Abstract
Manganese (Mn) is an essential trace metal nutrient, however, excess Mn can be neurotoxic. The degree to which chronic environmental or occupational exposures to Mn in adults cause neuropsychological dysfunction is of considerable interest. Descriptions of neuropsychological dysfunction following chronic Mn exposure have been somewhat inconsistent though, likely owing to different measures of exposure in different populations, complicated by factors of mixed exposures and differences in neuropsychological tests administered. We previously described up-regulation of the mRNA expression for amyloid-beta (A-beta) precursor-like protein 1 (APLP1) and the presence of A-beta diffuse plaques in frontal cortex of Mn-exposed monkeys. The present study examined Mn-induced changes in performance on a paired associate learning (PAL) task that has been suggested as a marker for preclinical Alzheimer’s disease. Aspects of performance of this task were affected early following initiation of Mn exposure. Thus, PAL performance may be a sensitive and valuable tool for the early, preclinical detection of incipient dementia and it may also be a sensitive tool for detecting cognitive dysfunction from Mn exposure. The current cognitive data, combined with our previous findings, suggest that frontal cortex may be a particularly sensitive target for the effects of Mn on cognition and that chronic Mn exposure may initiate or accelerate a process that could lead to or predispose to Alzheimer’s like pathology and cognitive dysfunction.
Keywords: manganese, cognition, monkeys
1. Introduction
Manganese (Mn) is an essential trace metal nutrient that plays important roles as a catalyst or cofactor for a variety of enzymatic processes in the brain (Hurley, 1987) as well as influencing synaptic transmission (Takeda, 2003) and the synthesis and metabolism of proteins, lipids, and carbohydrates (Keen, 1984; Wedler, 1993). However, excess Mn can be neurotoxic (Greger, 1998). The degree to which chronic environmental or occupational exposures to Mn in adults cause neuropsychological dysfunction is an area of considerable interest. It has been suggested that long-term, continuing exposure at very high levels is necessary to cause motor function deficits (Gibley, 2001; Hathaway, 1996) however, a number of studies have recently reported neuropsychological dysfunction resulting from lower level chronic exposures (Bowler et al., 2007a; Laohaudomchok et al., 2011) and dose-related cognitive deficits have been reported (Bowler et al., 2007b; Ellingsen et al., 2008).
The descriptions of neuropsychological dysfunction following chronic Mn exposure have been somewhat inconsistent, likely owing to different measures of exposure in different populations, complicated by factors of mixed exposures (i.e., Mn plus other metals such as lead and cadmium in various industrial exposures (for example, exposure to welding fumes) and differences in neuropsychological tests administered and neuropsychological domains assessed. However, recent studies have more often than not reported deficits in areas of fronto-executive functioning such as attention, working memory, and cognitive flexibility (Bowler et al., 2007a; Chang et al., 2010; Laohaudomchok et al., 2011).
We have previously described subtle deficits in spatial and non-spatial working memory and as well as subtle fine motor deficits in non-human primates exposed to different levels of Mn, with exposures lasting 227–272 days (Schneider et al., 2006, 2009). At a lower exposure level, only subtle neuropsychological changes were evident (Schneider et al., 2006) while at a higher exposure level, in the range of levels reported for human environmental, medical or occupational exposures, significant deficits in non-spatial working memory appeared relatively early and remained for the duration of the study (Schneider et al., 2009). Since these prior studies focused primarily on working memory, the goal of the present work, part of a large, longitudinal study of effects of chronic Mn exposure on various aspects of cognition, was to investigate the effects of chronic exposure to Mn, at a level previously shown to affect non-spatial working memory (Schneider et al., 2009), on a complex fronto-executive task. The task used for this study was visuospatial paired associate learning. This task, which requires both visual pattern and visuospatial memory, is known to be dependent on the functional integrity of frontal lobes (Owen et al., 1995) and to be particularly sensitive to, and specific for, detecting symptoms of neurocognitive decline associated with mild cognitive impairment and development of Alzheimer’s disease (Fowler et al., 1997; Fowler et al., 1995; Swainson et al., 2001).
2. Material and methods
2.1 Animals
Seven adult adult male M. fascicularis macaques, 5–6 years old at the start of the study, were used; four received Mn exposure and three served as control animals that were treated exactly the same as the Mn-exposed animals but received vehicle injections. Animals were fed standard lab chow (LabDiet ®5045, Animal Specialties and Provisions, Quakertown, PA, USA) supplemented with fruits or vegetables and water was available ad libitum in the home cage. Animals were food restricted in order to achieve training and stable responding on multiple tasks and restriction parameters and body weights were monitored regularly to ensure maintenance of good body condition scores. All animal studies were reviewed and approved by the Animal Care and Use Committee at Thomas Jefferson University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Behavioral training and testing
Following quarantine, animals were adapted to sitting in a primate chair and trained to perform cognitive tasks while sitting in front of a automated test panel attached to the front wall of a sound-attenuating, dimly lit, well-ventilated enclosure. Cognitive testing was achieved using the non-human primate Cambridge Neuropsychological Test Automated Battery (CANTAB) and the CANTAB Intellistation with pellet reward (Lafayette Neuroscience, Lafayette, IN, USA).
2.3 Paired Associate Learning (PAL)
The PAL task involves learning to associate visual stimuli with distinct spatial locations on a trial-by trial basis (see Schneider et al., 2013). This complex task has attentional, working memory, and executive components. At the easiest level, a single stimulus is presented in different possible locations on the screen and in the response phase, the animal must touch the stimulus in the same location in which was originally shown. On more difficult trials (containing 2 or 3 different stimuli), each stimulus is presented consecutively with a 1 second delay between presentations. After all stimuli have been presented, one of the sample stimuli is presented again in 2, 3, or 4 different locations on the screen. The animal must touch the target location in which the stimulus was originally presented in order to receive a reward. If an animal fails to successfully complete a trial, the same trial is presented again, up to six times and if still performed incorrectly, the trial is aborted and the system moves to the next trial (also referred to as a sequence). Each testing session consisted of 10 trials (or sequences) each at the following levels of task difficulty (varied slightly depending on different animal’s capabilities): 2 stimuli, 2 locations; 3 stimuli, 3 locations; 3 stimuli, 4 locations; or 2 stimuli, 2 locations; 2 stimuli, 3 locations; 3 stimuli, 3 locations. The measures analyzed were: the number of trials (sequences) successfully completed (within 6 presentations per level of difficulty); the number of trials (sequences) completed on the first attempt; and, the average number of attempts needed to successfully complete a trial (sequence) at each level of difficulty.
2.4 Mn Exposure
Once animals achieved a stable performance baseline on all measures, they were transported to Johns Hopkins University for in vivo imaging studies (and received additional imaging studies performed approximately midway through the Mn exposure period, results to be reported elsewhere). After return from the baseline imaging study, animals underwent a surgical procedure for placement of an indwelling jugular catheter attached to a titanium vascular access port secured to muscle parallel to the thoracic spine (MIDA-CBAS-C50, Instech Laboratories, Inc., Plymouth Meeting, PA, USA). Following an approximate two week post-surgery recovery period, behavioral testing was reinitiated and once a stable level of performance was confirmed, Mn exposure was initiated. For access to the ports, skin overlying the port was shaved and disinfected with DuraPrep Surgical Solution (3M, St. Paul, MN, USA). Ports were flushed at least once per week prior to initiation of Mn exposure. Using aseptic technique, a Huber needle, attached to a syringe, was inserted through the skin into the septum of the port. Saline (approx. 3.0 ml) was infused to verify patency of the catheter and then the line was locked with heparin/dextrose locking solution (hep/dex, 0.3ml heparin (10,000U/ml in 3ml dextrose). For Mn administration, after an initial saline flush, the syringe was removed and replaced with one containing Mn solution (or saline vehicle). Mn was infused at a rate of approx. 0.5 ml/min. followed by a flush of approx. 3.0 – 5.0 ml of sterile saline and hep/dex locking solution. In order to minimize risk of injuries to staff and animals, the port was accessed while the animals were seated in chair and while under light gas sedation (isofluorane 4% to induce, 2% to maintain, oxygen 1%). Mn was administered as MnSO4 monohydrate (Sigma-Aldrich, St. Louis, Mo., USA; 15 mg/kg/week for 5 weeks and then 20 mg/kg/week for the remainder of the study period: in which the total dose per week was divided and given at two different times/week. Manganese sulfate was prepared fresh for each injection (50 mg/ml in sterile saline), pH adjusted to 7.0, filtered, and warmed to 37 °C prior to use.
2.5 Data Analysis
Means and standard deviations were calculated for cognitive outcome measures at baseline (i.e., 3–4 weeks prior to the first Mn exposure) and at approximately 1 month intervals after the first Mn exposure. One-way analysis of variance followed by post hoc comparisons using Newman-Keuls test was used to analyze baseline performance. Task performance prior to and following Mn exposure was compared by repeated measures analysis of variance for each measure with appropriate post hoc comparisons (Dunnett’s post hoc t test) made to assess changes between baseline and each post Mn observation period. Additional one-way analysis of variance with post hoc Newman-Keuls test compared performance between conditions (i.e., vehicle vs. Mn-exposed). Statistical significance was defined at p<0.05.
3. Results
3.1 Manganese administration
Although all animals were implanted with vascular access ports for administration of Mn, these ports failed in most of the animals. In the 4 animals receiving Mn, ports failed at 72, 86 and 262 days after implantation; one animal maintained the port throughout the study. In the control group, the ports failed in 2 of the 3 animals (at 154 and 288 days). After ports failed, they were removed surgically and Mn or vehicle was administered intravenously by slow infusion, under the same anesthesia conditions used for port access. The mean ± s.e.m. cumulative amount of Mn administered was 330.28 ± 0.35 mg Mn/kg body weight (1016.23 ± 1.09 mg MnSO4/kg). Animals in this study received Mn over a 52 week period. Mean baseline blood Mn levels in the control group were 11.7 ± 3.0 μg/L and 11.0 ± 3.1 μg/L in the Mn exposure group. At the end of the approximate 52 week observation period, blood Mn levels in the control animals were 8.9 ± 1.1 μg/L (7, 8, and 11 μg/L) and 109.9 ± 15.3 μg/L (94, 94, 96, and 156 μg/L) in Mn-exposed animals. Brain Mn levels are not yet available form the animals described in this report as these animals are involved in ongoing studies.
3.2 Effects of Chronic Mn Exposure on Paired Associate Learning (PAL)
On the PAL task, in the baseline condition, the animals easily completed the number of trials (sequences) at all levels of task difficulty (9.8 ± 0.1, 9.3 ± 0.2, 8.3 ± 0.4 at 2 stimuli 2 locations (level 1), 2 stimuli 3 locations or 3 stimuli 3 locations (level 2), and 3 stimuli 3 locations or 3 stimuli 4 locations (level 3), respectively). Analysis of variance showed a statistically significant effect of task difficulty on the number of sequences completed at baseline (F(2,33) = 8.62, p = 0.001). Posthoc comparisons showed significant differences between performance at level 1 and level 3 (p < 0.001) and between level 3 and level 2 (p<0.01), but no difference between performance at level 1 and level 2. There were no significant effects of Mn exposure on the number of sequences completed at any level of task difficulty, compared to baseline performance (Figure 1A). However, by 12 months of Mn exposure there was no longer a significant effect of task difficulty on the number of sequences completed (F(2,33) = 1.69, p = 0.20).
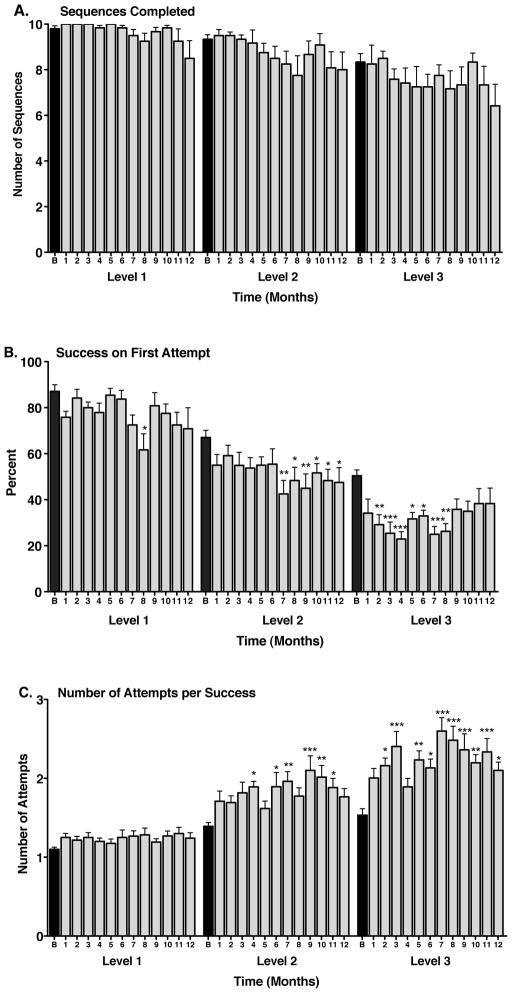
Figure 1.
Paired associate learning (PAL) performance in Mn-exposed animals. A. Manganese exposure had no significant effect on the number of sequences completed at any level of difficulty. Task difficulty levels (from easiest to most difficult) are designated as 1 (2 stimuli, 2 locations), 2 (2 stimuli, 3 locations or 3 stimuli, 3 locations), and 3 (3 stimuli, 3 locations or 3 stimuli, 4 locations). B. The number of trials (sequences) successfully completed on the first attempt (percent first attempt success) decreased with increasing duration of manganese exposure and this effect was related to the difficulty of the task (*p < 0.05, **p < 0.01, ***p < 0.001 vs. respective baseline (B) performance). C. The average number of attempts needed to successfully complete a trial (sequence) increased significantly with manganese exposure at all but the easiest task level (*p < 0.05, **p < 0.01, ***p < 0.001 vs. respective baseline (B) performance). Black bars depict baseline performance; shaded bars depict post-Mn performance. Data are presented as means ± SEMs. At the 1 month observation period, 1 animal was experiencing complications from port removal surgery and its data were omitted from the analysis.
At baseline, animals completed significantly fewer PAL trials on the first attempt at the more difficult levels compared to the easier levels of the task. One way analysis of variance showed a significant effect of task difficulty on the number of sequences completed on the first attempt (F(2,33) = 42.12, p < 0.0001). Pairwise comparisons showed a significant difference between performance at the level 1 and level 2, level 1 and level 3, and level 2 and level 3 (p < 0.001 for each). Mn exposure had significant effects on the number of sequences completed on the first attempt at all but the easiest level of task performance (Figure 1B). At level 2, there was a significant effect of time of Mn exposure on the number of sequences completed on the first attempt (F(12,11) = 1.99, p < 0.03) with significant decreases in performance beginning at the 7th month of Mn exposure (Figure 1B). There was also a significant effect of time of Mn exposure on the number of sequences completed on the first attempt at level 3 (F(12,11) = 3.35, p < 0.0003). At this most difficult task level, performance was affected by the first month of Mn exposure (Figure 1B). Lack of statistical significance at the last few time points might be related to the increased variability in performance across the small number of Mn-treated animals (average SEM for months 9–12 = 5.52; average SEM for months 2–8 = 3.48) or less likely, to a learning effect only observed in this parameter. By 12 months of Mn exposure, there was still a significant effect of task difficulty on the number of sequences completed on the first attempt (F(2,33) = 4.95, p = 0.01). However, in contrast to baseline, there was no longer a significant difference between performance at levels 2 and 3.
Analysis of variance showed a significant effect of task difficulty on the number of attempts needed to successfully complete a sequence in the baseline condition (F(2,33) = 14.92, p < 0.0001). Animals required more attempts to successfully complete trials at level 1 vs. level 3 (p < 0.001) and at level 1 vs. level 2 (p < 0.01) (Figure 1C). Mn exposure caused a significant increase in the number of attempts needed to successfully complete a sequence at levels 2 (F(12,11) = 2.59, p < 0.004) and 3 (F(12,11) = 4.38, p < 0.0001) of the task (Figure 1C). The influence of Mn was somewhat more variable at level 2 but at the more difficult level 3, consistent decreases in performance began to be observed by the 5th month of Mn exposure (Figure 1C).
3.3 Performance of Control Animals
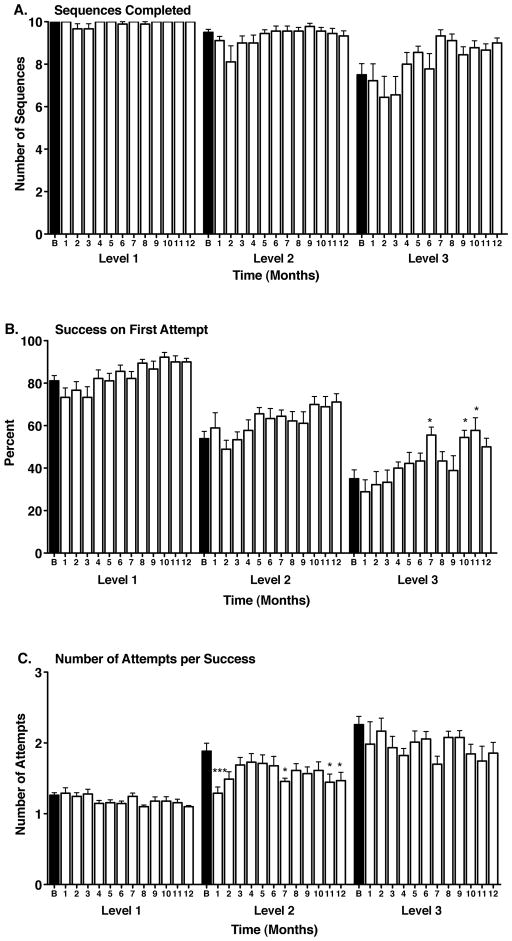
In the baseline condition, the control animals also easily completed the sequences at all levels of task difficulty (10.0 ± 0.0, 9.5 ± 0.1, 7.5 ± 0.5 at levels 1, 2 and 3, respectively). There was also an effect of task difficulty on number of sequences completed (F(2,24) = 17.58, p < 0.0001), the number of sequences completed on the first attempt (F(2,24) = 45.92, p < 0.0001), and the number of attempts need to successfully complete a sequence (F(2,24) = 27.53, p < 0.0001) (Figure 2A, B, C). These effects remained significant after 12 months of testing. Similar to the Mn-treated animals, there were no significant effects of time (saline injections) on the number of sequences completed at any level of task difficulty, compared to baseline performance (Figure 2A). At levels 1 and 2 of the task, there was no effect of time on the number of sequences completed on the first attempt compared to baseline, although there was trend towards improved performance over time. At level 3, there were significant improvements in performance at some of the later time points (Figure 2B). At level 1, there was no difference in the number of attempts needed to successfully complete a sequence at any time point, compared to baseline (Figure 2C). At level 2, there were some improvements in performance at some time points and at level 3 there were no significant differences from baseline, although again there was trend toward improved performance over time.
Figure 2.
Paired associate learning (PAL) performance in control, vehicle-exposed animals. A. Manganese exposure had no significant effect on the number of sequences completed at any level of difficulty, although there was a trend towards improved performance over time at the most difficult task level. Task difficulty levels (from easiest to most difficult) are designated as 1 (2 stimuli, 2 locations), 2 (2 stimuli, 3 locations or 3 stimuli, 3 locations), and 3 (3 stimuli, 3 locations or 3 stimuli, 4 locations). B. The number of trials (sequences) successfully completed on the first attempt (percent first attempt success) tended to increase over time (*p < 0.05 vs. respective baseline (B) performance). C. The average number of attempts needed to successfully complete a trial (sequence) either did not change or decreased over time (*p < 0.05, ***p < 0.001 vs. respective baseline (B) performance). Black bars depict baseline performance; open bars depict performance at time points similar to those in the Mn-exposed group. Data are presented as means ± SEMs.
4. Discussion
The current findings show that performance of non-human primates on a task that has been suggested as a marker for preclinical Alzheimer’s disease (Blackwell et al., 2004) is disrupted by chronic administration of moderate levels of Mn exposure. For example, using the human version of the PAL task on a CANTAB system, subjects with ‘questionable dementia’ exhibited significant deterioration over time in PAL performance, while maintaining performance on other neuropsychological measures (Fowler et al., 2002). At the end of a 2 year study period, all of the ‘questionable dementia’ subjects studied fulfilled the NINCDS–ADRDA criteria for probable dementia of the Alzheimer type. Performance on the PAL identified the onset of a progressive memory deterioration in this group (Fowler et al., 2002) and in almost all cases, deficits in PAL performance were detected well in advance of deterioration on other neuropsychological measures (Fowler et al., 2002). While PAL performance may be a sensitive and valuable tool for the early, preclinical detection of incipient dementia (Fowler et al., 2002), it may also be a sensitive tool for detecting cognitive dysfunction from Mn exposure.
Compared to their baseline pre-Mn performance, animals that received chronic exposure to Mn developed significant deficits on performance of the PAL task. In contrast, control animals that received vehicle administration according to the same schedule as Mn administration, either showed no change over time or improvement in performance, compared to their baseline level of task performance. Two main aspects of PAL performance, the number of attempts needed to successfully complete a sequence and the percent of sequences that were successfully completed on the first attempt, were disrupted within the first months of Mn exposure. Although Mn exposure did not have a significant long-term effect on the overall ability of animals to complete the task (i.e., the number of sequences completed per level of difficulty did not significantly change over the course of the study), Mn exposure did interfere with incremental learning aspects of the task (animals needed more attempts to complete a sequence at the intermediate and more difficult levels of the task). On these measures, performance at the easiest level of the task was unaffected by Mn exposure, suggesting that the effect of Mn on performance was associated with trial difficulty and that Mn interfered with task performance in a memory-load specific manner. These data also suggest that the results were not due to a Mn-induced impairment in motor function that could have interfered with task performance, as all levels of the task required the same exact motor response. In studies with human subjects with preclinical Alzheimer’s disease, errors to criterion or the number of errors made before the completion of the test (a measure similar to our number of attempts needed to successfully complete a sequence) were increased compared to controls (Fowler et al., 1995).
We previously reported that Mn administered at the same dose and according to the same exposure paradigm as used in this study resulted in mean blood Mn levels (measured several weeks after the last Mn administration) of 89.9 ± 10.5 μg/L (range 63.5 to 134.1 μg/L) after approximately 14 weeks of Mn administration and 70.8±15.6 μg/L (range 33.3 to 118.8 μg/L) after 28 weeks of Mn administration. Frontal cortical Mn levels these animals ranged from 0.34 to 0.55 μg/g tissue after 28 weeks of Mn administration and were within the range of known environmental/occupational exposures (Schneider et al., 2009). The blood Mn levels reported currently are somewhat higher than the previously reported figures (primarily due to one outlier which may reflect contamination of the sample) but still consistent with the upper range of environmental, medical and occupational exposures (ex., Takser et al., 2003). Blood collection for analysis also occurred within 48 hrs of Mn administration in the current study and several weeks after Mn administration in the previous study, making direct comparisons between the two studies difficult. It is worth noting however, that blood Mn levels may not necessarily accurately reflect Mn concentration in the brain, due to the ways in which absorbed Mn is sequestered and transported (Zheng et al., 2011). A number of studies have shown that blood Mn is a poor biomarker of Mn exposure or toxicity, especially under conditions of chronic exposure (Laohaudomchok et al., 2011; Zheng et al., 2011). Blood Mn levels may reflect exposure over a short and recent period of time (hours to days) but may not provide important information on the relationship between exposure and a biological effect in a target tissue like brain for chronic exposures (Laohaudomchok et al., 2011; Zheng et al., 2011).
The current findings on the sensitivity of the PAL task for detecting cognitive disturbance in Mn-exposed monkeys are particularly interesting in view of previous findings from our group in which we described up-regulation of the mRNA for amyloid-beta (A-beta) precursor-like protein 1 (APLP1) gene and increased APLP1 protein expression and the presence of A-beta diffuse plaques in frontal cortex of Mn-exposed monkeys (Guilarte, et al., 2008). The observation that Mn-exposed monkeys exhibited diffuse amyloid-β plaques and a significant level of neuropathology was unexpected since these animals were of an age (6–8 years at the end of the study) where we would not have expected to find evidence of plaque formation. The current cognitive data, combined with our previous findings, suggest that frontal cortex may be a particularly sensitive target for the effects of Mn on cognition and that chronic Mn exposure may initiate or accelerate a process that could lead to or predispose to Alzheimer’s like pathology and cognitive dysfunction.
Highlights.
Paired associate learning is a marker for preclinical Alzheimer’s disease
Paired associate learning performance is impaired in chronic Mn-exposed monkeys
Vehicle control animals showed no change or improved performance over time
Some paired associate learning deficits occurred soon after start of Mn exposure
Paired associate learning is sensitive for detecting Mn-related cognitive change
Acknowledgments
This work was funded by NIH grant R01 ES010975.
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17:42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, Roels HA, Park RM, Diamond E, Mergler D, Bouchard M, Bowler RP, Koller W. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007a;28:298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007b;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Lee JJ, Seo JH, Song HJ, Kim JH, Bae SJ, Ahn JH, Park SJ, Jeong KS, Kwon YJ, Kim SH, Kim Y. Altered working memory process in the manganese-exposed brain. Neuroimage. 2010;53:1279–1285. doi: 10.1016/j.neuroimage.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, Merkurjeva L, Chashchin M, Thomassen Y, Chashchin V. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology. 2008;29:48–59. doi: 10.1016/j.neuro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized delayed matching to sample and paired associate performance in the early detection of dementia. Appl Neuropsychol. 1995;2:72–78. doi: 10.1207/s15324826an0202_4. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized neuropsychological tests in the early detection of dementia: prospective findings. J Int Neuropsychol Soc. 1997;3:139–146. [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Gibley RL, Sullivan JB., Jr . Manganese. In: Sullivan JB, JGRK, editors. Clinical Environmental Health and Toxic Exposures. Lippincott; Philadelphia: 2001. pp. 930–937. [Google Scholar]
- Greger JL. Dietary standards for manganese: overlap between nutritional and toxicological studies. J Nutr. 1998;128:368S–371S. doi: 10.1093/jn/128.2.368S. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway GJ, Proctor NH, Hughes JP. Proctor and hughes’ chemical hazards of the workplace. 4. Van Nostrand Reinhold; New York: 1996. [Google Scholar]
- Keen CL, Lonnerdal B, Hurley LS. Manganese. In: Frieden E, editor. Biochemistry of the Essential Ultratrace Elements. Plenum Oress; New York: 1984. pp. 89–132. [Google Scholar]
- Hurley LS, CLK, editors. Manganese. Academic Press; San Diego: 1987. pp. 185–223. [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Shrairman R, Landau A, Christiani DC, Weisskopf MG. Neuropsychological effects of low-level manganese exposure in welders. Neurotoxicology. 2011;32:171–179. doi: 10.1016/j.neuro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, Weisskopf MG. Toenails, blood and urine as biomarkers of manganese exposure. J Occup Environ Med. 2011;53:506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of chronic manganese exposure on working memory in non-human primates. Brain Res. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Pioli EY, Jianzhong Y, Li Q, Bezard E. Effects of memantine and galantamine on cognitive performance in aged rhesus macaques. Neurobiol Aging. 2013;34:1126–1132. doi: 10.1016/j.neurobiolaging.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicol. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Wedler FC. Biological significance of manganese in mammalian systems. Prog Med Chem. 1993;30:89–133. doi: 10.1016/s0079-6468(08)70376-x. [DOI] [PubMed] [Google Scholar]
- Zheng W, Fu SX, Dydak U, Cowan DM. Biomarkers of manganese intoxication. Neurotoxicol. 2011;32:1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]