Abstract
The panic disorder (PD) literature provides evidence for both physiologic rigidity and instability as pathognomonic features of this disorder. This ambiguity may be a result of viewing PD at differential levels of temporal analysis. We assessed cardiac variability across three levels of temporal scale in PD patients, posttraumatic stress disorder (PTSD) patients, and healthy controls. Sixteen healthy controls, 14 PD patients, 23 PTSD patients, and 16 PTSD+PD patients presented for a polysomnogram. Differences were assessed in respiratory sinus arrhythmia (RSA), autoregressive stability of heart rate (HR), and the number of nonspecific accelerations in HR over the night. No differences in RSA were found between groups, however; PD patients exhibited significantly lower autoregressive HR stability and all patients had significantly more HR accelerations than controls. These data reinforce prior findings demonstrating physiologic instability in PD and indicate that prior equivocalities regarding physiologic variability in PD may be due to limited temporal scaling of measurements.
Descriptors: Heart rate variability, respiration, panic disorder, post-traumatic stress disorder, sleep
Heart rate variability (HRV) has been identified variously as a marker for anxiety disorders broadly (Licht, de Geus, van Dyck, & Penninx, 2009), for panic disorder (PD) specifically (Friedman, 2007), and for cardiovascular morbidity and mortality (Tsuji et al., 1996). Respiratory sinus arrhythmia (RSA) – the fluctuation in heart period resulting from gating of vagal input to the sinoatrial node during inspiration – has been given special attention as an index of self-regulatory capacity at physiological (Berntson, Cacioppo, & Quigley, 1993), psychological (Butler, Wilhelm, & Gross, 2006), and behavioral levels (Beauchaine, 2001). While a strong case has been made for impaired RSA in PD (Friedman, 2007), several studies have reported null findings in these patients (Asmundson & Stein, 1994; Slaap, Nielen, Boshuisen, van Roon, & den Boer, 2004; Stein & Asmundson, 1994; Woodward et al., 2009). Moreover, several authors have argued that HRV indices provide an incomplete quantitation of cardiac regulation given the multidimensional nature of the autonomic nervous system (Berntson, Cacioppo, & Quigley, 1991) and the chaotic structure of RR intervals (Lipsitz & Goldberger, 1992).
Nonlinear dynamical theories of physiologic organization posit that complex relationships can exist between subordinate and superordinate systems, such that relatively stable systems – e.g. heart rate (HR) – can be undergirded by complex or chaotic processes such as the fractal structure of RR intervals (Lipsitz & Goldberger, 1992). Moreover, this conceptualization of physiologic organization allows that differing amounts of variability versus stability may be observed at different levels of analysis. At the same time, proponents of complexity dynamics analyses have rarely articulated at what scalar levels complexity is prescribed versus contraindicated. Such considerations may be relevant to an understanding of PD, which is marked by rapid shifts in phenomenological experience (i.e. panic attacks) and for which several physiologic theories of both rigidity and instability have been proposed.
In the case of the former are those studies which posit that reduced RSA (i.e. high-frequency HRV) in PD reflects rigid regulatory control of HR and a decreased ability to flexibly respond to changing environmental demands (for a review see Friedman, 2007). Alternatively, several studies have implicated respiratory instability as a marker or potential vulnerability factor in PD during both waking (Gorman et al., 1988; Martinez et al., 2001; Wilhelm, Gevirtz, & Roth, 2001) and sleep (Martinez et al., 1995; Stein, Millar, Larsen, & Kryger, 1995). In addition to the distinct – if collaborative – physiologic systems utilized in these bodies of research, differences in scalar level of analysis should be emphasized as well; studies of RSA examine fluctuations in heart period oscillating at .15 to .40 Hz (i.e. the 3–5 second respiratory cycle), whereas those examining respiratory instability consider phenomena at considerably longer scales (range of above-cited studies = 3 – 30 minutes). Of note, only a handful of studies have examined the role of cardiac-related instability in PD at equivalent temporal scales (Meuret et al., 2011; Roth, Wilhelm, & Trabert, 1998; Wilhelm, Trabert, & Roth, 2001).
In a recent 24-hour ambulatory monitoring study, Meuret and colleagues (2011) demonstrated that the onset of naturally-occurring panic attacks in individuals with PD is immediately preceded by increased instability in cardiorespiratory variables such as tidal volume, end-tidal CO2 partial pressure, and HR. Compared to matched control periods, pre-panic periods exhibited increased variability in these measures – measured in one-minute epochs – as early as 47 minutes before the onsets of attacks. All but one of the 13 identified episodes in this study occurred during waking hours; thus, the role of cognitive processes in the generation or augmentation of these phenomena cannot be ruled out. This is a potentially important consideration given that cognitive-behavioral interventions have been shown to reduce panic symptomatology without mitigating underlying physiologic instability (Abelson, Weg, Nesse, & Curtis, 2001; Martinez et al., 2001).
The importance of identifying the presence, influence, and temporal expression of physiologic instability in PD is not limited to the elucidation of physiologic markers and vulnerability factors. Cognitive-behavioral theories of PD have posited interoceptive conditioning as an etiological factor in the development and maintenance of PD (Goldstein & Chambless, 1978). Such conceptualizations propose that otherwise innocuous variations in physiologic regulation can become panicogenic conditioned stimuli, resulting in a sensitivity to bodily arousal and catastrophic misinterpretation of physiologic sensations (Barlow, 2004; Clark, 1986). However, such models of interoception assume misinterpretation of normative physiologic variations. Absent from these conceptualizations is the possibility that individuals with PD actually experience physiologic sensations outside of normal limits, which provide a palette of experience with a greater variety of physiologic sensations and additional opportunities for panicogenic conditioning. Consequently, it remains important to investigate whether individuals with PD exhibit tonic elevations of physiologic variability at perceptible time scales – such as increases in HR over seconds or minutes.
The present study compared PD patients to healthy controls, post-traumatic stress disorder (PTSD) patients, and patients with PTSD and comorbid PD (PTSD+PD) during a single night's sleep in the laboratory. Both PTSD and PD have demonstrated dysphoric episodic sympathetic activation and increased noradrenergic sensitivity (Bremner, Krystal, Southwick, & Charney, 1996), as well as subjectively poor sleep (Sheikh, Woodward, & Leskin, 2003). Sleep studies help to attenuate effects of cognitive activity on physiologic systems, as waking data can be vulnerable to the effects of anxious states and anxiogenic mentation. We were interested in examining whether PD patients would exhibit significant physiologic instability compared to healthy controls and other anxious individuals. Moreover, given the scarcity of studies examining cardiac instability and the absence of sleep studies on this topic, we also sought to examine the presence of instability at varying scales of temporal measurement. Specifically, the present study assessed the relative degree of variability and stability at three different temporal scales: 1) in the variability of RR intervals in the .15 to .40 Hz range reflected by RSA, 2) in the autoregressive stability of adjacent 30-second epochs of HR assessed via vector-autoregressive modeling (see Method, below), and 3) in the number of nonspecific accelerations (e.g. sinus tachycardia) in HR observed over a single night’s sleep.
Method
Participants
Ninety-nine participants meeting screening and diagnostic criteria (see Procedure, below) were invited to participate in the current study. Of these, 17 declined to return for a screening polysomnography (PSG; which provided the data for this study); 10 participants who underwent PSG had apnea/hypopnea indices (AHIs) greater than 10 events per hour; and 3 participants were excluded due to periodic limb movement arousal indices (PLMAIs) greater than 20 events per hour. AHIs and PLMAIs were manually scored using the LifeShirt (VivoMetrics, Ventura, California) records and PSG records, respectively. A total of 69 participants were included in the present analyses, 16 healthy controls, 14 PD patients, 23 PTSD patients, and 16 PTSD+PD patients. Table 1 provides participant characteristics by group.
Table 1.
Participant characteristics, by group.
| Control (n=16) | PTSD (n=23) | PTSD+PD (n=16) | PD (n=14) | |
|---|---|---|---|---|
| Age | Mean: 36.82 SD: 12.54 |
Mean: 43.38 SD: 10.21 |
Mean: 41.96 SD: 11.54 |
Mean: 41.4 SD: 8.80 |
| Sex | Male: n=6 Female: n=10 |
Male: n=5 Female: n=18 |
Male: n=3 Female: n=13 |
Male: n=5 Female: n=9 |
| Education | Mean: 15.93 SD: 4.06 |
Mean: 15.50 SD: 2.31 |
Mean: 15.06 SD: 2.72 |
Mean: 14.62 SD: 3.25 |
| BMI | Mean: 22.65* SD: 1.98 |
Mean: 26.14 SD: 5.70 |
Mean: 26.91* SD: 4.85 |
Mean: 25.05 SD: 3.10 |
PTSD = post-traumatic stress disorder; PD = panic disorder; BMI = body mass index;
= significant difference at p < .05.
Participants were asked to maintain their nonhypnotic medications. Three tested participants reported having prescriptions for hypnotic medications, which they agreed to discontinue. Of the 69 participants, 19% (n=13) were taking selective serotonin reuptake inhibitors (SSRIs) or other antidepressant medications. These included paroxetine (n=3), fluoxetine (n=2), citalopram (n=1), sertraline (n=1), duloxetine (n=1), buproprion (n=3), nortriptyline (n=1), and venlafaxine (n=1). Two dichotomous dummy-coded variables (SSRI presence versus absence and all other antidepressants, presence versus absence) for antidepressant medication status were held as control variables in all analyses. SSRIs have been shown to alter vagal outflow both positively (Tucker et al., 1997; Tulen et al., 1994) and negatively (Adinoff, Mefford, Waxman, & Linnoila, 1992; Rissanen, Naukkarinen, Virkkunen, Rawlings, & Linnoila, 1998), whereas other antidepressants, such as tricyclics, have been shown to unidirectionally decrease vagal activity (Jakobsen, Hauksson, & Vestergaard, 1984; Mezzacappa, Steingard, Kindlon, Saul, & Earls, 1998; Yeragani et al., 1992).
Procedures
The present study was authorized by the Stanford/Veterans Affairs (VA) Palo Alto Human Research Protection Program. All participants provided written informed consent. Radio and newspaper advertisements were used to recruit participants from the community. These emphasized disturbances of sleep due to distressing experiences, fear and anxiety, and/or nightmares and nocturnal panic attacks. The recruitment of participants denying subjective sleep complaints relied principally on personal contacts between study participants and study staff and their respective social networks. Telephone screening excluded callers if they reported current medical illness, histories of central nervous system disease or injury, a Multivariate Apnea Prediction Index (MAPI) greater than .5, or a previously diagnosed medical sleep disorder (e.g., obstructive sleep apnea or periodic limb movement disorder). Persons were also excluded if they were currently abusing alcohol or other psychotropic substances or were unwilling to discontinue hypnotic medications. Provisional diagnoses were made during telephone screening requiring from 15 to 60 minutes. Participants passing screening traveled to the research setting and underwent structured interviews. Diagnoses of PTSD and PD were based on the Clinician-Administered PTSD Scale (CAPS) and the PD section of the Structured Clinical Interview for the DSM-IV (SCID), respectively. Screening of other Axis I psychopathology was confirmed using the Mini-International Neuropsychiatric Interview (MINI). Eight participants diagnosed with dysthymic disorder were retained. Of these eight participants, five were in the PTSD group, 2 in the PD group, and 1 in the PTSD+PD group.
Participants came to the study site in the afternoon to complete diagnostic interviews and self-report psychometrics. After dinner, they came to the sleep laboratory to prepare for the screening PSG. PSGs were manually staged by a single expert technician according to Rechtschaffen and Kales (1968) criteria applied to 30 second epochs. The PSG montage included EEG (Cz and Pz referenced to linked ears), electro-oculogram (EOG; vertical and horizontal), submental electromyogram (EMG), bilateral anterior tibialis EMG, and oral/nasal airflow (thermistor). Electrocardiogram and respiratory effort (RESP; via abdominal band, see below) were recorded via a VivoMetrics LifeShirt system. After completion of a pre-sleep startle protocol, participants went to sleep ad libitum.
Data Acquisition and Preparation
To facilitate manual sleep staging, EEG data were band-filtered to 0.1 – 50 Hz, EOG to 0.1 – 10 Hz, and EMG to 10 – 100 Hz. ECG was collected with Meditrace foam-backed ECG electrodes in the "lead 2" configuration (left ninth rib referenced to right subclavicular region). Respiratory effort was transduced using a Braebon piezoelectric strain-guage located around the abdomen. Data were amplified via a Grass 78 polygraph equipped with 7P511J AC-coupled amplifiers. Analog pre-filtering for both EGC and RESP was 1 – 100 Hz. All data were sampled at 600 Hz with 16 bits of amplitude resolution via a Kiethley 3708 data acquisition circuit using custom software developed in VisualBasic. ECG was later digitally filtered to 1–100 Hz and RESP .1 – .5 Hz prior to R-wave detection and RESP rate calculation, respectively, using phase-invariant time-domain FIR filters implemented in Matlab. RSA was estimated from the .15 – .40 Hz spectral band of HRV spectra. Spectra were calculated using the classical Welch periodogram analysis as implemented in Matlab (“pwelch”) applied to detrended, Hamming-windowed, 30-second epochs of interbeat intervals (IBI) resampled to real time at 4 Hz.
Of the physiological data collected during PSG, the three physiological foci of the present study were RSA, HR, and respiration rate, whereby the first two were examined for stability versus instability and the latter was held as a control variable. Continuous measurements of ECG and respiration were processed to create time series of 30-second epochs of HR, respiration rate, and RSA. Nonspecific accelerations in HR were identified via a moving, localized window of +/− five epochs (i.e. m=5 and width=11 epochs). Individual epoch-HR-estimates greater than 1.5 times the median window value were designated as nonspecific accelerations. Across participants, accelerations comprised a mean of 2.2% of epochs (range = 0% – 7.7%).
Approach to Modeling the Autoregressive Stability of Heart Rate
To assess physiologic instability at the intermediate scale, the present study employed dynamic factor modeling (Molenaar, 1985), a vector-autoregressive (VAR) methodology that utilizes a structural equation model framework to assess contemporaneous correlations and time-lagged regressions in multivariate time series. The dynamic factor model is a VAR model for measuring linear interdependencies in multivariate time series, and is a generalization of univariate autoregressive models and path models. The multivariate time series for HR, respiration rate, and RSA were used to estimate block-Toeplitz variance-covariance matrices for each participant. The block-Toeplitz matrix represents the contemporaneous and time-lagged covariance between variables at time (t) and times (t−n), where n is a designated number of lags. The present study employed a VAR (1) model, which estimated a single lag and utilized a block-Toeplitz matrix with variance-covariance relationships for time (t) and time (t−1). Thus, for each participant we generated a VAR (1) model containing a set of contemporaneous correlations between HR, respiration rate, and RSA at times (t) and (t−1), respectively, and regressions of all variables at time (t) on time (t−1). Consistent with other multivariate general linear model procedures – such as multiple regression – correlations and regression parameters are estimated simultaneously and thus control for the presence of each other.
Thus, the concurrent time series for HR, respiration rate, and RSA were modeled such that we controlled for contemporaneous correlations between HR, respiration rate, and RSA, as well as lagged autoregressive relationships within each variable, and cross-lagged (i.e. Granger-causal; Granger, 1969) relationships between variables. This allowed us to estimate the degree of epoch-to-epoch variability in HR while accounting for the effects of the interrelated cardiorespiratory system. Regarding the study hypotheses, HR instability can be considered the inverse of autoregressive stability. Dynamic factor models were constructed and analyzed for each individual separately, a potentially important extension of more traditional, aggregate methodologies that may not accurately represent individual-level phenomena (Fisher, Newman, & Molenaar, 2011; Molenaar, 2004). All dynamic factor analyses were carried out in LISREL (Version 8.80). Standardized coefficients were extracted from each individual model for use in regression analyses reported below.
Results
Preliminary Analyses
Two multivariate analyses of variance (MANOVA) were conducted to assess possible group differences in participant characteristics and the distribution of sleep stages and sleep duration across participants. The first MANOVA examined group differences in age, education, and body mass index (BMI). There were no significant group differences in age [F(3,65) = .91, p = .44] or years of education [F(3,65) = .37, p = .77]. However there was a significant between-subjects effect of group on BMI [F(3,65) = 3.96, p = .01]. Scheffe’s post hoc test revealed a significant difference between the PTSD+PD and healthy control groups (MD = 5.20, SE = 1.59, p = .02), whereby individuals in the PTSD+PD group exhibited significantly greater BMI. No other significant differences were observed between groups. The second MANOVA examined group differences in the distribution of sleep stage percentages and sleep duration across individuals. There were no significant differences between groups in the percentages of stage 1 [F(3,68) = .83, p = .49], stage 2 [F(3,68) = .55, p = .65], slow-wave sleep [F(3,68) = .278, p = .84], or REM sleep [F(3,68) = 2.21, p = .10], and although there was an effect of group on sleep duration at the alpha level of .05 [F(3,68) = 2.74, p = .05], Scheffe’s post hoc tests revealed no significant differences between any groups in sleep duration. Table 2 presents sleep percentages and sleep duration (in hours) by group.
Table 2.
Sleep stage percentages and hours of sleep, by group.
| Stage 1 Percent | Stage 2 Percent | SWS Percent | REM Percent | Hours of Sleep | |
|---|---|---|---|---|---|
| Controls | Mean: 8.01 SD: 6.33 |
Mean: 44.92 SD: 6.57 |
Mean: 18.05 SD: 8.16 |
Mean: 17.88 SD: 6.15 |
Mean: 7.06 SD: 1.31 |
| PTSD | Mean: 9.20 SD: 4.46 |
Mean: 47.89 SD: 8.77 |
Mean: 15.94 SD: 8.72 |
Mean: 17.65 SD: 7.24 |
Mean: 8.27 SD: 1.58 |
| PD | Mean: 7.37 SD: 3.38 |
Mean: 46.22 SD: 11.01 |
Mean: 17.89 SD: 11.85 |
Mean: 15.85 SD: 6.05 |
Mean: 7.34 SD: 1.58 |
| PTSD+PD | Mean: 7.86 SD: 4.98 |
Mean: 43.96 SD: 10.22 |
Mean: 15.99 SD: 9.63 |
Mean: 13.09 SD: 9.88 |
Mean: 7.46 SD: 1.43 |
SWS = slow-wave sleep; REM = rapid eye movement; PTSD = post-traumatic stress disorder; PD = panic disorder.
Regression of Respiratory Sinus Arrhythmia on Diagnostic Groups and Control Parameters
A regression analysis was conducted to examine the effect of diagnostic status on RSA. Age, gender, BMI, series length, and both SSRI and non-SSRI antidepressant medications were held as control parameters. Robust regression via M-estimation (Huber, 1964) – a generalization of maximum likelihood – was employed via the MASS package in R (version 2.15.1). Table 3 presents the results of this analysis. Gender and non-SSRI antidepressant medications were significant predictors of RSA, whereby females and individuals taking non-SSRI antidepressants exhibited greater RSA across the measurement period. No significant effects of diagnosis on RSA were observed.
Table 3.
Regression models for the effect of diagnostic groups and control parameters on respiratory sinus arrhythmia, epoch-to-epoch autoregressive stability, and number of nonspecific heart rate accelerations.
| Respiratory Sinus Arrhythmia1 | Autoregressive Stability of Heart Rate1 | Number of Heart Rate Accelerations2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | t | p | d | β | SE | t | p | d | β | SE | t | p | d | |
| Intercept | .223 | .003 | 72.33 | < .001 | -- | .623 | .036 | 17.51 | < .001 | -- | 3.222 | .063 | 50.90 | < .001 | -- |
| PTSD | −.002 | .005 | −.47 | .64 | .14 | −.052 | .048 | −1.08 | .28 | .31 | .193 | .093 | 2.08 | .04 | .60 |
| PTSD+PD | .0003 | .004 | .07 | .95 | .03 | −.056 | .051 | −1.09 | .28 | .38 | .498 | .096 | 5.16 | < .001 | 1.81 |
| PD | .0003 | .004 | .06 | .95 | .03 | −.112 | .050 | −2.28 | .03 | .87 | .645 | .087 | 7.41 | < .001 | 2.82 |
| Gender | .011 | .003 | 3.49 | .001 | .59 | .107 | .036 | 3.02 | .004 | .51 | −.241 | .063 | −3.81 | < .001 | .65 |
| Age | −.002 | .003 | −.98 | .33 | .17 | .005 | .002 | 3.18 | .002 | .54 | −.019 | .003 | −6.72 | < .001 | 1.14 |
| BMI | .004 | .003 | 1.01 | .31 | .17 | .004 | .004 | 1.05 | .30 | .18 | −.012 | .007 | −1.61 | .11 | .27 |
| SSRI | −.0002 | .003 | −.06 | .95 | .03 | .022 | .016 | 1.37 | .18 | .53 | −.208 | .037 | −5.66 | < .001 | 2.22 |
| AntiDep | .009 | .003 | 2.72 | .008 | 1.06 | .006 | .017 | .38 | .71 | .15 | −.118 | .039 | −3.00 | .003 | 1.17 |
| TS Length | .003 | .003 | .78 | .44 | .13 | .0001 | .0001 | .74 | .46 | .13 | .001 | .0002 | 5.50 | < .001 | .94 |
Reference group = healthy controls; PTSD = post-traumatic stress disorder; PD = panic disorder; BMI = body mass index; SSRI = selective serotonin reuptake inhibitor; AntiDep = non-SSRI antidepressant medications; TS Length = length of time series;
= estimated via robust linear regression;
= estimated via generalized linear regression with Poisson distribution; d = Cohen’s d (calculated as d = t*sqrt[2/n]).
Regression of Autoregressive Stability of 30-second Heart Rate Epochs on Diagnostic Groups and Control Parameters
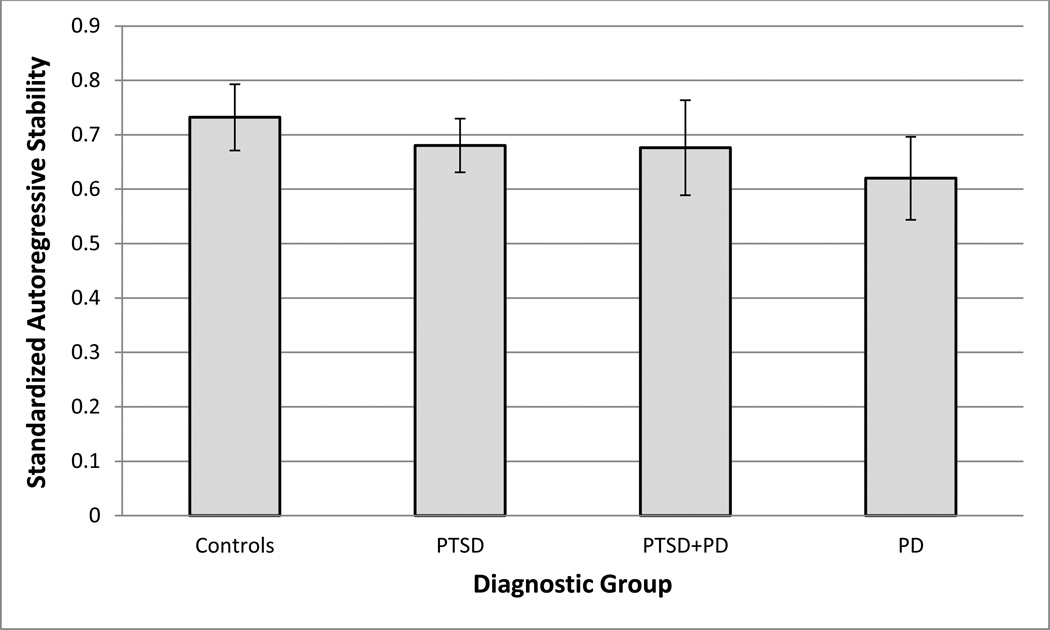
After conducting dynamic factor analyses for each participant, model parameters were extracted and aggregated across the study population. A regression was then conducted to examine the effect of diagnostic status on the epoch-to-epoch autoregressive stability of HR. Robust regression via M-estimation was again utilized. While medication status, age, gender, BMI, and series length were held as control parameters within the regression model, it should be reemphasized that the contributions of RSA and respiration to the broader cardiorespiratory system were controlled for within the person-specific dynamic factor models. Table 3 presents the results of this analysis. Gender and age significantly predicted autoregressive stability of HR, with both older and female participants exhibiting greater epoch-to-epoch stability. In addition, PD patients significantly differed from controls, exhibiting lesser autoregressive stability. No significant differences were found between the PTSD or PTSD+PD groups and controls. Figure 1 presents the autoregressive stability of HR by group.
Figure 1.
Autoregressive stability of heart rate across sleep period, by group.
Controls = healthy control group; PTSD = post-traumatic stress disorder; PD = panic disorder
Poisson Regression for N umber of Nonspecific Accelerations in HR on Diagnostic Groups and Control Parameters
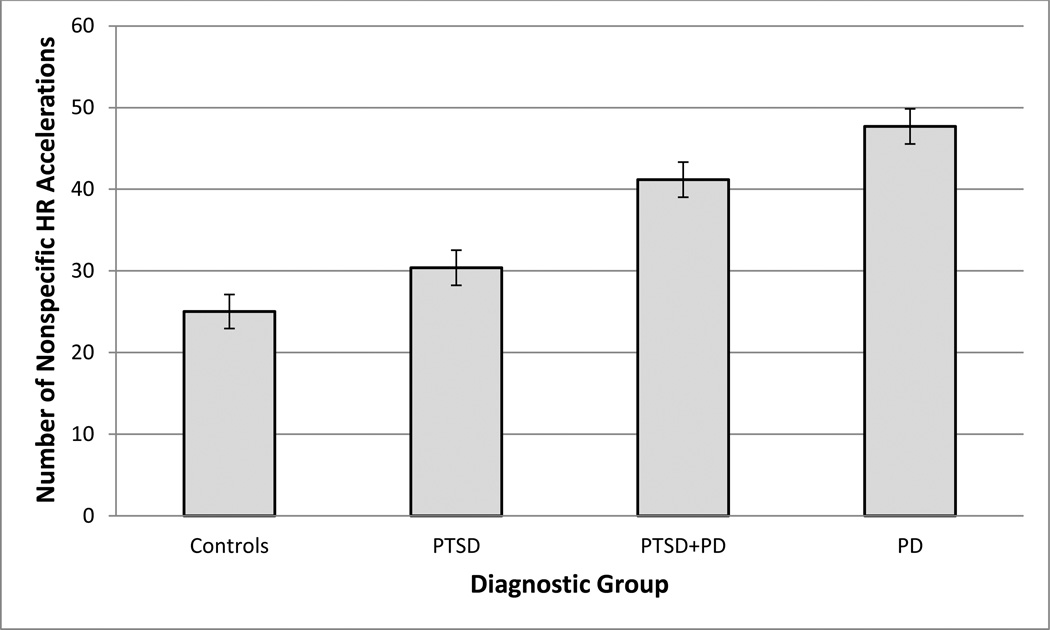
Finally, we examined the effect of diagnostic status on the number of nonspecific accelerations in HR observed in the epoch-level time series. Age, gender, BMI, series length, and antidepressant medication status were again held as control parameters in the model. A generalized linear regression with a Poisson distribution was utilized given the quantification of the dependent variable as counts. Table 3 presents the results of this analysis. All three patient groups – PTSD, PD, and PTSD+PD – exhibited a significantly greater number of accelerations compared to controls. In addition, both SSRI and non-SSRI antidepressant medications, age, gender, and time series length were significant predictors of the number of accelerations. Use of SSRI and non-SSRI antidepressant medications predicted fewer accelerations; females and older participants also exhibited fewer accelerations; and longer time series predicted a greater number of accelerations. Figure 2 presents the rates of nonspecific HR accelerations by group.
Figure 2.
Number of nonspecific heart rate accelerations across sleep period, by group.
HR = heart rate; Controls = healthy control group; PTSD = post-traumatic stress disorder; PD = panic disorder
Discussion
The present study assessed cardiac variability and stability at three, successively larger, levels of temporal analysis; in the 3–5 second respiratory range of parasympathetically-mediated HRV (RSA), in the autoregression of HR estimated across 30-second epochs within dynamic factor models, and in the number of nonspecific HR accelerations across a single night. We found that – compared to controls – PD patients exhibited increasing levels of variability at greater temporal scales. That is, no differences were seen in RSA, a medium effect was seen in autoregressive stability, and a large effect was seen in the number of nonspecific HR accelerations. In addition, while neither the PTSD nor PTSD+PD groups exhibited significant differences with controls in RSA or autoregressive stability, a medium effect was observed for the PTSD group and a large effect was observed for the PTSD+PD group in the number of HR accelerations. Of note, the PTSD patient group was the largest of the four groups, indicating that power was not likely implicated in the reduced effect in this group. These data reinforce prior findings demonstrating physiologic instability in PD and point to a set of physiologic processes either specific to or unmasked during sleep.
Although a strong case has been made for impaired RSA in PD (Friedman, 2007), several studies – including recent work by our group (Woodward et al., 2009) – have reported no significant difference between PD patients and healthy controls (Asmundson & Stein, 1994; Slaap et al., 2004; Stein & Asmundson, 1994). Arguments supporting impaired RSA in PD posit that PD is marked by rigid regulatory control of HR and an absence of adaptive variability in RR intervals. However, this hypothesis is contrasted by a body of literature demonstrating physiologic instability in PD across multiple cardiorespiratory indices (Abelson et al., 2001; Martinez et al., 2001; Martinez et al., 1995; Meuret et al., 2011; Roth et al., 1998; Stein et al., 1995; Wilhelm, Trabert, et al., 2001). Moreover, a recent study demonstrated that naturally-occurring panic attacks in individuals with PD were preceded by increased variability in minute-by-minute epochs of HR and respiration (Meuret et al., 2011). Our findings indicating decreased HR stability in 30-second epoch-to-epoch measurements and increased variability in mean HR across a single night’s sleep appear to strengthen the argument in favor of physiologic instability in PD. Additionally, observation of these phenomena during sleep attenuates the potential role of cognitive processes such as bodily scanning, sensitivity to somatic sensations, and catastrophic misinterpretation in the observed results.
Findings indicating autonomic rigidity and variability in PD may not be incompatible, as apparently divergent results may derive from methodologies addressing different temporal scales of analysis. Nonlinear dynamical theory predicts that complex or chaotic systems often undergird stable superordinate systems, such that nonlinear relationships can exist between variability at microscopic and stability at macroscopic levels of analysis. To this end, it has been argued that physiologic health is a function of complex or chaotic temporal variability, and pathology is marked by rigid periodicities and temporal invariance (Goldberger, 1992; Lipsitz & Goldberger, 1992). Here, high degrees of microscopic variability underlie more stable and adaptive macroscopic functioning. It is thus possible that in PD this system is disrupted, leading to decreased underlying variability and increased overt variability. Consequently, complexity – a measure of the chaotic, fractal-like nature of physiologic systems (Goldberger, 1990; Lipsitz & Goldberger, 1992) – has been shown to be reduced in HR time series in PD (Rao & Yeragani, 2001). It is possible that RSA reflects cardiac variation at a temporal scale that fails to embed the relevant pathognomic dynamics and variability in HR and respiration characterizing PD.
Cognitive-behavioral models of PD have posited sensitivity to bodily arousal and catastrophic misinterpretation of physiologic sensations as maintaining factors (Barlow, 2004; Clark, 1986) resulting from interoceptive conditioning (Goldstein & Chambless, 1978). Ehlers (1993) has proposed three hypotheses to account for increased sensitivity to and perception of bodily symptoms in PD: greater physiologic reactivity compared to other groups, an enhanced ability to perceive bodily sensations and changes, and heightened attentional focus on bodily functioning. A fourth possibility is that some individuals are subject to an increased level of physiologic variability, providing additional opportunities for maladaptive attentional focus or conditioning to interoceptive stimuli.
The findings of the present study point to a series of cardiac accelerations observed during sleep, presumably unrelated to cognition or reaction to environmental provocation. It should be noted that accelerations were observed in all groups, including healthy controls. However, in a clinical group sensitive to bodily changes, such perturbations could have an anxiogenic effect. Thus, a positive feedback loop may occur in PD, wherein tonic variation in physiologic stability leads to anxious arousal, which in turns promotes an increased likelihood of further perturbations through mechanisms such as adrenergic arousal. Consistent with this, the present study found that both PD groups exhibited a greater number of accelerations compared to controls. While the PTSD group also exhibited a significantly greater number of accelerations than controls, this effect was substantially smaller than in either the PD or PTSD+PD groups. Moreover, no significant difference was observed between the PTSD and control groups when not controlling for antidepressant medications, a finding consistent with Yehuda and colleague’s observations that while individuals with PTSD often exhibit increased circulating norepinephrine and adrenergic receptor reactivity (Yehuda, 2002), these effects are mitigated in PTSD patients with comorbid depression (Yehuda et al., 1998).
An important limitation of this study was the absence of discrete nocturnal panic attacks in either the PD or PTSD+PD groups. Prior research has sought to link physiologic instability in PD to the occurrence of such attacks. To this end, Meuret and colleagues (Meuret et al., 2011) demonstrated that increased variability in cardiorespiratory variables preceded the onset of panic attacks in PD patients. Moreover, the increases in physiologic variability observed by these authors were relative to within-person matched control periods (which were not followed by panic attacks). Thus, the findings of the present study would be strengthened by demonstrating significant relationships between decreased autoregressive stability of HR, increased nonspecific HR accelerations, and the onset and/or severity of panic attacks in PD patients. It remains an empirical question whether the phenomena observed in the present study represent group-level differences between PD and comparison groups, or whether exaggerated relationships exist in the presence of discrete panic attacks. Additionally, the data from the present study were taken from a single night, with no opportunity for subjects to acclimate to the setting or PSG montage. Thus, anxious participants may not have habituated to the potentially anxiogenic effects of sleeping in a novel environment and being monitored by PSG equipment. Thus, future research utilizing multiple recordings may be indicated to further validate the present results.
The present study provides evidence for the role of elevated physiologic variability, or instability, in PD. Moreover, the present findings demonstrate increasing separation between healthy controls and PD patients in the level of physiologic variability at increasingly larger time scales. While we have contrasted this variability with the construct of physiologic rigidity in PD, it may be heuristic to defer such a comparison until a fuller description of autonomic function in PD is achieved. Given the complexity of physiological regulation and the multiplicity of underling control systems (c.f. Goldberger et al., 2002), it would not be surprising if the flexibility/rigidity framework was superseded by models incorporating variability at multiple time scales. Research in PD may be accelerated by regularly assessing a range of time scales and also incorporating the coordinated measurement of mood and behavior.
Acknowledgments
This research was funded by Grant number MH64724 from the National Institute of Mental Health to Javaid I. Sheikh.
We are grateful for the administrative support provided by the Research Service of the Veterans Affairs Palo Alto Health Care System and the Sponsored Projects Office, Stanford University. We are likewise grateful for the technical support provided by Ned J. Arsenault, Karin Voelker, Tram Nguyen, Janel Lynch, Karyn Skultety, Erika Mozer, and Gregory A. Leskin.
Footnotes
All authors reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Aaron J. Fisher, University of California, Berkeley
Steven H. Woodward, Dissemination and Training Division, National Center for PTSD, Palo Alto
References
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biological Psychiatry. 2001;49(7):588–595. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Mefford I, Waxman R, Linnoila M. Vagal tone decreases following intravenous diazepam. Psychiatry Research. 1992;41(2):89–97. doi: 10.1016/0165-1781(92)90101-8. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Stein MB. Vagal attenuation in panic disorder: an assessment of parasympathetic nervous system function and subjective reactivity to respiratory manipulations. Psychosomatic Medicine. 1994;56(3):187–193. doi: 10.1097/00006842-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. the Guilford Press; 2004. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98(4):459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23(1):39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Clark DM. A cognitive approach to panic. Behaviour Research and Therapy. 1986;24(4):461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Ehlers A. Somatic symptoms and panic attacks: a retrospective study of learning experiences. Behaviour Research and Therapy. 1993;31(3):269–278. doi: 10.1016/0005-7967(93)90025-p. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Newman MG, Molenaar PCM. A quantitative method for the analysis of nomothetic relationships between idiographic structures: Dynamic patterns create attractor states for sustained posttreatment change. Journal of Consulting and Clinical Psychology. 2011;79(4):552–563. doi: 10.1037/a0024069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Goldberger AL. Nonlinear dynamics, fractals and chaos: applications to cardiac electrophysiology. Annals of Biomedical Engineering. 1990;18(2):195–198. doi: 10.1007/BF02368429. [DOI] [PubMed] [Google Scholar]
- Goldberger AL. Fractal mechanisms in the electrophysiology of the heart. IEEE Engineering in Medicine and Biology Magazine. 1992;11(2):47–52. doi: 10.1109/51.139036. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AJ, Chambless DL. A reanalysis of agoraphobia. Behavior Therapy. 1978;9(1):47–59. [Google Scholar]
- Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Klein DF. Ventilatory physiology of patients with panic disorder. Archives of General Psychiatry. 1988;45(1):31–39. doi: 10.1001/archpsyc.1988.01800250035006. [DOI] [PubMed] [Google Scholar]
- Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica: Journal of the Econometric Society. 1969;37(3):424–438. [Google Scholar]
- Huber PJ. Robust estimation of a location parameter. The Annals of Mathematical Statistics. 1964;35(1):73–101. [Google Scholar]
- Jakobsen J, Hauksson P, Vestergaard P. Heart rate variation in patients treated with antidepressants. An index of anticholinergic effects? Psychopharmacology. 1984;84(4):544–548. doi: 10.1007/BF00431464. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, van Dyck R, Penninx BW. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA) Psychosomatic Medicine. 2009;71(5):508–518. doi: 10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Goldberger AL. Loss of 'complexity' and aging. Journal of the American Medical Association. 1992;267(13):1806. [PubMed] [Google Scholar]
- Martinez JM, Kent JM, Coplan JD, Browne ST, Papp LA, Sullivan GM, Klein DF. Respiratory variability in panic disorder. Depression and Anxiety. 2001;14(4):232–237. doi: 10.1002/da.1072. [DOI] [PubMed] [Google Scholar]
- Martinez JM, Papp LA, Coplan JD, Anderson DE, Mueller CM, Klein DF, Gorman JM. Ambulatory monitoring of respiration in anxiety. Anxiety. 1995;2(6):296–302. [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Wilhelm FH, Zhou E, Conrad A, Ritz T, Roth WT. Do unexpected panic attacks occur spontaneously? Biological Psychiatry. 2011;70(10):985–991. doi: 10.1016/j.biopsych.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Steingard R, Kindlon D, Saul JP, Earls F. Tricyclic antidepressants and cardiac autonomic control in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(1):52–59. doi: 10.1097/00004583-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Molenaar PCM. A dynamic factor model for the analysis of multivariate time series. Psychometrika. 1985;50(2):181–202. [Google Scholar]
- Molenaar PCM. A Manifesto on Psychology as Idiographic Science: Bringing the Person Back Into Scientific Psychology, This Time Forever. Measurement: Interdisciplinary Research and Perspectives. 2004;2(4):201–218. [Google Scholar]
- Rao RK, Yeragani VK. Decreased chaos and increased nonlinearity of heart rate time series in patients with panic disorder. Autonomic Neuroscience. 2001;88(1–2):99–108. doi: 10.1016/S1566-0702(01)00219-3. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Los Angeles: Brain Research Institute, University of California; 1968. [Google Scholar]
- Rissanen A, Naukkarinen H, Virkkunen M, Rawlings RR, Linnoila M. Fluoxetine normalizes increased cardiac vagal tone in bulimia nervosa. Journal of Clinical Psychopharmacology. 1998;18(1):26–32. doi: 10.1097/00004714-199802000-00005. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Trabert W. Autonomic instability during relaxation in panic disorder. Psychiatry Research. 1998;80(2):155–164. doi: 10.1016/s0165-1781(98)00066-3. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Woodward SH, Leskin GA. Sleep in post-traumatic stress disorder and panic: Convergence and divergence. Depression and Anxiety. 2003;18(4):187–197. doi: 10.1002/da.10066. [DOI] [PubMed] [Google Scholar]
- Slaap BR, Nielen MM, Boshuisen ML, van Roon AM, den Boer JA. Five-minute recordings of heart rate variability in obsessive-compulsive disorder, panic disorder and healthy volunteers. Journal of Affective Disorders. 2004;78(2):141–148. doi: 10.1016/s0165-0327(02)00240-9. [DOI] [PubMed] [Google Scholar]
- Stein MB, Asmundson GJ. Autonomic function in panic disorder: cardiorespiratory and plasma catecholamine responsivity to multiple challenges of the autonomic nervous system. Biological Psychiatry. 1994;36(8):548–558. doi: 10.1016/0006-3223(94)90619-x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Millar TW, Larsen DK, Kryger MH. Irregular breathing during sleep in patients with panic disorder. American Journal of Psychiatry. 1995;152(8):1168–1173. doi: 10.1176/ajp.152.8.1168. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Tucker P, Adamson P, Miranda R, Jr, Scarborough A, Williams D, Groff J, McLean H. Paroxetine increases heart rate variability in panic disorder. Journal of Clinical Psychopharmacology. 1997;17(5):370–376. doi: 10.1097/00004714-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Tulen JH, Mulder G, Pepplinkhuizen L, Man in 't Veld AJ, van Steenis HG, Moleman P. Effects of lorazepam on cardiac vagal tone during rest and mental stress: assessment by means of spectral analysis. Psychopharmacology. 1994;114(1):81–89. doi: 10.1007/BF02245447. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gevirtz R, Roth WT. Respiratory dysregulation in anxiety, functional cardiac, and pain disorders. Assessment, phenomenology, and treatment. Behavior Modification. 2001;25(4):513–545. doi: 10.1177/0145445501254003. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Physiologic instability in panic disorder and generalized anxiety disorder. Biological Psychiatry. 2001;49(7):596–605. doi: 10.1016/s0006-3223(00)01000-3. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Arsenault NJ, Voelker K, Nguyen T, Lynch J, Skultety K, Sheikh JI. Autonomic activation during sleep in posttraumatic stress disorder and panic: a mattress actigraphic study. Biological Psychiatry. 2009;66(1):41–46. doi: 10.1016/j.biopsych.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. New England Journal of Medicine. 2002;346(2):108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Siever LJ, Teicher MH, Levengood RA, Gerber DK, Schmeidler J, Yang R-K. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biological Psychiatry. 1998;44(1):56–63. doi: 10.1016/s0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Weinberg P, Merlos B. Effect of imipramine treatment on heart rate variability measures. Neuropsychobiology. 1992;26(1–2):27–32. doi: 10.1159/000118892. [DOI] [PubMed] [Google Scholar]