Abstract
Context
Although partial nephrectomy is the preferred treatment for many patients with early-stage kidney cancer, recent clinical trial data demonstrating better survival for patients treated with radical nephrectomy has generated new uncertainty regarding the comparative effectiveness of these treatment options.
Objective
We sought to clarify this issue by performing an instrumental variable analysis comparing long-term survival after partial versus radical nephrectomy among a population-based patient cohort whose treatment reflects contemporary surgical practice.
Design, Setting, and Patients
We performed a retrospective cohort study of Medicare beneficiaries with clinical stage T1a kidney cancer treated from 1992 through 2007 with partial or radical nephrectomy. Using an instrumental variable approach to account for measured and unmeasured differences between treatment groups, we fit a two-stage residual inclusion model to estimate the treatment effect of partial nephrectomy on long-term survival.
Main outcome measures
Overall and kidney cancer-specific survival.
Results
Among 7,138 Medicare beneficiaries with early-stage kidney cancer, we identified 1,925 (27.0%) patients treated with partial nephrectomy, and 5,213 (73.0%) patients treated with radical nephrectomy. During a median follow-up of 62 months, 487 (25.3%) and 2,164 (41.5%) patients died following partial or radical nephrectomy, respectively. Kidney cancer was the cause of death for 37 (1.9%) patients treated with partial nephrectomy, and 222 (4.3%) patients treated with radical nephrectomy. Patients treated with partial nephrectomy had a significantly lower risk of death (HR 0.54, 95% CI 0.34-0.85). This corresponded to a predicted survival increase with partial nephrectomy of 5.6 (95% CI 1.9-9.3), 11.8 (95% CI 3.9-19.7), and 15.5 (95% CI 5.0-26.0) percentage points at 2-, 5-, and 8-years post-treatment (p<0.001). No difference was noted in kidney cancer-specific survival (HR 0.82, 95% CI 0.19-3.49).
Conclusions
Among Medicare beneficiaries with early-stage kidney cancer who were candidates for either surgery, treatment with partial rather than radical nephrectomy was associated with improved survival.
Keywords: kidney neoplasm, nephrectomy, partial nephrectomy, survival
Introduction
The incidence of kidney cancer has risen inexorably over the last two decades due mainly to an increasing number of patients diagnosed with small (i.e., ≤ 4 cm) renal tumors.1-3 Although radical nephrectomy had long been the standard treatment for these patients, partial nephrectomy (i.e., surgical removal of the tumor only) is now the preferred treatment option based on its provision of equivalent cancer control and better preservation of long-term renal function.4-8 Several observational studies have also demonstrated better survival following partial versus radical nephrectomy, a finding that is generally attributed to the avoidance of chronic kidney disease-related morbidity and mortality.9-11
More recently, however, long-term data from a multi-center, randomized trial comparing outcomes among patients treated for small kidney cancers identified a survival benefit for those treated with radical (versus partial) nephrectomy.12 Nonetheless, this study—conducted by the European Organization for Research and Treatment of Cancer (EORTC)—had several notable limitations (e.g., accrual difficulties, premature closure) and occurred in an era when most surgeons rarely performed partial nephrectomy.13 As such, many argue that the EORTC trial is not generalizable to contemporary practice.
Because the likelihood of better designed trials is low, we performed an instrumental variable analysis using linked Surveillance, Epidemiology, and End Results (SEER)-Medicare data to compare long-term survival among patients treated with partial versus radical nephrectomy. Instrumental variable analysis is an econometric method that leverages naturally-occurring variation within observational data to balance both measured and unmeasured variables among treatment groups.14,15 By applying this technique to a population-based patient cohort, we can clarify the comparative effectiveness of partial versus radical nephrectomy in the treatment of patients with early-stage kidney cancer.
Methods
Data source
After this study was deemed exempt by the Institutional Review Board at the University of Michigan, we used linked data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Medicare and Medicaid Services (Medicare) to identify patients diagnosed with incident kidney cancer from 1992 through 2007. SEER is a nationally-representative, population-based registry that collects data regarding cancer incidence, treatment, and mortality.16 Successful linkage with hospital and physician claims is achieved for over 90% of patients whose primary health insurance is provided by the Medicare program.16,17
Cohort identification
After limiting our sample to patients with Medicare fee-for-service coverage, we identified a preliminary cohort of 9,111 patients diagnosed with localized, non-urothelial kidney tumors less than 4 cm in size (i.e., clinical stage T1a kidney cancer).18 We then excluded patients lacking claims for kidney cancer surgery, and those with claims suggesting a solitary kidney, bilateral tumors, and/or multi-focal disease. This process yielded a sample comprising 7,398 patients with early-stage kidney cancer.
Treatment variable and patient covariates
Next, we used a validated claims-based algorithm to identify patients treated with partial or radical nephrectomy by either an open or laparoscopic approach.19 This served as the treatment variable for our analyses.
For each patient, we used SEER data to ascertain demographic information including age, gender, race/ethnicity, marital status, income and education, and cancer severity (i.e., grade and histology).20 We also assigned each patient to a rural or urban locale using rural-urban commuting area (RUCA) codes.21 We measured pre-existing comorbidity using a modification of the Charlson index based on inpatient and outpatient claims submitted during the 12 months prior to surgery.22 We also used established claims algorithms to identify post-operative complications that occurred during the index hospitalization or within 30 days of surgery (please see eMethods 1 for additional details).23-26
Outcome measures
Our primary outcome was overall survival. We ascertained the occurrence of death from any cause based on the date of death provided in the Medicare files. We defined survival time as the interval from the date of surgery until the date of death or until May 31, 2010 (the last month for which vital status data were available). Using cause of death codes available through SEER for patients who died on or before December 31, 2008, we measured kidney cancer-specific survival as a secondary outcome.
Statistical Methods
We used chi-squared tests to evaluate associations between surgical treatment (i.e., partial versus radical nephrectomy) and patient-level covariates. Next, we calculated Kaplan-Meier estimates for all-cause and kidney-cancer specific mortality, stratified by treatment. We compared mortality between treatment groups using the log-rank test.
One important concern with studies based on observational data is the potential for residual confounding due to unmeasured patient characteristics (or other relevant variables). If present, such confounding can lead to incorrect inferences regarding the effectiveness of different treatments. One strategy to address this limitation is the use of an instrumental variable analysis that is designed to balance both measured and unmeasured variables between treatment groups.14
To be considered valid, an instrumental variable must satisfy two conditions: 1) the variable must be highly associated with the treatment of interest (in this case receipt of partial nephrectomy); and 2) the variable cannot be associated with the outcome (in this case survival) except through its effect on the treatment received. Once a suitable instrument is identified, it can be used to generate pseudo-randomization thereby allowing estimation of the treatment effect. However, in contrast to a randomized control trial that identifies the average treatment effect, an instrumental variable analysis estimates the treatment effect for the “marginal” patient—or the patient in whom the likelihood of undergoing the treatment is based on the instrumental variable.14,15
Guided by the published literature, we selected the differential distance to a partial nephrectomy provider as our instrumental variable; we defined this as the distance from the patient’s residence to the nearest provider performing at least one partial nephrectomy in the year of treatment minus the distance from the patient’s residence to the nearest surgeon performing any kidney cancer surgery.14 We calculated distances using the linear distance function in SAS version 9.2 (SAS Institute, Cary, NC), which measures the number of miles between the centers of two zip codes. We were able to calculate differential distance for 7,138 patients (97% of our preliminary sample) (please see eMethods 2 for additional details).
For this group of patients, we created a 4-category instrumental variable by assigning patients with a differential distance of zero (i.e., the closest kidney cancer surgeon was also a partial nephrectomy surgeon) to a single category, and partitioning the remaining patients into three equally-sized terciles. To assess its validity as an instrument, we confirmed that differential distance was highly correlated with receipt of partial nephrectomy (F-statistic greater than 10),27 but not associated with survival in a standard multivariable proportional hazards model. We also examined covariate balance across the differential distance categories; we noted greater balance in patient-level covariates across the categories of our instrument compared with the pooled sample (eMethods 2).
We utilized a two-stage residual inclusion estimation framework for the instrumental variable analysis.28,29 The residual inclusion approach has been shown to generate more consistent (and less biased) estimates for a variety of non-linear models and has been applied specifically to non-parametric survival models using a Weibull distribution.28,29 In the first-stage model, we measured the association between partial nephrectomy and our instrument, adjusting for patient-level covariates including surgical approach (i.e., laparoscopic versus open). From this model, we determined the raw residual for each patient by calculating the difference between the model-predicted probability of receiving partial nephrectomy and the actual treatment received. The residuals were then included as an additional covariate in our second-stage survival model.
In the second-stage model, we specified a Weibull distribution and estimated the association between treatment and survival (both overall and kidney-cancer specific), adjusting for patient-level covariates, surgical approach, and post-operative complications. We then calculated model-derived estimates (i.e., predicted probabilities) of 2-, 5-, and 8-year survival for patients treated with partial or radical nephrectomy. Using the estimated differences in survival between treatment groups, we also calculated the number needed to treat (with partial rather than radical nephrectomy) to avoid one death following kidney cancer surgery.
We performed several additional analyses to more clearly identify patient subgroups (based on age and comorbidity status) that may derive particular benefit from partial nephrectomy. To assess the robustness of our findings, we also performed three sensitivity analyses. First, because a small proportion of patients who undergo treatment are found to have less common pathological diagnoses (e.g., oncocytoma, lymphoma, nephroblastoma),3,9,12,30 we repeated our analyses after limiting our sample to patients with histologically-confirmed renal cell carcinoma. Second, because access to partial nephrectomy may differ across urban versus rural environments (a consideration that could influence our instrumental variable),31 we also fit separate models for these patient groups. Finally, to better estimate the contemporary treatment effect, we fit separate models for patients treated from 1992-1999 and from 2000-2007.
All statistical testing was 2-sided, completed using SAS version 9.2 (SAS Institute, Cary, NC) and STATA version 11.0 (STATA Corp, College Station, Texas), and carried out at the 5% significance level.
Results
Among 7,138 patients treated surgically for clinical stage T1a kidney cancer, we identified 1,925 (27.0%) and 5,213 (73.0%) patients treated with partial or radical nephrectomy, respectively. Patients treated with partial nephrectomy were younger, more often male, and resided in census tracts with higher levels of average income and education than those treated with radical nephrectomy (p-values<0.001) (Table 1).
Table 1.
Patient Characteristics
| Partial (N=1,925) | Radical (N=5,213) | P-valuea | |
|---|---|---|---|
| Age (%) | |||
| 65-69 | 632 (32.8) | 1336 (25.6) | <0.001 |
| 70-74 | 571 (29.7) | 1465 (28.1) | |
| 75-79 | 476 (24.7) | 1369 (26.3) | |
| 80-84 | 205 (10.7) | 761 (14.6) | |
| 85 or older | 41 (2.1) | 282 (5.4) | |
|
| |||
| Race (%) | |||
| Caucasian | 1584 (82.3) | 4362 (83.7) | 0.005 |
| African-American | 150 (7.8) | 404 (7.8) | |
| Hispanic | 99 (5.1) | 289 (5.5) | |
| Other | 92 (4.8) | 158 (3.0) | |
|
| |||
| Female (%) | 803 (41.7) | 2419 (46.4) | <0.001 |
|
| |||
| Married (%) | 1250 (64.9) | 3206 (61.5) | 0.008 |
|
| |||
| Incomeb (%) | |||
| Low | 584 (30.3) | 1735 (33.3) | <0.001 |
| Intermediate | 599 (31.1) | 1717 (32.9) | |
| High | 698 (36.3) | 1620 (31.1) | |
|
| |||
| Educationb (%) | |||
| Low | 569 (29.5) | 1754 (33.6) | <0.001 |
| Intermediate | 594 (30.9) | 1722 (33.0) | |
| High | 718 (37.3) | 1596 (30.6) | |
|
| |||
| Rural Residence (%) | 301 (15.6) | 910 (17.5) | 0.07 |
|
| |||
| Charlson index score (%) | |||
| 0 | 1108 (57.6) | 3017 (57.9) | 0.96 |
| 1 | 468 (24.3) | 1264 (24.2) | |
| ≥ 2 | 349 (18.1) | 932 (17.9) | |
|
| |||
| Tumor Histology (%) | |||
| Clear cell | 1421 (73.8) | 4391 (84.2) | <0.001 |
| Papillary | 282 (14.7) | 404 (7.7) | |
| Chromophobe | 126 (6.5) | 192 (3.7) | |
| Oncocytoma | 11 (0.6) | 19 (0.4) | |
| Other histology | 85 (4.4) | 207 (4.0) | |
|
| |||
| Tumor Grade (%) | |||
| Well-differentiated | 364 (18.9) | 921 (17.7) | 0.004 |
| Mod-differentiated | 803 (41.7) | 2027 (38.9) | |
| Poorly differentiated | 228 (11.8) | 581 (11.1) | |
| Undifferentiated | 17 (0.9) | 66 (1.3) | |
| Unknown | 513 (26.7) | 1618 (31.0) | |
|
| |||
| Laparoscopic Surgery (%) | 527 (27.4) | 1468 (28.2) | 0.51 |
|
| |||
| Post-operative Complication (%) | 645 (33.5) | 1801 (34.5) | 0.41 |
|
| |||
| Year of Surgery (%) | |||
| 1992 – 1995 | 82 (4.2) | 589 (11.3) | <0.001 |
| 1996 – 1999 | 119 (6.2) | 699 (13.4) | |
| 2000 – 2003 | 610 (31.7) | 1806 (34.6) | |
| 2004 – 2007 | 1114 (57.9) | 2119 (40.7) | |
Comparisons between treatment groups performed using chi-squared testing.
Income and education terciles are based on the median census-tract income and percentage of non-high school graduates, respectively. Income and education data were not available for 185 patients.
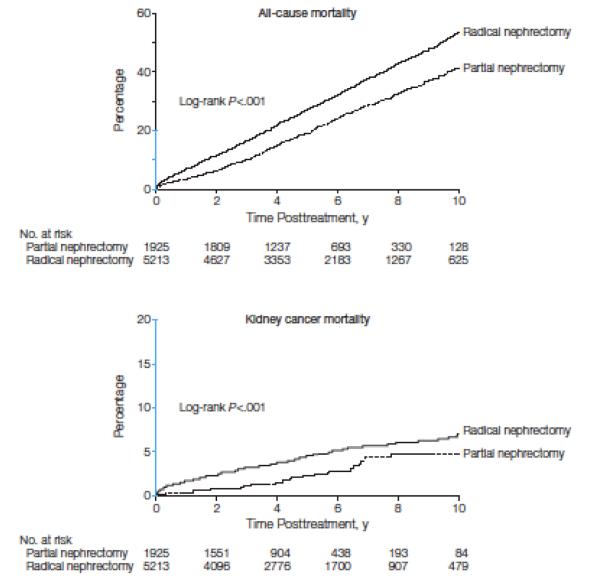
During a median follow-up of 62 months (interquartile range 39 – 92 months), 487 (25.3%) and 2,164 (41.5%) patients died from any cause after partial or radical nephrectomy, respectively. Kidney cancer was identified as the cause of death for 37 (1.9%) patients treated with partial nephrectomy, and 222 (4.3%) patients treated with radical nephrectomy (eTable 1). Figure 1 presents Kaplan-Meier estimates of overall and kidney cancer-specific mortality. In these unadjusted analyses, patients treated with partial nephrectomy had lower overall and kidney cancer-specific mortality (p-values<0.001).
Figure 1.
Kaplan-Meier estimates of all-cause and kidney cancer-specific mortality for patients treated with partial versus radical nephrectomy. Kaplan-Meier mortality estimates are compared using the log-rank test. The y-axis regions shown in blue indicate the range from 0% to 20%.
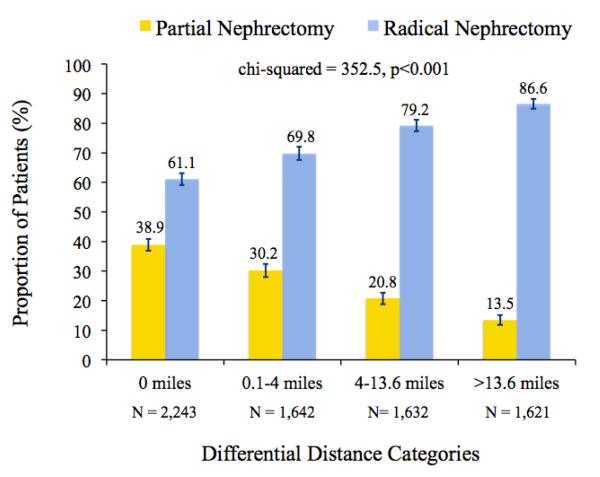
The differential distance instrument was strongly associated with receipt of partial nephrectomy (chi-squared test, p<0.001); patients living closer to a partial nephrectomy provider were more likely to receive this treatment (Figure 2). This relationship persisted in a multivariable model that adjusted for all measured patient characteristics (F-statistic = 97.3). Furthermore, in a standard proportional hazards survival model, we observed no independent association between the instrument and overall survival (HR 1.03, 95% CI 0.99-1.07). Taken together, these findings suggest strongly that differential distance satisfies the two principal conditions for a valid instrument.
Figure 2.
Proportion of patients treated with partial or radical nephrectomy according to differential distance category. The reported chi-squared statistic is for the unadjusted association between differential distance and type of surgical treatment. Error bars depict the 95% confidence interval for each proportion. The number of patients within each differential distance category is also reported.
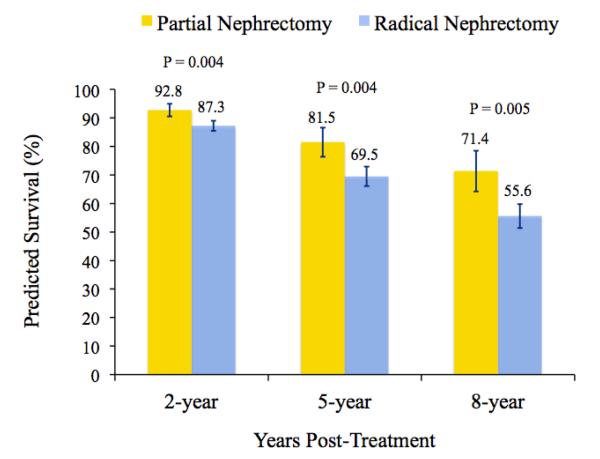
The two-stage residual inclusion model estimates based on this instrument indicate that patients treated with partial nephrectomy had a significantly lower likelihood of death than those treated with radical nephrectomy (HR 0.54, 95% CI 0.34-0.85). We found no difference in kidney cancer-specific survival between treatment groups (HR 0.82, 95% CI 0.19-3.49). Figure 3 presents model-predicted survival probabilities for patients treated with partial versus radical nephrectomy. The predicted survival improvement for patients treated with partial nephrectomy was 5.6 (95% CI 1.9-9.3), 11.8 (95% CI 3.9-19.7), and 15.5 (95% CI 5.0-26.0) percentage points at 2-, 5-, and 8-years following surgery, respectively. This corresponds to a number needed to treat of 18, 9, and 7 patients at 2-, 5-, and 8-years post-treatment, respectively. In other words, treating 7 patients with partial rather than radical nephrectomy would avoid one death during eight years of follow-up.
Figure 3.
Predicted survival probabilities at 2-, 5-, and 8-years after treatment with partial or radical nephrectomy. Probability estimates are derived from a two-stage residual inclusion model, adjusting for patient demographics, cancer severity, surgical approach, and the occurrence of post-operative complications. Statistical significance was determined by assessing the predicted marginal difference in survival between treatment groups at each time point. Error bars depict the 95% confidence interval for each survival estimate.
In subgroup analyses, we observed that the survival benefit associated with partial nephrectomy may be greatest for patients younger than 75 years of age, and for those with a Charlson comorbidity index score of 1 or higher. Our findings did not change substantively when we limited our sample to patients with renal cell carcinoma, those living in urban settings, and patients treated in more recent years (Table 2).
Table 2.
Estimated treatment effect of partial versus radical nephrectomy
| Partial nephrectomy No. of deaths / No. of patients |
Radical nephrectomy No. of deaths / No. of patients |
Hazard ratio for overall survival |
95% confidence interval |
|
|---|---|---|---|---|
| Primary analysis | ||||
| All patients | 487 / 1925 | 2164 / 5213 | 0.54 | 0.34 – 0.85 |
|
| ||||
| Subgroup analyses | ||||
| Patients < 75 years of age | 259 / 1203 | 962 / 2801 | 0.47 | 0.24 – 0.92 |
| Patients ≥ 75 years of age | 228 / 722 | 1202 / 2412 | 0.63 | 0.34 – 1.17 |
| Charlson score = 0 | 215 / 1108 | 1042 / 3017 | 0.75 | 0.38 – 1.45 |
| Charlson score 1 or more | 272 / 817 | 1122 / 2196 | 0.40 | 0.21 – 0.75 |
|
| ||||
| Sensitivity analyses | ||||
| Renal Cell Carcinoma onlyb | 457 / 1829 | 2059 / 4987 | 0.53 | 0.33 – 0.84 |
| Urban residence | 412 / 1624 | 1801 /4303 | 0.56 | 0.33 – 0.92 |
| Rural residence | 75 / 301 | 363 / 910 | 0.45 | 0.16 – 1.30 |
| Treatment years 1992 – 1999 | 126 / 201 | 938 / 1288 | 0.58 | 0.28 – 1.27 |
| Treatment years 2000 – 2007 | 361 / 1724 | 1226 / 3925 | 0.48 | 0.27 – 0.86 |
Hazard ratios are derived from our two-stage residual inclusion model using a Weibull distribution.
Renal Cell Carcinoma only includes clear cell, papillary, and chromophobe based on histology provided within SEER. Patients with oncocytoma or other histology were excluded.
Comment
Current treatment guidelines for patients with early-stage kidney cancer are informed mainly by observational studies suggesting that partial nephrectomy yields oncologic outcomes that are equivalent to those for radical nephrectomy,4,7,10,32 while at the same time reducing the risk of subsequent chronic kidney disease.5,7,33 It is presumed—but not established—that the downstream sequelae of chronic kidney disease lead to excess mortality and therefore less favorable survival outcomes among patients treated with radical nephrectomy.8-11,33 The face validity of this reasoning has yielded widespread acceptance of partial nephrectomy as the preferred treatment option for the rapidly growing population of patients with small, early-stage kidney cancers.6
However, because the data supporting a survival advantage for partial nephrectomy are observational, the potential for selection bias and residual confounding limit causal inference regarding the relationship between treatment with partial nephrectomy and long-term survival. This concern is accentuated by recent data from a randomized trial demonstrating improved survival for patients treated with radical nephrectomy.12 Indeed, despite its many flaws—including accrual difficulties, protocol revisions, higher rates of crossover for the partial nephrectomy group, and premature closure—the EORTC study has generated new uncertainty regarding the comparative effectiveness of treatment with partial versus radical nephrectomy.
We sought to clarify this issue by performing an instrumental variable analysis based on a large population-based cohort of patients whose treatment more accurately reflects contemporary practice patterns and surgical techniques. With this approach, we did not replicate findings from the EORTC trial. Instead, we found that for patients with early-stage kidney cancer, treatment with partial nephrectomy was associated with better overall and equivalent cancer-specific survival. Based on a predicted survival difference of 15.5 percentage points at 8-years, one life would be saved for every 7 patients treated with partial rather than radical nephrectomy. Accordingly, our findings support partial nephrectomy as the preferred treatment option for the ever-expanding pool of patients with kidney tumors measuring 4 cm or smaller.
There are several possible reasons why our results contradict evidence from the randomized EORTC trial. It is plausible that, in the absence of true randomization, the survival advantage with partial nephrectomy reflects residual unmeasured differences between treatment groups. The degree to which an instrumental variable analysis alleviates this concern depends on the selection of an instrument that induces meaningful variation in the treatment without independently impacting the outcome of interest. Consistent with previous work,14 differential distance met these criteria convincingly in our analysis. As such, our methods should have effectively balanced both measured and unmeasured variables between the treatment groups, mollifying concerns that the observed findings are due to bias or confounding.
Instead, the discordance with trial results likely reflects the influence of distinct treatment eras. At the outset of the EORTC study, there were widespread concerns regarding the oncologic effectiveness of partial nephrectomy. Outside of the trial setting, therefore, this procedure was reserved mainly for patients with a solitary kidney or chronic renal insufficiency who were treated at a relatively limited number of centers.13,31,34 It was not until very late in the trial’s accrual period that partial nephrectomy was utilized with any frequency as an elective procedure among patients with a normal contralateral kidney, suggesting that many treating surgeons and hospitals possessed limited experience with this complex surgical procedure.13,31,33 In the last decade, however, partial nephrectomy has been more widely adopted and the surgical technique has been modified in ways that reduce complication risks, ease convalescence, and better preserve function of the remnant kidney.30,35-38 Accordingly, both the patients receiving partial nephrectomy, and the operation itself, are likely quite different now than during the clinical trial. In this context, rather than viewing them as contradictory, we believe results from the EORTC study provide mainly historical context, while our findings reflect the current comparative effectiveness of partial versus radical nephrectomy.
Our study has several limitations. Because the sample includes only Medicare beneficiaries, our results may not be generalizable to younger patients with kidney cancer. The analysis is also limited to patients with the smallest kidney tumors (i.e., ≤ 4 cm); as such, our findings may not pertain to patients with larger masses. In addition, our analyses did not account for potential treatments for recurrent or metastatic kidney cancer. However, because surgical cure rates exceed 90% for patients with early-stage tumors, the number of patients that received these treatments is likely to be small and evenly-distributed among patients treated with partial or radical nephrectomy.4,7,10,12,32,39 Additionally, it is plausible that our instrument (differential distance) may serve as a proxy for quality of care: namely, patients living closer to a partial nephrectomy provider may have access to better health care services that ultimately influence their survival after kidney cancer surgery. That being said, the survival advantage with partial nephrectomy was maintained for patients residing in urban areas, where access to care is presumably less sporadic than rural settings. Although our analysis identifies a survival advantage with partial nephrectomy, the mechanisms underlying this finding (e.g., a reduction in post-operative chronic kidney disease and its attendant morbidity and excess mortality) require further clarification. The yet to be released renal functional outcomes from the EORTC trial may provide invaluable insights regarding the links between kidney cancer surgery, renal function, and non-oncologic morbidity and mortality. Finally, because we used an instrumental variable approach rather than actual randomization, our study identifies the treatment effect in the “marginal” rather than the average patient. Although characterizing this subpopulation can be difficult in the clinical setting,14,40 our subgroup analyses offer some insight into who may benefit most from partial nephrectomy.
These limitations notwithstanding, our findings have important implications for the care of patients with kidney cancer. By demonstrating that patients treated with partial nephrectomy live longer than those treated with radical nephrectomy, these data suggest that—despite findings from the EORTC trial—partial nephrectomy is the best treatment for many patients with small, localized kidney cancers. Although utilization of partial nephrectomy has been increasing for the last decade,35,36 there are still many suitable patients who do not receive this operation, highlighting the need for continued efforts to accelerate its adoption.41Because our subgroup analyses suggest that partial nephrectomy may be most beneficial for patients younger than 75 years of age and those with significant comorbidity, surgeons should pay particular attention to expanding the use of partial nephrectomy in patients meeting these clinical criteria.41,42
At the same time, however, we acknowledge that partial nephrectomy remains a technically challenging operation with potentially significant complications (e.g., hemorrhage, urinary fistula) that are seen less frequently with radical nephrectomy.43 This concern cannot be ignored when making treatment decisions. Indeed, the benefits of partial nephrectomy must always be weighed against the risk of acute surgical morbidity. In certain scenarios, some patients may be better served with an uncomplicated radical nephrectomy. Likewise, alternative treatment options—including active surveillance and ablative therapies—may be particularly prudent for patients in whom the benefits of surgical removal are less apparent.
Nevertheless, surgical management is undertaken in the vast majority of patients diagnosed with this increasingly common malignancy.2,3,6 Our findings suggests that by judiciously expanding the use of partial nephrectomy, clinicians can optimize survival outcomes among patients seeking treatment for early-stage kidney cancer.
Supplementary Material
Table: Claims-based definitions for post-operative complications
Table: Covariate balance for pooled sample and across differential distance categories using chi-squared testing
eTable 1: Cause of Deatha
Acknowledgements
For this study, the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database was used. The interpretation and reporting of these data are the sole responsibility of the authors. We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Funding and support This research was supported by funding from the Agency for Healthcare Research and Quality (K08 HS018346-01A1), the Edwin Beer Research Fellowship in Urology and Urology-Related Fields from the New York Academy of Medicine, and the University of Michigan Comprehensive Cancer Center (all to DCM).
Role of the sponsor The sponsors had no input on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author contributions Conception and design – Tan, Norton, Ye, Hafez, Gore, Miller
Acquisition of data – Tan, Ye, Miller
Analysis and interpretation of data – Tan, Norton, Ye, Hafez, Gore, Miller
Drafting of the manuscript – Tan, Norton, Ye, Miller
Critical revision of the manuscript for important intellectual content – Hafez, Gore
Statistical Analysis – Tan, Norton, Ye
Obtaining funding – Miller
Administrative, technical or material support – Tan, Norton, Ye, Gore, Miller
Supervision – Norton, Hafez, Gore, Miller
Conflicts of interest and financial disclosures Dr. John L. Gore is a member of the paid advisory board for Blue Cross Blue Shield of America. Dr. David C. Miller is a paid consultant for United HealthCare.
Online only material eMethods 1, eMethods 2, eTable 1
References
- 1.Chow WH, Devesa SS, Warren JL, Fraumeni JF. Rising incidence of renal cell cancer in the United States. JAMA. 1999 May 5;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. JNCI. 2006 Sep 20;98(18):1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute; Bethesda, MD: [Accessed January 30, 2012]. Nov 10, 2011. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 4.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000 Feb;163(2):442–445. [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006 Sep;7(9):735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novick AC, Campbell SC, Belldegrun A, et al. [Accessed January 30, 2012];Guideline for Management of the Clinical Stage 1 Renal Mass. 2009 http://www.auanet.org/content/media/renalmass09.pdf.
- 7.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000 Dec;75(12):1236–1242. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 8.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009 Jan;181(1):55–61. doi: 10.1016/j.juro.2008.09.017. discussion 61-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009 Apr 1;115(7):1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008 Feb;179(2):468–471. doi: 10.1016/j.juro.2007.09.077. discussion 472-463. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. NEJM. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 12.Van Poppel H, Da Pozzo L, Albrecht W, et al. A Prospective, Randomised EORTC Intergroup Phase 3 Study Comparing the Oncologic Outcome of Elective Nephron-Sparing Surgery and Radical Nephrectomy for Low-Stage Renal Cell Carcinoma. Eur Urol. 2010 Dec 22; doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006 Mar;175(3 Pt 1):853–857. doi: 10.1016/S0022-5347(05)00422-2. discussion 858. [DOI] [PubMed] [Google Scholar]
- 14.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007 Jan 17;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999 Dec;8(12):1117–1121. [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed Springer; New York: 2010. [Google Scholar]
- 19.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9:92–98. doi: 10.1186/1472-6963-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002 Aug;40(8 Suppl):IV-19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 21.Morrill R, Cromartie J, Hart G. Metropolitan, urban, and rural communting areas: toward a better depiction of the United States settlement system. Urban Geogr. 1999;20:727–748. [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Weingart SN, Iezzoni LI, Davis RB, et al. Use of administrative data to find substandard care: validation of the complications screening program. Med Care. 2000 Aug;38(8):796–806. doi: 10.1097/00005650-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994 Jul;32(7):700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality [Accessed January 30, 2012];Guide to the Patient Safety Indicators. 2007 http://www.qualityindicators.ahrq.gov/Downloads/Software/SAS/V30/psi_guide_v30.pdf.
- 26.Tan HJ, Wolf JS, Ye Z, Wei JT, Miller DC. Complications and failure to rescue after laparoscopic versus open radical nephrectomy. J Urol. 2011 Oct;186(4):1254–1260. doi: 10.1016/j.juro.2011.05.074. [DOI] [PubMed] [Google Scholar]
- 27.Staiger D, Stock JH. Instumental variables regression with weak instruments. Econometrica. 1997;65(3):557–586. [Google Scholar]
- 28.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008 May;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: a cautionary note. Health Serv Res. 2008 Jun;43(3):1102–1120. doi: 10.1111/j.1475-6773.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007 Jul;178(1):41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006 Feb;67(2):254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 32.Russo P, Jang TL, Pettus JA, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008 Jul 1;113(1):84–96. doi: 10.1002/cncr.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008 Feb 1;112(3):511–520. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 34.Novick AC. The role of renal-sparing surgery for renal cell carcinoma. Semin Urol. 1992 Feb;10(1):12–15. [PubMed] [Google Scholar]
- 35.Dulabon LM, Lowrance WT, Russo P, Huang WC. Trends in renal tumor surgery delivery within the United States. Cancer. 2010 May 15;116(10):2316–2321. doi: 10.1002/cncr.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooperberg MR, Mallin K, Kane CJ, Carroll PR. Treatment trends for stage I renal cell carcinoma. J Urol. 2011 Aug;186(2):394–399. doi: 10.1016/j.juro.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 37.Lane BR, Russo P, Uzzo RG, et al. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol. 2011 Feb;185(2):421–427. doi: 10.1016/j.juro.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 38.Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010 Sep;58(3):340–345. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Saigal CS, Deibert CM, Lai J, Schonlau M. Disparities in the treatment of patients with IL-2 for metastatic renal cell carcinoma. Urol Oncol. 2010 May-Jun;28(3):308–313. doi: 10.1016/j.urolonc.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res. 1998 Dec;33(5 Pt 1):1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 41.Miller DC, Ruterbusch J, Colt JS, et al. Contemporary clinical epidemiology of renal cell carcinoma: insight from a population based case-control study. J Urol. 2010 Dec;184(6):2254–2258. doi: 10.1016/j.juro.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007 May 1;109(9):1763–1768. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 43.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2007 Jun;51(6):1606–1615. doi: 10.1016/j.eururo.2006.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table: Claims-based definitions for post-operative complications
Table: Covariate balance for pooled sample and across differential distance categories using chi-squared testing
eTable 1: Cause of Deatha