Abstract
The subventricular zone (SVZ) continuously supplies new interneurons that incorporate into preexisting olfactory bulb circuitry. Khodosevich et al showthat connective tissue growth factor (CTGF) regulates a multicellular signaling cascade determining the number of postnatally-born inhibitory interneurons in odor-activated glomeruli.
Robust neurogenesis takes place in subventricular zone (SVZ) of the adult mouse brain(Luskin, 1993). The neuroblasts, generated in the SVZ, travel along the rostral migratory stream, arrive at the core of the olfactory bulb (OB) and migrate radially until their appearance in either the granule cell layer or the glomerular layer(Lois and Alvarez-Buylla, 1994; Luskin, 1993). Subsequently, the neuroblasts, differentiate in their respective layers into the two major interneurons: granule cells andperiglomerular cells. Each day, thousands of neuroblasts migrate to the OB and integrate into pre-existing circuits after differentiating. The majority end up in the granule layer, while the rest end their journey in the glomerular layer based on their fate that was established in the SVZ(Luskin, 1993; Merkle et al., 2007). This continual flux of cells provides the substrate to selectively incorporate new cells and remodel the olfactory circuitry that processes sensory input. In this issue of Neuron, Khodosevich et al show that odorant-activated expression of the previously characterized connective tissue growth factor, CTGF, controls the survival of periglomerular cells by potentiating TGFβ2 activity and activating an apoptotic pathway in periglomerular cells in selective glomeruli (Khodosevich et al., 2013). This regulation is important in odorant-mediated behaviors.
Olfactory stimuli are transduced by the sensory neurons (OSNs) located in the sensory epithelium of the nasal cavity. Each OSN expresses exactly one allelefrom a repertoire of ~1000 olfactory receptor (OR) genes(Buck and Axel, 1991). Axons from OSNs that choose the same OR converge into a common glomerulus located in the glomerular layer of the OB. These sensory processes make excitatory synapses with mitral and tufted cells, the major excitatory neurons of the OB. Within the glomerulus, OSNs also synapse with periglomerular cells, the major glomerular layer inhibitory interneuron(Lledo et al., 2008; Mombaerts, 2006).
CTGF wasinitially highlightedin microarray studies in which the authors observed high expression of CTGF in the OB, but low or undetectable levelsin the SVZ and the rostral migratory stream leading to the OB(Khodosevich et al., 2007; Khodosevich et al., 2009). While the role of CTGF in wound healing and fibrosis is established, little is known about its role under normal physiological conditions(Shi-Wen et al., 2008).In the OB, CTGF is detectedin the OB glomerular layer at postnatal day 3 (P3), peaks at P5 and continues to be expressed in into adulthood(Khodosevich et al., 2013). This coincides with a time of rapid cellular and anatomical expansion of the sensory epithelium. The CTGF-positive cells co-express cholecystokinin (CCK) and are glutamatergic, characteristic of external tufted cells(Liu and Shipley, 1994; Ohmomo et al., 2009). Although the CTGF was detected primarily postnatally, the external tufted cells are primarily born embryonically during bulb development. Consistent with their identification as external tufted cells,CTGF-positive neurons were generated during the peak of OB development (E16-18), and their birth completed by P0.Together these observations suggest that earlier-born external tufted cells adopt a new and selective role that involves CTGF in the postnatal and adult animal.
Rather than depend on tissue-specific conditional knock-outs, the authors utilized adeno-associated virus (AAV) that robustly infects all cell types - dividing and nondiving in the OB. Their experiments have revealed an interesting intercellular control mechanism that modulates the number of inhibitory interneurons. The authors combined expression manipulations that decreased CTGF expression with retroviral EGFP reporter-marking of SVZ neuroblasts at P3 and observed an increase in the number of EGFP-positive cells in the glomerular layer. This effect was reversed when ashRNA-resistant form of CTGF mRNA was injected. Morphological analysis identified EGFP-positive cells in the glomerular layer as periglomerular neurons. Why were more periglomerular cells present in the CTGF knockdown brains? During the first few weeks after the newborn neurons reach the OB, roughly half of them undergo apoptosis. The authors hypothesized that knock down of CTGF selectively altered apoptosis of periglomerularbut not granule cells. The number of apoptotic cells in the glomerular layer but not the granule cell layer decreased in the CTGF knockdown mice. Injecting shRNA-resistant CTGF increased the number of apoptotic cells. Thus, CTGF seemed to play a role in promoting apoptosis of periglomerular cells.
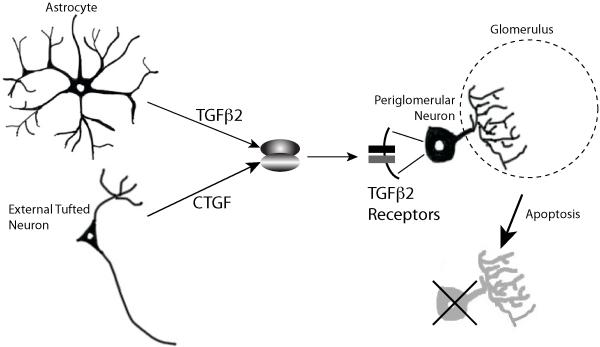
Although the role of CTGF in inhibitory interneuron survival was clear, the signaling pathway that mediated the effects of the tufted cell-derived factor was more enigmatic. In particular, the receptors for canonical CTGF signaling are not expressed in the maturing neuroblasts of the olfactory bulb. CTGF is also known to bind to other growth factors and modulate their activity(Cicha and Goppelt-Struebe, 2009). The authors beautifully demonstrate this ligand complex mechanism and identified TGFβ2 as a potential candidate involved in the CTGF downstream signaling. Each of the proteins in this putative pathway, CTGF, TFGβ2, and its receptors TGFβRI and TGFβRII, were expressed in the glomerular layer.TFGβ2 was secreted by GFAP-positive astrocytes, while its receptors – TGFβRI and TGFβRII – were expressed in a subpopulation of newly-born GAD-positive periglomerular neurons. In vivo evidence for CTGF/TGFβ2 interaction was provided by knocking down TGFβRI selectively in postnatally born neuroblasts via viral injection. TGFβRI knockdown led to an increase in the number of neurons located in the glomerular layer, indicating a reduction in apoptosis. Furthermore, the effect of knocking down CTGF in OB, shown in the initial experiments to effect cell survival, could be abrogated by the simultaneous knockdown ofTGFβRI receptor in the targetneuroblasts. Together, these data indicated that CTGF acts in a complexwith TGFβ2 toactivate a TGFβ signaling pathway in postnatally-bornperiglomerular cells that leads to activation of apoptosis in these cells (figure 1).
Figure 1.
Schematic of the Cells and Molecules Contributing to Regulation of Periglomerular Survival.
Knockdown of CTGF led to an increased number of periglomerular cells. Did this affect olfactory information processing at the level of OB circuitry and electrophysiology?In the CTGF knockdown OB, the frequency but not the amplitude of spontaneous inhibitory postsynaptic currents (sIPSC) increased in both prenatally and postnatally-generated populations of periglomerular interneurons. The frequency and the amplitude of spontaneous excitatory postsynaptic current (sEPSC) in these cells, however, did not change significantly.Therefore, the sEPSC:sIPSC (excitation:inhibition ratio) decreased in postnatally and prenatally-born CTGF-knockdown periglomerular cells. These results indicated that CTGF expression level impacts local circuit activity andthe presence of an increased number of periglomerular neurons resulted in stronger inhibition on the mitral cells.
Do the alterations in the number of inhibitory cells have a consequence in mouse olfactory behavior? To understand its role, odorant detection, discrimination, and long-term memory were examined in mice that were subject to CTGF knockdown in the olfactory bulb. Compared to control mice, CTGF knockdown mice displayed a decrease in odorant detection threshold, i.e. the CTGF knockdown mice were more sensitive to odors than control mice. In the odorant discrimination test, CTGF knockdown mice performed better than control mice. The only test in which CTGF knockdown and control mice performed equally was the long-term memory test using supra-threshold odorant stimuli.
The mammalian olfactory bulb is subject to dynamic and variable changes throughout adult life. New OSNs are continually re-innervating the OB as a result of normal turnover of these cells and traumatic or pathogenic lesions in the sensory epithelium. Furthermore, the odor environment is constantly changing in intensity and quality. The authors therefore tested whether the expression of CTGF was dependent on olfactory experience. OSNs were chemically ablated, and CTGF expression was examined at various time points post-ablation (during regeneration of OSNs). CTGF expression was the lowest in the glomerular layer when sensory input was lacking and expression gradually increased with OSN re-innervation of the OB. Conversely, lack of sensory input led to a strong increase in TGFβ2 expression. Since lack of sensory input led to a decrease in CTGF expression, the authors wondered whether olfactory enrichment could increase CTGF expression. Elucidating the effects of individual odors or even simple mixtures on the entire population of olfactory glomeruli is problematic.The authors took advantage of genetically modified mice where the target glomeruli for a well characterized olfactory receptor (MOR23) could be visualized. Exposure to the odorant lyral, that activates the MOR23-IRES-tauGFP OSNs, resulted in decreasedperiglomerularneuronal survival in the two glomeruli activated by the odor.Adjacent glomeruli were unaffected. Additionally, after CTGF knockdown in MOR23-IRES-tauGFP mice, lyral was unable to decrease neuronal survival in these glomeruli. Taken together, their observations indicate that olfactory activity modulates number of inhibitory interneurons present in the odorant-specific glomeruli through a CTGF-dependent mechanism.
The maintenance of olfactory bulb organization and function requires the exquisite balance of inhibitory cells and connections in the face of dynamic changes in excitatory inputs and stimulus. On one hand, homeostasis is essential to provide appropriate signal processing and output. In contrast, when the odor environment is modulated over short time spans, the novelty of the resulting signals in the bulb could provide additional cues to drive sensory behaviors. How these two opposing processes are regulated and resolved remains largely unanswered.
In their paper, the authors identified a new and exciting role of CTGF under physiological conditions. CTFG acts as a regulator of survival of postnally born periglomerular cells in the OB. In addition, they identified a pathway that is involved in the neuronal survival process. The model that they propose is that CTGF, derived from prenatally-born external tufted cells, potentiates the activity of astrocyte-derived TGFβ2. TGFβ2 binds to its receptors TGFβ2RI and TGFβ2RII, expressed bypostnatally-born periglomerular, and activates SMAD3 toturn on the apoptotic pathway in periglomerular cells. The overall modest decrease in number of periglomerular cells leads to greater olfactory sensitivity and selective changes in OB circuitry in specific glomeruli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009;35:200–208. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- Khodosevich K, Inta D, Seeburg PH, Monyer H. Gene expression analysis of in vivo fluorescent cells. PloS one. 2007;2:e1151. doi: 10.1371/journal.pone.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodosevich K, Lazarini F, von Engelhardt J, Kaneko H, Lledo PM, Monyer H. Connective Tissue Growth Factor Regulates Interneuron Survival and Information Processing in the Olfactory Bulb. Neuron. 2013 doi: 10.1016/j.neuron.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Khodosevich K, Seeburg PH, Monyer H. Major signaling pathways in migrating neuroblasts. Frontiers in molecular neuroscience. 2009;2:7. doi: 10.3389/neuro.02.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WL, Shipley MT. Intrabulbar associational system in the rat olfactory bulb comprises cholecystokinin-containing tufted cells that synapse onto the dendrites of GABAergic granule cells. The Journal of comparative neurology. 1994;346:541–558. doi: 10.1002/cne.903460407. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends in neurosciences. 2008;31:392, 400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annual review of cell and developmental biology. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Ohmomo H, Ina A, Yoshida S, Shutoh F, Ueda S, Hisano S. Postnatal changes in expression of vesicular glutamate transporters in the main olfactory bulb of the rat. Neuroscience. 2009;160:419, 426. doi: 10.1016/j.neuroscience.2009.02.048. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine & growth factor reviews. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]