google Scholar]
- Basu AK, Marnett LJ. Molecular requirements for the mutagenicity of malondialdehyde and related acroleins. Cancer Res. 1984;44:2848–2854. [PubMed] [Google Scholar]
- Bieler GS, Williams RL. Ratio estimates, the delta method, and quantal response tests for increased carcinogenicity. Biometrics. 1993;49:793–801. [PubMed] [Google Scholar]
- Blakey DH, Maus KL, Bell R, Bayley J, Douglas GR, Nestmann ER. Mutagenic activity of 3 industrial chemicals in a battery of in vitro and in vivo tests. Mutat Res. 1994;320:273–283. doi: 10.1016/0165-1218(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Boorman GA, Morgan KT, Uriah L. Pathology of the Fischer Rat. Academic Press; San Diego, California: 1990. [Google Scholar]
- Boorman GA, Eustis SL, Ellwell MR, McKenzie WF, editors. Pathology of Fischer Rat: Reference ans Atlas. Academic Press, Inc; San Diego, California: 1990. [Google Scholar]
- Brown HR, Monticello TM, Maronpot RR, Randall HW, Hotchkiss JR, Morgan KT. Proliferative and neoplastic lesions in the rodent nasal cavity. Toxicol Pathol. 1991;19:358–372. doi: 10.1177/0192623391019004-105. [DOI] [PubMed] [Google Scholar]
- Dow Chemical Company . EPA/OTS Document No. 86800030. Dow Chemical Research Laboratory; 1964. Results of repeated exposure of male and female rats to 80 ppm of propargyl alcohol in air; p. 11. [Google Scholar]
- Dahl AR, Hadley WM. Nasal cavity enzymes involved in xenobiotic metabolism: effects on the toxicity of inhalants. Crit Rev Toxicol. 1991;21:345–372. doi: 10.3109/10408449109019571. [DOI] [PubMed] [Google Scholar]
- Dahl AR, Lewis JL. Respiratory tract uptake of inhalants and metabolism of xenobiotics. Annu Rev Pharmacol Toxicol. 1993;33:383–407. doi: 10.1146/annurev.pa.33.040193.002123. [DOI] [PubMed] [Google Scholar]
- DeMaster EG, Dahlseid T, Redfern B. Comparative oxidation of 2-propyn-1-ol with other low molecular weight unsaturated and saturated primary alcohols by bovine liver catalase in vitro. Chem Res Toxicol. 1994;7:414–419. doi: 10.1021/tx00039a020. [DOI] [PubMed] [Google Scholar]
- Dix KJ, Coleman DP, Fossett JE, Gaudette NF, Jr., Stanley AP, Thomas BF, Jeffcoat AR. Disposition of propargyl alcohol in rat and mouse after intravenous, oral, dermal and inhalation exposure. Xenobiotica. 2001;31:357–375. doi: 10.1080/00498250110053228. [DOI] [PubMed] [Google Scholar]
- Dunn OJ. Multiple Comparisons Using Rank Sums. Technometrics. 1964;6:241. [Google Scholar]
- Dunnett CW, Crisafio R. The operating characteristics of some official weight variation tests for tablets. The Journal of pharmacy and pharmacology. 1955;7:314–327. doi: 10.1111/j.2042-7158.1955.tb12043.x. [DOI] [PubMed] [Google Scholar]
- Green T, Toghill A, Foster JR. The role of cytochromes P-450 in styrene induced pulmonary toxicity and carcinogenicity. Toxicology. 2001;169:107–117. doi: 10.1016/s0300-483x(01)00488-7. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric Estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Lewis RJ., Sr. Hawley's Condensed chemical Dictionary. Twelfth ed. Van Nostrand Reinhold Company; New York: 1993. [Google Scholar]
- Li AA, Fowles J, Banton MI, Picut C, Kirkpatrick DT. Acute inhalation study of allyl alcohol for derivation of acute exposure guideline levels. Inhal Toxicol. 2012;24:213–226. doi: 10.3109/08958378.2012.661801. [DOI] [PubMed] [Google Scholar]
- Lington AW, Bevan C. Patty's Industrial Hygiene and Toxicology. 4th revised edition John Wiley and Sons; New York: 1994. [Google Scholar]
- Morgan KT, Harakema JM. Neoplasms: Nasal Neoplasia. Springer Verlag; New York: 1996. The Upper Respiratory System. [Google Scholar]
- Morgan KT, Monticello TM. Airflow, gas deposition, and lesion distribution in the nasal passages. Environmental Health Perspectives. 1990;85:209–218. doi: 10.1289/ehp.85-1568327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moridani MY, Khan S, Chan T, Teng S, Beard K, O'Brien PJ. Cytochrome P450 2E1 metabolically activates propargyl alcohol: propiolaldehyde-induced hepatocyte cytotoxicity. Chemico-Biological Interactions. 2001;130–132:931–942. doi: 10.1016/s0009-2797(00)00246-5. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Wang RS, Elovaara E, Park SS, Gelboin HV, Vainio H. Cytochrome P450-related differences between rats and mice in the metabolism of benzene, toluene and trichloroethylene in liver microsomes. Biochemical pharmacology. 1993;45:1079–1085. doi: 10.1016/0006-2952(93)90252-r. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program Technical Report Series). [accessed on 7 Aug, 2013];Toxicology and carcinogenesis studies of propargyl alcohol (CAS No. 107-19-7) in F344/N rats and B6C3F1 mice (inhalation studies) 2008 :1–172. Available at http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr552.pdf, [PubMed]
- Ott MG, Teta MJ, Greenberg HL. Assessment of exposure to chemicals in a complex work environment. American Journal of Industrial Medicine. 1989a;16:617–630. doi: 10.1002/ajim.4700160602. [DOI] [PubMed] [Google Scholar]
- Ott MG, Teta MJ, Greenberg HL. Lymphatic and hematopoietic tissue cancer in a chemical manufacturing environment. American Journal of Industrial Medicine. 1989b;16:631–643. doi: 10.1002/ajim.4700160603. [DOI] [PubMed] [Google Scholar]
- Portier CJ, Bailer AJ. Testing for increased carcinogenicity using a survival-adjusted quantal response test. Fundamental and Applied Toxicology. 1989;12:731–737. doi: 10.1016/0272-0590(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Rowe VK, McCollister SB. Patty's Industrial Hygiene and Toxicology. John Wiley and Sons; New York: 1982. Alcohols. [Google Scholar]
- Schmidt E, Schmidt FW. Sex differences of plasma cholinesterase in the rat. Enzyme. 1978;23:52–55. doi: 10.1159/000458549. [DOI] [PubMed] [Google Scholar]
- U. Mohr CC, Capen DL, Dungworth RA, Greismer NI, V.S. Turusov E. Respiratory System. International Agency for Research on Cancer Scientific Publications; Lyon, France: 1992. International Classification of Rodent Tumors, Part I: The Rat1. [Google Scholar]
- Vernot EH, MacEwen JD, Haun CC, Kinkead ER. Acute toxicity and skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicology and Applied Pharmacology. 1977;42:417–423. doi: 10.1016/0041-008x(77)90019-9. [DOI] [PubMed] [Google Scholar]
- Ward JM, Uno H, Kurata Y, Weghorst CM, Jang JJ. Cell proliferation not associated with carcinogenesis in rodents and humans. Environ Health Perspect. 1993;101(Suppl 5):125–135. doi: 10.1289/ehp.93101s5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA. A Note on Shirley Nonparametric Test for Comparing Several Dose Levels with a Zero-Dose Control. Biometrics. 1986;42:183–186. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


