Abstract
During chronic infections and cancer, T cells progressively lose function and become exhausted. However, effective T-cell responses are necessary to ultimately control viral infections and tumors. Hence, strategies that either restore endogenous immune responses or provide functional T cells by adoptive immunotherapy need to be explored. CD8 T cells play a prominent role in viral infections, as well as cancer, but CD4 T cells are necessary to support CD8 T-cell function. In addition, CD4 T cells exert direct effector functions, induce optimal B-cell responses and orchestrate innate immunity. Therefore, we propose that adoptive transfer strategies should exploit CD4 T cells alone or in combination with CD8 T cells, for the treatment of chronic infections and cancer. Furthermore, since adoptively transferred cells are subject to exhaustion, combining adoptive transfer therapy with immunotherapies that inhibit T-cell exhaustion should maximize the longevity and success rate of responses.
Keywords: adoptive cell transfer, cancer, CD4 T cells, chronic infection, exhaustion, immunotherapy, PD-1
CD4 T cells greatly impact the course of viral infections [1], and the role of CD4 T cells in anti-tumor responses is receiving growing attention [2]. CD4 T cells secrete multiple cytokines that can have direct effector functions, but also activate other immune cells. In addition, activated CD4 T cells express CD40L, which provides important signals to B cells, dendritic cells (DCs) and even CD8 T cells. Since both CD8 T cells and B cells rely on CD4 help for optimal responses, CD4 T cells have the unique role to coordinate cellular and humoral responses [1].
In contrast to acute infections that are resolved by effective immune responses, during chronic infections and cancer, T cells progressively lose function and become exhausted. High levels of continuous antigen stimulation can even culminate in physical deletion of exhausted antigen-specific T cells [3]. T-cell exhaustion has been best described in CD8 T cells, but CD4 T cells also become exhausted due to persistent antigen stimulation [4–7]. Both extrinsic and intrinsic factors drive a specific gene-expression program characteristic of exhausted T cells. Immunosuppressive cytokines (e.g., TGF-β and IL-10) and cells (e.g., Tregs and myeloid suppressor cells), as well as altered antigen presentation, are all extrinsic factors that can promote T-cell dysfunction. In addition, persistent antigen stimulation induces sustained expression of inhibitory receptors, such as PD-1, which intrinsically regulate T-cell exhaustion [3,8].
To ultimately control and achieve resolution of chronic viral infections or cancer, it is necessary to restore functional T-cell responses. Alternatively to immunotherapies that rescue endogenous immune responses, adoptive transfer of T cells can be employed. Viral-specific T cells can confer immunity to patients that have failed to generate efficient viral immune responses, and antitumor T cells can mediate cancer regression. CD8 T cells are generally thought to be essential for viral and cancer control, hence adoptive transfer strategies have largely focused on the transfer of CD8 T cells. Nonetheless, adoptive transfer of CD4 T cells has shown success for the treatment of tumor-bearing recipients [2,9–12]. Additionally, we have recently shown that adoptive transfer of lymphocytic choriomeningitis virus (LCMV)-specific CD4 T cells to chronically infected mice rescues endogenous LCMV-specific CD8 T-cell responses and mediates viral control [13].
In this article, we summarize and discuss the role of CD4 T cells in chronic viral infections, which have many parallels to cancer immunity. We also present recent advances and future perspectives regarding adoptive cell transfer of CD4 T cells for the treatment of chronic infections and cancer.
Central role of CD4 T cells in antiviral immune responses
The magnitude and quality of CD4 T-cell responses may determine the outcome of viral infections. In HCV infection, robust CD4 T-cell responses are associated with resolution [14]. Similarly, in HIV infection, a sufficient number of functionally active CD4 T cells correlates to viral control [15]. Interestingly, in resolving acute hepatitis A virus (HAV) infection in chimpanzees, anti-HAV CD4 T-cell responses are of higher magnitude and functionality than CD8 T-cell responses, and viral control is temporally associated to the anti-HAV CD4 T-cell response [16].
CD4 T cells are able to instruct and coordinate several components of the immune system, including CD8 T cells, B cells, DCs, macrophages and NK cells. In the following sections, we will review the mechanisms whereby CD4 T cells are able to engage such diverse immune responses, with emphasis on the control of chronic viral infections.
CD8 T cells rely on CD4 help during chronic infections
In the absence of CD4 help, chronic infections lead to more pronounced CD8 T-cell exhaustion. Infection of mice with LCMV clone 13 leads to a chronic infection that lasts more then 3 months, but is eventually cleared from most organs. However, if CD4 T cells are transiently depleted during LCMV clone 13 infection, mice do not develop LCMV-specific CD4 T cells and have multiorgan life-long viremia. In the absence of CD4 help, LCMV-specific CD8 T cells become deeply exhausted and do not recover function [17,18]. Without CD4 help, B-cell responses are also impaired and antibodies also play a role in viremia control during chronic LCMV infection [19], but this aspect will be addressed in the following section.
CD4 T cells are crucial to support CD8 T-cell responses to several viruses, including HCV and HIV. CD4 memory T cells are required in HCV-immune chimpanzees to ensure optimal CD8 T-cell responses that can rapidly eliminate virus and control escape mutants during reinfection [20]. Moreover, in humans, IL-2 production by HCV-specific CD4 T cells correlates with immune protection and recovery from infection [14]. In HIV long-term nonprogressor patients, in vitro proliferation of CD8 T cells to HIV peptides is abrogated when CD4 T cells are depleted, showing that CD4 T cells sustain anti-HIV CD8 T-cell responses [15].
In many situations CD8 T cells rely on CD4 T-cell help in the form of DC licensing (Figure 1A) in order to undergo efficient priming and appropriate differentiation into memory cells. Shortly after antigen recognition, CD4 T cells express CD40L and activate DCs presenting cognate antigen through CD40 cross-linking [21–23]. Additionally, direct CD40 ligation on CD8 T cells by cognate CD40L+ CD4 T cells may also play a role on CD8 T-cell activation (Figure 1A) [24]. Still, it is well established that CD4-licensed DCs become activated and better APCs due to increased expression of costimulatory molecules and enhanced ability to secrete cytokines such as IL-1, IL-6, TNF-α and IL-15 [1]. Although some reports argue that viral infections may provide enough inflammatory signals to directly induce optimal DC activation, in a chronic infection setting, when acute inflammatory signals have waned, CD4 help may be critical to activate DCs presenting viral antigens to promote rescue of exhausted CD8 T cells.
Figure 1. CD4 T cells can reinvigorate immune responses by activating different arms of the immune system.
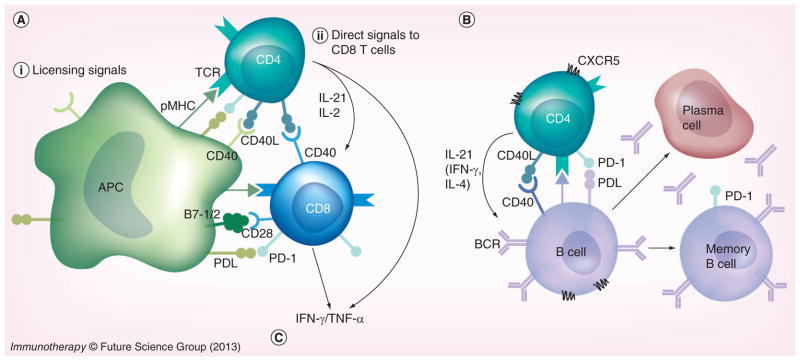
During chronic lymphocytic choriomeningitis virus (LCMV) infection, virus-specific CD8 T cells are exhausted and B cells are not engaged in effective antiviral responses. (A) CD8 help. Adoptive transfer of LCMV-specific CD4 T cells into chronically infected mice rescues CD8 T cells. CD4 T cells may provide help to cognate CD8 T cells by licensing APCs. Activated CD4 T cells express CD40L, which crosslinks CD40 on the surface of APCs (i), and it may also provide direct signals to CD8 T cells (ii). (i) CD40 crosslinking on APCs induces an increase in MHC class I expression and costimulatory B7 molecules, which enhance antigen presentation and CD8 T-cell activation. (ii) Activated CD4 T cells secrete cytokines that directly enhance function of exhausted CD8 T cells (e.g., IL-21 and IL-2). (B) B-cell help. CD4 T cells provide help to cognate B cells in the form of CD40 ligation and IL-21. B cells that receive T-cell help in the germinal center reaction are selected to become memory B cells or plasma cells. Hence, adoptive transfer of LCMV-specific CD4 T cells can orchestrate and restore endogenous antiviral responses to promote viral control. (C) Pleiotropic antiviral effects. Increased production of IFN-γ and TNF-α (derived from both transferred CD4 T cells and rescued endogenous CD8 T cells) can recruit and activate effector cells from the innate immune system (e.g., macrophages, monocytes, eosinophils, neutrophils and NK cells). In addition, IFN-γ and TNF-α can have direct effects on infected cells, by inhibiting viral replication and also promoting cell death. Importantly, during chronic infections, exhausted CD8 T cells express the inhibitory receptor PD-1, and PD-1 ligands are ubiquitously expressed during persistent inflammation. Therefore, CD8 T-cell rescue is limited by the PD-1 pathway. In addition, CD4 T cells also express PD-1 upon activation and are not optimally functional in chronically infected hosts. Thus, blockade of the PD-1 pathway in combination with adoptive transfer of CD4 T cells results in enhanced function of transferred CD4 T cells and improved rescue of CD8 T cells that ultimately leads to superior viral control. Similar scenarios could also be envisaged for the treatment of other chronic infections, as well as cancer.
For more information, please see [1,13,50].
pMHC: Peptide–MHC complex; BCR: B-cell receptor; TCR: T-cell receptor.
CD4 T cells can also modulate CD8 T-cell recruitment and migration. Activated CD4 T cells and CD4-licensed DCs produce chemokines, such as CCL3 (MIP-1α) and CCL4 (MIP-1β) that attract CD8 T cells to sites where APCs have cognate antigen [25]. In addition, IFN-γ production by activated CD4 T cells can trigger the surrounding tissue to secrete CXCL9 and CXCL10, which are required to attract effector CD8 T cells to some infection sites, as demonstrated for vaginal HSV-2 infection in mice [26].
CD4 helper T cells also secrete cytokines that can directly impact exhausted CD8 T cells (Figure 1A). IL-2 administration to LCMV chronically infected mice, induces proliferation of LCMV-specific CD8 T cells and results in decreased viral burden [27]. IL-2 production by CD4 T cells has also been shown to play an important role for antiviral CD8 T-cell function in HIV and HCV infection [14,28]. In addition, IL-21 production by CD4 T cells sustains LCMV-specific CD8 T-cell responses during chronic infection [29–31]. Likewise, in vitro experiments have shown that IL-21 can enhance functionality of exhausted HIV-specific CD8 T cells [32]. The mechanisms whereby IL-21 supports CD8 T-cell function during chronic infections are still ill-defined. A recent study has shown that IL-21 can induce T-bet expression in CD8 T cells [33]. This is an interesting finding, since T-bet promotes expression of several genes important for cytotoxic activity, as well as repressing PD-1 expression during chronic LCMV infection [34].
Activated CD4 T cells can differentiate into different CD4 T-cell subsets that exhibit distinct features and cytokine patterns. Viral infections induce type I interferons or IL-12 during priming and thus antiviral CD4 T cells mostly differentiate into Th1 cells. Th1 CD4 T cells express the transcription factor T-bet, and are characterized by high IFN-γ production, as well as TNF-α and IL-2 (polyfunctional Th1 cells) [1]. However, it is not clear if a particular CD4 T-cell subset would be responsible or specialized to provide CD8 help. While IL-2 production is associated with Th1 cells, highest IL-21 secretion is detected in T-follicular helper cells – the CD4 T-cell subset specialized for B-cell help. Thus, CD4 T cells with a mixed phenotype or different subsets of CD4 T cells might be needed to optimally engage CD8 T-cell responses. In conclusion, CD4 T cells ensure optimal CD8 T-cell responses and cognate CD4 help is specially required during chronic infections, when conditions for CD8 T-cell priming are suboptimal.
CD4 T cells are necessary for B-cell responses
Antibodies play an essential role in the prevention of most viral infections. The majority of successful vaccines and acute-resolving infections induce neutralizing antibodies that prevent or greatly diminish subsequent infections with the same pathogen. Even though B-cell responses are not sufficient for acute viral control in primary LCMV infection, antibodies are needed to ensure long-term and complete virus elimination by complementing CD8 T-cell-mediated virus control [19,35]. Furthermore, in chronic LCMV infection, the appearance of neutralizing antibodies temporally correlates with resolution [19] and passive administration of potent antiviral antibodies can reduce HIV burden in infected humanized mice [36]. In addition to viral neutralization, antibodies can opsonize infected cells to induce their destruction and elimination by phagocytes or the complement system [37]. Conversely, the role of antibodies in cancer regression is much less explored. It has long been established that transfer of immune cells to tumor-bearing recipients could mediate tumor regression while serum could not. Still, antibodies against tumor antigens can activate the complement system to lyse tumor cells and recruit innate effector cells, mediate antibody-dependent cell-mediated cytoxicity, promote Fc-mediated phagocytosis, as well as enhance antigen presentation of tumor antigens by DCs [38].
CD4 T cells are required to generate high-affinity and long-lived antibody responses. CD4 T cells drive affinity maturation and direct class switch recombination in germinal center (GC) B cells. B cells undergo somatic hypermutation and the affinity of the B-cell receptor determines the amount of antigen captured and presented into peptide–MHC class II complexes. Therefore, B cells bearing a high-affinity B-cell receptor have a greater likelihood to interact with limiting cognate CD4 T cells [39]. CD4 T cells provide IL-21 and also CD40 ligation to cognate B cells. GC B cells that receive T-cell help, survive and differentiate into long-lived memory B cells or plasma cells (Figure 1B) [40,41].
T-follicular helper (Tfh) cells are the CD4 T-cell subset responsible for B-cell help. DC priming can initiate Tfh cell differentiation program through induction of the transcriptional repressor Bcl6. The cues required for early Tfh cell differentiation remain ill-defined, but both IL-6 and IL-12 have been shown to promote Bcl6 expression, while IL-2 signaling represses Bcl6 through Blimp-1 induction [40,42]. Primed CD4 T cells downregulate CCR7 and move closer to the B-cell follicle. Subsequent interactions with cognate B cells are required to stabilize Bcl6 expression, ensure CXCR5 expression and entrance into the GC for the maintenance of a Tfh cell differentiation program. Tfh cells are CCR7− CXCR5+Bcl6+, express SAP and have high expression of PD-1 and inducible T-cell costimulator. In addition, it has been suggested that GL7 expression demarks Tfh cells present in the GC, while GL7− Tfh cells localize in the follicle [42]. Interestingly, IL-21 and CD40L, the two most important T-cell features for B-cell help, are not exclusively dependent on Bcl6, and can be expressed by non-Tfh cells [40]. Still, Tfh cells are the main producers of IL-21. Besides IL-21, GC Tfh cells secrete other cytokines that direct B-cell class switch recombination; for example, whereas IFN-γ favors IgG2a, IL-4 favors IgG1 class switching [43].
Accumulation of Tfh cells has been reported during chronic viral infections with LCMV in mice [44], SIV in macaques [45,46] and HIV in humans [47]. In chronic LCMV infection, late IL-6 production by GC follicular DCs was shown to be responsible for Tfh cell induction and was required for eventual control of LCMV infection [48]. Likewise, IL-6 levels are also increased during SIV chronic infection [45]. Remarkably, in HIV and SIV infection, Tfh cells appear defective in providing adequate B-cell help, but the mechanisms involved in this deregulation still need to be better defined. Interestingly, a recent study has shown that during chronic HIV infection a higher frequency of GC B cells express PD-L1. The authors in this study propose that excessive PD-1 signaling in Tfh cells during HIV infection may compromise Tfh function, including IL-21 production [49]. Therefore, even though Tfh cells accumulate during chronic viral infections, their function might be impaired and effective B-cell responses cannot be sustained.
In conclusion, CD4 T cells play a major role in humoral responses. Even though recent studies have defined the CD4 T-cell differentiation program necessary to help B-cell responses, much remains to be understood in terms of specific molecules and pathways involved in CD4 T-cell differentiation for B-cell help.
CD4 T cells can mediate direct antiviral effects
Cytokines derived from CD4 T cells, such as IFN-γ and TNF-α can have pleiotropic antiviral effects [50]. IFN-γ and TNF-α can directly reduce viral replication in infected cells and may also induce cell death. In addition, IFN-γ plays a pivotal role in the activation of macrophages and NK cells, which then exert antiviral activity (Figure 1C) [2,51]. Thus, besides orchestrating cellular and humoral responses, CD4 T cells can also impact viral innate immunity.
In addition, CD4 T cells can participate in viral control by direct killing of infected cells. Several different cytotoxic mechanisms have been reported in CD4 T cells, such as FAS ligand and TRAIL-mediated killing, as well as degranulation of granzymes and perforin [51–53]. For example, in CMV infection, the presence of CMV-specific CD4 T cells producing IFN-γ correlates positively with virus control [54], and it has been shown that CMV-specific CD4 T cells can have cytolytic activity [55]. In West Nile virus infection in mice, CD4 T cells also confer protection that is at least partially mediated by direct cytotoxic activity [56]. Lung epithelial cells express MHC class II during influenza virus infection in mice, and CD4 T cells in the lungs have a IFN-γ-independent perforin-dependent cytolytic phenotype, which contributes to recovery after infection [57].
Professional APCs, such as DCs, are very efficient in capturing either soluble or cell-associated antigens for presentation to MHC class II molecules. Thus, viruses do not need to infect cells that express MHC class II to trigger activation of antiviral CD4 T cells. On the other hand, a significant role of cytolytic CD4 T cells in reduction of viral burden is constrained by MHC class II expression on infected cells. Nonetheless, in humans, besides professional APCs, both epithelial and endothelial cells can express MHC class II, depending on cytokine stimulation [51]. In conclusion, cytotoxic CD4 T cells may play a role in viral control in some infections, especially when evasion mechanisms include downregulation of MHC class I presentation. Similarly to the situation in viral infections, CD4 T cells that recognize tumor antigens can also mount effective immune responses against tumors that do not express MHC class II molecules, either by secretion of cytokines that can directly affect tumor cells or by orchestrating activation of other immune cells [58].
Adoptive T-cell immunotherapy
Adoptive transfer of virus-specific T cells has been successfully employed in hematopoietic cell transplantation (HCT) patients to prevent reactivation of endogenous latent viruses or acute infections from opportunistic viruses. Early studies have shown that bulk T-cell transfer from EBV-immune bone marrow donors could treat EBV-associated B-cell lymphoma in transplant recipients [59]. However, transfer of bulk T cells was also associated with increased graft-versus-host disease. Posterior studies then showed that transfer of EBV-specific T cells into immunocompromised patients provided long-term protection with minimal side effects [60–62]. Likewise, transfer of CMV-specific T-cell clones also provided protection to allogeneic bone marrow transplant recipients [63,64].
Adoptive T-cell therapy has also been successfully used to treat cancer. Adoptive therapy with transfer of expanded tumor-infiltrating T cells can mediate objective responses in approximately half of stage IV melanoma patients and even durable complete responses in some individuals [65].
Adoptive transfer of CD4 T cells
Transfer of CMV-specific CD8 T-cell clones can provide protection to allogeneic bone marrow transplant recipients against CMV infection, but CMV-specific CD4 T cells are required for the persistence of transferred CD8 T cells [64]. Similarly, control of chronic LCMV infection was only achieved with adoptive transfer of both CD8 and CD4 T cells from LCMV-immune mice [66,67]. It has been reported that 20 out of 31 HBV chronically infected patients that received HCT from HBV-immune donors cleared the virus, and none of those individuals developed liver cirrhosis [68]. Importantly, in HBV infection, both CD8 and CD4 T-cell responses have been associated with viral control. In addition, adoptive transfer of HIV-specific CD8 T-cell clones to HIV-infected patients only provided a transient decrease in viral burden, consistent with the disappearance of the transferred cells [69]. This is probably not surprising, since HIV chronically infected patients have a paucity of CD4 T cells, and CD8 T cells are particularly dependent on CD4 T-cell help during chronic infections. Importantly, CD4 T cells isolated during acute HIV infection can support ex vivo proliferation of HIV-specific CD8 T cells from chronically infected individuals [28]. In summary, there is broad evidence that adoptive T-cell therapy could be an effective treatment for chronic viral infections, and the presence or transfer of viral-specific CD4 T cells may be essential to support antiviral responses.
Nevertheless, clinical trials with adoptive transfer of CD4 T cells are scarce and studies in animal models have been mostly restricted to cancer therapy [2]. Interestingly, one of the early landmark papers in adoptive T-cell therapy described that CD4 T cells – and not CD8 T cells – mediated efficient control of established murine leukemia, in combination with cyclophosphamide chemotherapy [9]. Several studies have now shown that adoptive transfer of CD4 T cells specific for tumor antigens can mediate elimination of tumor cells. In a mouse model of melanoma expressing the tumor antigen p97, adoptive transfer of CD4 T-cell clones specific for p97 were able to eradicate melanoma metastasis in the lungs. CD4 T cells did not mediate direct tumor lysis, but secreted cytokines that could activate macrophages with tumoricidal activity [10]. Activation of NK cells by tumor-specific CD4 T cells also mediated efficient MB49 bladder carcinoma rejection [58]. In another mouse model, transfer of activated CD4 T-cell clones specific for a protein expressed by a sarcoma tumor cell line elicited endogenous CD8 T-cell responses and promoted tumor control [11]. An interesting case report described complete clinical remission of metastatic melanoma in a patient that received autologous CD4 T-cell clones specific for the tumor antigen NY-ESO-1. Adoptively transferred CD4 T cells persisted for at least 3 months and triggered endogenous T-cell responses to other melanoma-associated tumor antigens [12]. Thus, protocols assessing transfer of T cells for cancer therapy, should carefully analyze the cells selected for adoptive transfer (CD8 or CD4 T cells, or a mixture of both T-cell subsets) to evaluate the efficacy of tumor regression and establish immune correlates for partial and complete responses.
In mice chronically infected with LCMV, we have shown that adoptive transfer of naive or effector CD4 T cells specific for a single LCMV epitope, was able to stimulate endogenous responses to control viremia. Importantly, CD4 help rescued exhausted CD8 T cells specific to major and minor LCMV epitopes. This is an important aspect, because induction of immune responses with increased breadth are thought to be especially required for the control of highly mutagenic viruses [70]. In addition to CD8 T-cell responses, adoptive transfer of LCMV-specific CD4 T cells to chronically infected mice also restored B-cell responses to LCMV antigens. Thus, provision of T-cell help can stimulate GC reactions and induce antibody responses during chronic infections [13]. In conclusion, adoptively transferred CD4 T cells can coordinate effective, broad and long-lasting immune responses.
Strategies to improve adoptive T-cell immunotherapy
Protocols that improve ex vivo generation of a large number of T cells with the desired phenotype, as well improve expansion and function of the transferred cells in the hosts are still being optimized (Figure 2) [71]. In the following sections, we have chosen to highlight a few of the recent advances in the knowledge and techniques that may positively impact the success of adoptive T-cell immunotherapy.
Figure 2. Strategies to improve adoptive T-cell immunotherapy.
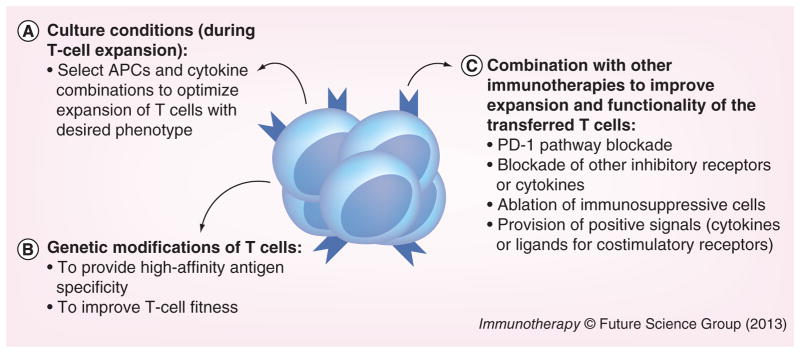
We propose that adoptive T-cell immunotherapy could be improved with: (A) optimization of culture conditions for ex vivo T-cell expansion in order to generate T cells with the desired phenotype. Novel types of APCs, as well as cytokine combinations have been able to generate functional T cells with specific phenotypes. (B) Genetic modifications of T cells, to generate T cells with high affinity to the desired antigen (by introducing a specific T-cell receptor or even chimeric antigen receptor) or with special features (e.g., for HIV-infected patients, T cells that are deficient in HIV coreceptors can be generated). (C) Combination with other immunotherapies to improve in vivo expansion and function of the transferred T cells. Chronically infected hosts or cancer patients have several immunosuppressive mechanisms in place that dampen immune responses. Thus, to overcome inhibitory signals and avoid exhaustion of the transferred T cells, adoptive immunotherapy should be combined with other strategies that remove immunosuppressive pathways (e.g., blockade of the PD-1 pathway). Nevertheless, to be able to improve adoptive immunotherapy at the steps we propose, future studies will need to define the ideal T-cell specificity and phenotype to be transferred (which will vary according to the infection/cancer to be treated). In addition, an analysis of the immunosuppressive mechanisms in place in each patient may guide which pathways need to be modulated to increase the efficiency of adoptively transferred T cells as well as improve endogenous responses.
Expansion & differentiation of effective T cells
High numbers of viral-specific T cells suitable for adoptive transfer can be generated by ex vivo expansion of autologous cells. This methodology is based on the premise that antiviral T cells are present in chronically infected hosts and can be expanded given the right conditions. It has the disadvantage of relying on small numbers of antiviral T cells and also that the cells of interest might be exhausted and, thus, less permissive to proliferation and acquisition of effector functions. Nonetheless, in cancer patients, this method has worked remarkably well for melanoma treatment [65], demonstrating that T cells bearing the right specificity are present within tumors, and isolation and culture of T cells can remove immunosuppression.
Antigen-specific CD4 T cells can be expanded with peptide-pulsed APCs (when relevant peptides have been identified), APCs fused to or fed with infected/tumor cells, or even polyclonal stimulation, when the initial T-cell population is enriched for the desired specificity. For example, high-dose IL-2 can expand tumor-infiltrating lymphocytes with multiple specificities to the tumor and, combined with short-term anti-CD3 stimulation, can generate enough cells for reinfusion [72].
Not many CD4 T-cell epitopes shared between patients with a particular cancer have been described, thus, personalized immunotherapies involving expansion of tumor-infiltrating lymphocytes have prevailed in cancer immunotherapy. Nonetheless, it would be highly advantageous to have defined peptides that would stimulate antitumor T cells, and many research groups are actively engaged in this purpose. For example, a considerable percentage of melanoma patients have CD4 T cells (and also CD8 T cells) that recognize the tyrosinase antigen [73]. In addition, MART-1, a differentiation antigen expressed in the majority of melanomas, contains epitopes for both CD8 and CD4 T cells, and MART-1 phosphoepitopes are more restricted to malignant cells [74]. Also in melanoma, the common B-raf V599E mutant protein can be presented onto MHC class II molecules [75].
While one of the challenges in cancer immunotherapy resides in the identification of antigens that are different from the ‘normal-self’, in viral infections, the difficulty resides in finding epitopes conserved between different viral isolates. In this regard, T-cell therapies for chronic infections might need to be personalized in a similar way as cancer immunotherapies. Alternatively, it could be envisaged that by eliciting an immune response to multiple conserved epitopes that are important for viral fitness, it could be possible to control chronic infections with highly mutagenic viruses such as HIV, HCV and HBV, without selection of escape variants.
Another essential aspect for the efficacy of adoptive immunotherapy, is conditioning of the host. Adoptive transfer into lymphopenic environments (e.g., recipients of HCT) facilitates expansion of the transferred T-cell population. Reduced competition for cytokines such as IL-15 and IL-7 promotes memory T-cell proliferation and survival [76]. In addition, depletion of suppressive cells, such as Tregs and myeloid-derived suppressor cells may also play a role. Importantly, conventional antitumor treatments consisting of chemotherapy or irradiation promote lymphodepletion in cancer patients and thus facilitate subsequent engraftment and survival of adoptively transferred T cells [77].
In chronic infections, short persistence of the transferred cells is probably one of the major obstacles to successful adoptive transfer. For adoptive transfer of CD8 T cells, it has been shown that effector memory cells have a dramatically reduced survival when compared with central memory CD8 T cells [78], but similar analyses for CD4 T cells are still lacking. It is worth noting that in our study, adoptive transfer of naive CD4 T cells was more efficient than transfer of effector cells [13], probably due to differences in expansion and survival. Although costly, multiple T-cell transfers could be performed to overcome the short survival of adoptively transferred cells. However, ideally, the method used for T-cell expansion or host conditioning could be tailored to enhance survival and expansion of adoptively transferred T cells.
The diversity in CD4 T-cell phenotypes is another important aspect to be considered in adoptive T-cell therapy. Upon activation, CD4 T cells differentiate into distinct subsets with specialized functions, and whether a particular CD4 T-cell subset would be more desirable to aid in the control of chronic viral infections is an important question that still needs to be addressed. Tfh cells are necessary to provide B-cell help, and IL-21 production by CD4 T cells plays a critical role to limit CD8 T-cell exhaustion and promotes rescue [30–32]. However, Th1 cells have been classically considered to be the CD4 T cells involved in viral control [1]. Although, there is considerable plasticity in cytokine production by different CD4 T-cell subsets. For example, both Tfh and Th1 cells are generated after acute LCMV infection and both subsets can produce IL-21 and IFN-γ (albeit at different levels) [79]. Hence, it is conceivable that either Tfh or Th1 cells might be able to provide CD8 help in viral infections. Nonetheless, B-cell help might be constrained to Tfh cells that express CXCR5 and can enter the B-cell follicle.
Future studies need to evaluate the efficacy of adoptive transfer of different CD4 T-subsets for chronic viral infections and cancer. Recent advances in T-cell culture techniques have improved the quality of APCs. In addition, manipulation of culture conditions with cytokines (or cytokine signaling blockade) can allow expansion of a particular subset of CD4 T cells. Moreover, methods that avoid transfer of suppressive CD4 Tregs should improve efficacy of adoptive T-cell transfer [71].
Genetic modifications of T cells for adoptive transfer
Alternatively to antigen-driven expansion of T cells with the desired specificity, T cells can be engineered to express a new T-cell receptor (TCR) or chimeric antigen receptor (CAR). Even though introduction of a new TCR can confer high avidity and specificity to viral antigens, mispairing with endogenous TCR chains can result in T cells with undesired specificity and autoimmune reactivity. Strategies that optimize exogenous TCR-chain pairing or use γδ-T cells as recipients can minimize off-target activity, but MHC restriction is still a limitation for the widespread use of antiviral TCR gene introduction. CARs have the advantage to overcome MHC restriction, but can only be directed to native proteins expressed on the surface of target cells, such as proteins from enveloped viruses. CARs are composed of a single-chain antibody-binding domain fused to the CD3ζ chain alone or combined with the signaling domain of costimulatory molecules (e.g., 4-1BB or CD28) [80]. Impressive results in the treatment of acute and chronic B-cell lymphocytic leukemia have recently been reported with the use of adoptive T-cell therapy consisting of autologous T cells expressing CD19-specific CARs [81–83]. Interestingly, those studies have used a mixture of T cells and the mean frequency of CD4 T cells among the reinfused CD19-specific T cells has varied between 46 to 83% in different clinical trials, but the consequences of different CD8 to CD4 T-cell ratios has not been explored [84–86].
Recent advances regarding genetic modifications of T cells have also prompted clinical trials with adoptive CD4 T-cell transfer for the treatment of HIV-infected patients. HIV infects CD4 T cells that coexpress CCR5, but some virus strains can also use CXCR4 as a coreceptor. Hence, strategies that reduce expression of those chemokine receptors in CD4 T cells have been envisaged to control HIV spread and restore CD4 T-cell counts. It was first reported that ex vivo costimulation of CD4 T cells with anti-CD28 reduced expression of CCR5. HIV-infected individuals that received CD28 costimulated autologous CD4 T cells had a sustained increase in CD4 T-cell counts and no increase in HIV burden [87]. Novel technologies using zinc-finger nucleases (ZFNs) that produce DNA double-strand breaks at sites recognized specifically by zinc-finger protein motifs, can achieve genetic disruption of CCR5 or CXCR4 expression in CD4 T cells [88,89]. Primary CD4 T cells transduced with adenovirus vector expressing CCR5 ZFNs had a survival advantage to HIV-1 infection in vitro. Moreover, adoptive transfer of CCR5-disrupted CD4 T cells increased CD4 T-cell counts and lowered plasma viremia in a mouse model of HIV-1 infection [88]. Based on these results, one clinical trial involving autologous CD4 T cells modified with ZFN-mediated CCR5 gene disruption for the treatment of HIV-infected patients has been completed and other studies have been planned or are ongoing [101–103].
Improving T-cell function by blockade of the PD-1 pathway
Similar to CD8 T cells, CD4 T cells become exhausted during chronic antigen stimulation and inhibitory receptors play a major role in CD4 dysfunction. Exhausted CD4 T cells express PD-1 and LAG-3, but unlike CD8 T cells, do not express 2B4 or CD160 [5,7].
PD-1 engagement attenuates TCR and CD28-activating signals thereby preventing T-cell effector functions [90]. In HIV infection, PD-1 expression on CD4 T cells positively correlates with viral load and inversely correlates with CD4 T-cell counts [4]. Also, in vitro blockade of PD-1 signaling in exhausted CD4 T cells from HIV-infected patients improves not only proliferation but also secretion of cytokines [4,5]. In CMV-seropositive transplant patients, a large fraction of virus-specific CD4 T cells express high levels of PD-1, which coincides with lower IFN-γ production and a lack of IL-2 secretion. Consistent with an exhausted phenotype, in vitro blockade of the PD-1 pathway enhances proliferation of CMV-specific CD4 T cells [6]. In a mouse model of chronic malaria, Plasmodium-specific CD4 T cells become dysfunctional as measured by impaired cytokine production upon restimulation. In this model, exhausted CD4 T cells express PD-1 and the inhibitory molecule LAG-3. Importantly, blockade of PD-1 and LAG-3 inhibitory pathways during murine chronic malaria increased the frequency and functionality of Plasmodium-specific CD4 T cells, induced robust antibody responses and resulted in accelerated parasite clearance [7]. Thus, similar to CD8 T cells [91,92], exhausted CD4 T-cell responses can be rescued by blockade of the PD-1 pathway.
T cells transferred into chronically infected patients also become exhausted if viral control is not achieved in a timely manner. LCMV- specific CD4 T cells upregulate and maintain PD-1 expression when adoptively transferred into chronically infected mice. Even though transferred CD4 T cells produced cytokines to LCMV antigens, the functionality of those cells was much inferior to effector CD4 T cells obtained during acute LCMV infection. Importantly, blockade of PD-1 signaling improved polyfunctionality of LCMV-specific CD4 T cells transferred into chronically infected mice. Therefore, combining CD4 T-cell adoptive transfer with blockade of the PD-1 inhibitory pathway improved function in both LCMV-specific CD4 and CD8 T cells and resulted in a highly significant reduction in viral load [13].
Similarly, it was recently reported that adoptive CD4 T-cell therapy can promote eradication of established melanoma in mice, but transferred CD4 T cells acquire an exhausted phenotype and melanoma reoccurrence can occur. The authors then show that combining blockade of the PD-1 and LAG-3 pathways could successfully treat mice with melanoma reoccurrence [93].
In conclusion, we propose that blockade of inhibitory pathways, such as PD-1, should be combined with adoptive transfer therapies. PD-1 blockade will not only enhance function of the transferred cells, but also improve rescue of endogenous T-cell responses. Notably, blockade of the PD-1 pathway as a single therapy has shown promising results in Phase I clinical trials for advanced cancer patients [94–96]. Hence, combination treatments should increase the success rate and longevity of immunotherapies.
Conclusion
CD4 T cells play a key role during chronic viral infections. CD8 T cells are particularly dependent on CD4 T cells during chronic stimulation as CD4 help reduces the extent of CD8 T-cell exhaustion. In addition, B-cell responses, which also rely on cognate interactions with CD4 T cells, supplement CD8-mediated viral control. Importantly, when antiviral immune responses have failed and chronic infection is established, adoptive transfer of antiviral CD4 T cells can reinvigorate endogenous responses. Likewise, transfer of CD4 T cells specific for tumor antigens can promote regression of established tumors. Thus, transfer of CD4 T cells can orchestrate broad and long-lasting immune responses that enable viral and cancer control.
Future perspective
Personalized therapy in the form of adoptive T-cell transfer has shown impressive results in some patients with treatment-refractory metastatic cancer. In the same way, patients with chronic viral infections should also benefit from T-cell therapy. Current therapies for HBV and HCV are limited and true cure is a rare event. For HIV patients, drugs that inhibit viral replication have drastically improved the lives of chronically infected patients, but lifelong treatment is needed. Recent advances regarding the generation of CD4 T cells refractory to HIV infection, and ongoing clinical trials with HIV chronically infected patients, should boost the field of CD4 T-cell therapy for chronic viral infections. Long-term B-cell cancer remission in patients treated with T cells bearing anti-CD19 CARs should further encourage the use of CAR technology to create potent antitumor and antiviral T cells. Furthermore, it will be important to dissect the specific roles of CD4 T cells and CD8 T cells within adoptively transferred T cells. CD4 T cells need to be moved from a coadjuvant role to a leading role in orchestrating effective immune responses. Further studies evaluating the advantages of CD4 T-cell transfer as a single therapy or in conjunction to CD8 T cells should be performed. Importantly, the concept that multiple immunotherapies should be combined to increase treatment success rate is a recurrent theme. Thus, we anticipate that immunotherapies that block inhibitory pathways (e.g., the PD-1 pathway) in combination with adoptive T-cell therapy will greatly contribute to the long-term maintenance of effective immune responses to achieve resolution of chronic infections and cancer.
Executive summary.
Central role of CD4 T cells in antiviral immune responses
CD4 T cells play a central role in orchestrating immune responses, but CD4 T cells become exhausted during chronic infections and cancer.
CD4 help is particularly important to sustain CD8 T-cell responses during chronic antigen stimulation.
Adoptive T-cell immunotherapy
Adoptive transfer of CD4 T cells can rescue exhausted CD8 T-cell responses, as well as B-cell responses.
Strategies to improve adoptive T-cell immunotherapy
More studies are needed to define features of CD4 T cells required for CD8 help, and whether a particular subset or CD4 differentiation program would be more desirable for CD4 T-cell immunotherapy.
Exhaustion of adoptively transferred T cells reduces the efficiency of adoptive immunotherapy for treatment of chronic infections, as well as cancer.
Combining adoptive T-cell immunotherapy to PD-1 pathway blockade improves function of adoptively transferred T cells, as well as endogenous T-cell responses.
Acknowledgments
The authors thank A Wieland for comments on the manuscript.
Financial & competing interests disclosure
This work was supported by grants from the NIH R01 AI030048 and P01 A1080192 (R Ahmed) and by the Irvington Institute Fellowship Program of the Cancer Research Institute (AO Kamphorst). R Ahmed holds patents for the PD-1 inhibitory pathway. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪▪.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4( +) T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. Recent review regarding the central role of CD4 T cells to mount efficient immune responses to viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21(2):200–208. doi: 10.1016/j.coi.2009.02.004. Important review that discusses the use of CD4 T cells in adoptive immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4▪▪.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. Demonstrates the importance of the PD-1 pathway in HIV infection and shows that PD-1 controls CD4 T-cell exhaustion. [DOI] [PubMed] [Google Scholar]
- 5.Porichis F, Kwon DS, Zupkosky J, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118(4):965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus-specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8(7):1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 7.Butler NS, Moebius J, Pewe LL, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13(2):188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg PD, Cheever MA, Fefer A. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2- lymphocytes. J Exp Med. 1981;154(3):952–963. doi: 10.1084/jem.154.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn M, Sugawara H, McGowan P, et al. CD4+ T cell clones specific for the human p97 melanoma-associated antigen can eradicate pulmonary metastases from a murine tumor expressing the p97 antigen. J Immunol. 1991;146(9):3235–3241. [PubMed] [Google Scholar]
- 11.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164(2):562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. Impressive clinical case of melanoma regression by transfer of CD4 T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪▪.Aubert RD, Kamphorst AO, Sarkar S, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108(52):21182–21187. doi: 10.1073/pnas.1118450109. Demonstrates that established CD8 T-cell exhaustion can be rescued by CD4 help. Also shows that adoptive transfer of CD4 T cells can elicit B-cell responses during chronic lymphocytic choriomeningitis virus infection. Importantly, this study demonstrates that blockade of the PD-1 pathway can improve function of adoptively transferred CD4 T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyk-Pearson S, Tester IA, Klarquist J, et al. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82(4):1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T-cell responses associated with control of viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Callendret B, Xu D, et al. Dominance of the CD4( +) T helper cell response during acute resolving hepatitis A virus infection. J Exp Med. 2012;209(8):1481–1492. doi: 10.1084/jem.20111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matloubian M, Concepcion RJ, Ahmed R. CD4 + T cells are required to sustain CD8 + cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68(12):8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Bergthaler A, Flatz L, Verschoor A, et al. Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol. 2009;7(4):e1000080. doi: 10.1371/journal.pbio.1000080. Highlights the importance of antibody responses to completely clear viral infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302(5645):659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 21.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 22.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4 + T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 23.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 24.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8 + T cells in the generation of CD8 + T cell memory. Science. 2002;297(5589):2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 25.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8 + T cells to sites of CD4 + T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8( +) T lymphocyte mobilization to virus-infected tissue requires CD4( +) T-cell help. Nature. 2009;462(7272):510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9(5):540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 28▪.Lichterfeld M, Kaufmann DE, Yu XG, et al. Loss of HIV-1-specific CD8 + T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4 + T cells. J Exp Med. 2004;200(6):701–712. doi: 10.1084/jem.20041270. Elegant work shows that functional CD4 T cells can contribute to maintaining HIV-specific CD8 T-cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Frohlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324(5934):1576–1580. doi: 10.1126/science.1172815. Uncovered the importance of IL-21 signals to maintaining CD8 T-cell function during chronic viral infections. [DOI] [PubMed] [Google Scholar]
- 30▪.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. Uncovered the importance of IL-21 signals to maintaining CD8 T-cell function during chronic viral infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. Uncovered the importance of IL-21 signals to maintaining CD8 T-cell function during chronic viral infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevalier MF, Julg B, Pyo A, et al. HIV-1-specific interleukin-21 + CD4 + T-cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85(2):733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland AP, Joller N, Michaud M, Liu SM, Kuchroo VK, Grusby MJ. IL-21 Promotes CD8 + CTL activity via the transcription factor T-bet. J Immunol. 2013 doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- 34.Kao C, Oestreich KJ, Paley MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8 + T-cell responses during chronic infection. Nat Immunol. 2011;12(7):663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachmann MF, Hunziker L, Zinkernagel RM, Storni T, Kopf M. Maintenance of memory CTL responses by T helper cells and CD40–CD40 ligand: antibodies provide the key. Eur J Immunol. 2004;34(2):317–326. doi: 10.1002/eji.200324717. [DOI] [PubMed] [Google Scholar]
- 36.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2(9):706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 38.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35(5):671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 42.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 43.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10(4):385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, Mcgavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208(5):987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrovas C, Yamamoto T, Gerner MY, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122(9):3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4( +) T follicular helper, B, and CD8( +) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188(7):3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindqvist M, van Lunzen J, Soghoian DZ, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122(9):3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334(6057):825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cubas RA, Mudd JC, Savoye AL, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19(4):494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 51.Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol. 2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174(2):614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 53.Brown DM. Cytolytic CD4 cells: direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262(2):89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4 + T cells in protection against CMV disease. Blood. 2003;101(7):2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 55.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4 + T lymphocytes with cellular maturation. J Exp Med. 2006;203(13):2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181(12):8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown DM, Lee S, de Garcia-Hernandez ML, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86(12):6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Diez A, Joncker NT, Choi K, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109(12):5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein–Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330(17):1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 60.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 61.Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2(5):551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 62.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 64.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 65▪▪.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. Recent paper that describes successful adoptive T-cell transfer immunotherapy for the treatment of melanoma patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunziker L, Klenerman P, Zinkernagel RM, Ehl S. Exhaustion of cytotoxic T cells during adoptive immunotherapy of virus carrier mice can be prevented by B cells or CD4 + T cells. Eur J Immunol. 2002;32(2):374–382. doi: 10.1002/1521-4141(200202)32:2<374::AID-IMMU374>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 67.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212(1):244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 68.Hui CK, Lie A, Au WY, et al. A long-term follow-up study on hepatitis B surface antigen-positive patients undergoing allogeneic hematopoietic stem cell transplantation. Blood. 2005;106(2):464–469. doi: 10.1182/blood-2005-02-0698. [DOI] [PubMed] [Google Scholar]
- 69.Brodie SJ, Lewinsohn DA, Patterson BK, et al. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5(1):34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 70.Koenig S, Conley AJ, Brewah YA, et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1(4):330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 71.Paulos CM, Suhoski MM, Plesa G, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42(1–3):182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Topalian SL, Rivoltini L, Mancini M, et al. Human CD4 + T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci USA. 1994;91(20):9461–9465. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Depontieu FR, Qian J, Zarling AL, et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc Natl Acad Sci USA. 2009;106(29):12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharkey MS, Lizée G, Gonzales MI, Patel S, Topalian SL. CD4( +) T-cell recognition of mutated B-RAF in melanoma patients harboring the V599E mutation. Cancer Res. 2004;64(5):1595–1599. doi: 10.1158/0008-5472.can-03-3231. [DOI] [PubMed] [Google Scholar]
- 76.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 77▪▪.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T-cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. Recent review on adoptive T-cell immunotherapy for cancer that discusses current advances, as well as further avenues for improvements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8 + T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hale JS, Youngblood B, Latner DR, et al. Distinct memory CD4 T Cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after Acute viral infection. Immunity. 2013;38(4):805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turtle CJ, Riddell SR. Genetically retargeting CD8 + lymphocyte subsets for cancer immunotherapy. Curr Opin Immunol. 2011;23(2):299–305. doi: 10.1016/j.coi.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85▪.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10(5):267–276. doi: 10.1038/nrclinonc.2013.46. This review summarizes the impressive data from different clinical trials that used adoptive transfer of T cells expressing anti-CD19 chimeric antigen receptors for treatment of B-cell malignancies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4 + T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8(1):47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 88.Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4 + T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilen CB, Wang J, Tilton JC, et al. Engineering HIV-resistant human CD4 + T cells with CXCR4-specific zinc-finger nucleases. PLoS Pathog. 2011;7(4):e1002020. doi: 10.1371/journal.ppat.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 92.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4 + T cells during recurrence of melanoma. J Immunol. 2013 doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Autologous T-cells genetically modified at the CCR5 gene by zinc finger nucleases SB-728 for HIV (zinc-finger). http://clinicaltrials.gov/show/NCT00842634
- 102.Phase 1 Dose escalation study of autologous T-cells genetically modified at the CCR5 gene by zinc finger nucleases in HIV-infected patients. http://clinicaltrials.gov/show/NCT01044654
- 103.Study of autologous T-cells genetically modified at the CCR5 gene by zinc finger nucleases in HIV-infected subjects. http://clinicaltrials.gov/show/NCT01252641


