Abstract
As the racial composition of the population changes, intergroup interactions are increasingly common. To understand how we perceive and categorize race and the attitudes that flow from it, scientists have used brain imaging techniques to examine how social categories of race and ethnicity are processed, evaluated and incorporated in decision-making. We review these findings, focusing on black and white race categories. A network of interacting brain regions is important in the unintentional, implicit expression of racial attitudes and its control. On the basis of the overlap in the neural circuitry of race, emotion and decision-making, we speculate as to how this emerging research might inform how we recognize and respond to variations in race and its influence on unintended race-based attitudes and decisions.
A new picture of the US has emerged from the recent 2010 census data. Non-white minorities now comprise 36% of the US population, up 29% since the 2000 census1. More than ever, intergroup interactions are a ubiquitous element of daily life in the US, ushering in a greater need to explore the mechanisms involved in intergroup attitudes and beliefs. For the past few decades, scientists interested in the mental representation of race and ethnicity have increasingly turned to electrophysiology, functional magnetic resonance imaging (fMRI) and other physiological methods to address how individuals process, evaluate and utilize human variation along the social dimension of race in decision-making in everyday interactions. These new tools push the frontiers of our understanding of how humans perceive and evaluate race and how these processes relate to the type of social behaviors that have consequential effects for the perceiver and the perceived. They yield insights into how to address the unintended expressions of racial attitudes, including those that diverge from conscious attitudes.
Here we review all of the studies using fMRI to examine responses to black and white race categories in American participants. These studies build on a rich literature, which began in the 1990s with electrophysiological techniques being used to examine the neural systems of race2. We selectively incorporate this literature, as well as the more sparse research using patients with brain lesions, but focus on studies examining blood oxygenation level–dependent (BOLD) signal throughout the brain. These studies most consistently report activation in a network of brain regions that includes the amygdala, anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC) and fusiform face area (FFA) (Table 1). We sought to integrate the modest, but growing, fMRI research on race with the aim of characterizing what the results mean about intergroup perception, and how these findings may link to real-world decision-making and potential prospects for social change.
Table 1.
Studies, stimuli, tasks, Montreal Neurological Institute coordinates and cortical regions involved in either viewing black and white faces or correlated with measures of racial bias
| Reference | Stimuli | fMRI task | x | y | z | Amygdala | DLPFC | ACC | FFA |
|---|---|---|---|---|---|---|---|---|---|
| 10 | Black and white faces | View faces, judge if male or female | −15.4 | −6.5 | −20.5 | X | |||
| 10.6 | −6.4 | −20.9 | |||||||
| 18 | Black and white faces | N-back | −18.1 | −5.0 | −15.9 | X | |||
| 35.5 | −2.5 | 8.7 | |||||||
| 16.8 | −0.06 | 35.6 | X | ||||||
| 56 | Black and white faces | View faces and remember faces | −34.8 | −42.6 | −26.7 | X | |||
| 11 | Black and white faces | Face to the left or right of fixation | 43.4 | 55.5 | 19.4 | X | X | ||
| 36.7 | 64.1 | 8.6 | |||||||
| −47.5 | 47.5 | 8.3 | |||||||
| 7.8 | 46.4 | 27.7 | |||||||
| 14.1 | 35.3 | 11.9 | |||||||
| 7.9 | 23.7 | 26.5 | |||||||
| 12 | Black and white faces | Face to the left or right of fixation (face shown 30 ms or 525 ms) | 18 | −6 | −12 | X | |||
| 27 | 48 | 24 | X | ||||||
| 33 | 48 | 36 | |||||||
| 24 | 60 | 27 | |||||||
| −57 | 18 | 36 | |||||||
| −6 | 36 | 24 | X | ||||||
| −9 | 18 | 33 | |||||||
| 3 | 18 | 33 | |||||||
| −30 | −36 | −21 | X | ||||||
| −45 | −42 | −21 | |||||||
| 51 | −42 | −24 | |||||||
| 20 | Black and white faces | Categorize race of face using pictures, categorize race of face using words or categorize shape of face | 28.9 | 2.2 | −21.0 | X | |||
| 18.1 | −10.1 | −26.3 | |||||||
| 18.1 | 6.2 | −23.4 | |||||||
| 15.9 | −0.4 | −25.0 | |||||||
| 13 | Black and white faces | Yes or no: over 21, vegetable preference or presence of a dot | −20.7 | −10.7 | −18.9 | X | |||
| 23.6 | −9.5 | −19.7 | |||||||
| 14 | Black and white faces | Social categorization task: age Nonsocial categorization task: object visible in array |
26.8 | −1.0 | −20.6 | X | |||
| 26.8 | −3.3 | −22.6 | |||||||
| 36.7 | −44.6 | −17.6 | X | ||||||
| 44 | Black and white faces | Race IAT | 30 | 0 | −14 | X | |||
| 30 | 58 | 14 | X | ||||||
| 32 | 56 | 14 | |||||||
| 34 | 48 | 18 | |||||||
| −8 | 32 | 24 | X | ||||||
| 8 | 40 | 14 | |||||||
| 8 | 34 | 28 | |||||||
| 15 | Black and white faces, dark or light skin | Face to the left or right of fixation | −27 | −0.2 | −20.9 | X | |||
| 17.5 | 59.5 | 29.6 | X | ||||||
| −27.6 | 34.7 | 42.9 | |||||||
| 72 | Black and white faces | Categorize by group or by race | |||||||
| 16 | Faces (same- and other-race and self-resembling) | Cyberball | −2 | 46 | 12 | X | |||
| 6 | 44 | 2 | |||||||
| 16 | 20 | 34 | |||||||
| 99 | Black and white faces | View individuals in painful or neutral situations and participant’s rate their empathy | −21.7 | −12.3 | −13.1 | X | |||
| 1.7 | −6.9 | 43.1 | X | ||||||
| 17 | Black and white faces | Primed with music: rap, metal or none, view faces (at 32 ms or 525 ms) | −20 | −4 | −12 | X | |||
| 32 | −2 | −18 | |||||||
| 36 | 2 | −24 | |||||||
| 22 | −4 | −24 | |||||||
| −48 | 34 | 22 | X | ||||||
| −44 | 36 | 22 | |||||||
| 28 | −2 | 56 | |||||||
| −42 | 2 | 46 | |||||||
| 38 | 36 | 34 | |||||||
| −18 | 40 | 26 | |||||||
| −48 | 18 | 42 | |||||||
| −32 | 30 | 40 | |||||||
| −12 | 38 | 44 | |||||||
| 22 | 44 | 28 | |||||||
| 22 | 32 | 34 | |||||||
| 18 | 46 | 28 | |||||||
| −22 | 10 | 60 | |||||||
| 28 | 26 | 44 | |||||||
| 22 | 24 | 54 | |||||||
| 83 | Black and white faces | View faces and trait words and remember them | 40.1 | 54.5 | 20.7 | X | |||
| −31.6 | 60.7 | −12.3 | |||||||
| 100 | Black and white faces | View individuals in painful or neutral situations and participant’s rate their empathy | 40.1 | 42.6 | 19.7 | X | |||
| 1.2 | 61.8 | 20.6 | X | ||||||
| −1.9 | 31.0 | 24.9 | |||||||
| 46.5 | −68.8 | −12.0 | X | ||||||
| 79 | Black and white faces | Trust game | 27 | 2 | −23 | X | |||
| −15 | −1 | −14 | |||||||
| 39 | 20 | 40 | X | ||||||
| −6 | −22 | 40 | X | ||||||
| −3 | 41 | 7 |
Regions commonly implicated in the study of race
Amygdala
The amygdala, a subcortical structure in the anterior-temporal lobe, is the brain area that has been reported with greatest frequency in studies of black-white race attitudes, beliefs and social decision-making. The amygdala comprises a small group of nuclei that are critical for the acquisition, storage and expression of classical fear conditioning3. In humans, the role of the amygdala in emotional learning is extended to include social means of fear learning, such as verbal instruction and observation4. In addition, the amygdala has widespread connections with the cortex. These connections link to the amygdala’s broader role in detecting emotionally relevant stimuli in the environment, including salient positive stimuli, and modulating cognitive functions, such as attention and memory5–8.
It is the amygdala’s role in the detection of emotional relevance that inspired the first investigations of its contribution to race processing. The history of race relations in the US, especially black-white relations, is fraught with complex emotions, including fear, hostility and lack of trust. Consistent with the emotional salience of race in American culture and, increasingly, elsewhere, a number of studies have found greater amygdala BOLD activity to outgroup race faces (that is, faces judged as belonging to a race group different from oneself) than to ingroup faces9–17. Although this pattern is generally observed, inconsistencies exist, with some studies failing to report an overall effect for black versus white in white American participants11,18, and others finding that black American participants show either greater amygdala activity to ingroup19 or outgroup faces10. The psychological study of race provides some potential insights into these inconsistent findings.
For almost a century, psychologists studied the feelings and beliefs that white Americans have about black Americans, and the data demonstrate a consistent and marked drop in negative attitudes and stereotypes20,21. However, for the past three decades, dissatisfaction with survey-type measures of explicitly reported race attitudes and stereotypes grew. This led investigators to develop indirect measures of social cognition. Among the more widely used measures of implicit social cognition is the implicit association test22 (IAT), which measures the strength of association between concepts, such as white and black, and attributes, such as good and bad. Using response latencies to classify concept and attribute pairings, the IAT provides an index of individual differences in the ease of association, such as black and good versus white and good. When such measures of social cognition that bypass access to conscious control are used, the findings stand in contrast to the picture obtained from the self-report measures of attitudes and stereotypes. Specifically, for white Americans, even when weak or no race preference is apparent on explicit, self-report measures, substantial levels of preference for positive stereotypes of white rather than black are observed on the IAT23. For black Americans, the pattern is more complex, with 40% of black Americans showing a pro-white preference on the IAT, 40% showing pro-black attitudes and 20% neutral with regard to race23. This variability in race attitudes across and within race groups is thought to reflect cultural and social learning of race attitudes and stereotypes24–26. Notably, implicit attitudes show predicative validity; the magnitude of preference exhibited on the test predicts a host of discriminative behaviors, from nonverbal avoidance to evaluating an individual’s work27.
This dissociation between the explicit reporting of race attitudes and implicit race preference as assessed with the IAT mirrors an early finding from research in patients with amygdala damage who demonstrate disruption of the indirect, physiological expression of fear memories, leaving the explicit knowledge of these memories intact28,29. If the amygdala is linked more strongly to implicit measures of emotional learning, variability in implicit measures of race attitudes might mediate amygdala responses to ingroup versus out-group faces. In an initial demonstration of this relationship, participants were asked to view pictures of black and white faces while BOLD responses were measured and implicit and explicit assessments of race attitudes were conducted18. Although this study did not find an overall BOLD signal difference between black and white faces in white American participants, this BOLD difference correlated with two implicit measures of pro-white race preference across participants: the IAT and eyeblink startle to black versus white faces, a physiological assessment of negative affect to outgroup versus ingroup faces30. In contrast, none of the explicit measures of race attitudes correlated with amygdala activation9,12.
This link between the implicit expression of race attitudes and amygdala activation to black and white faces implies that the manner in which race stimuli are interpreted may be more important than other, perceptual distinguishing characteristics. For instance, equivalent amygdala activation to light- and dark-skinned faces labeled as black has been observed, suggesting that differential amygdala BOLD responses to black and white faces cannot be attributed to color or contrast differences14. In addition, the variability in amygdala activation across white American participants9,18, as well as inconsistent reports of amygdala activation to ingroup versus outgroup races among black participants10,19, is indicative of variability in implicit attitudes. Investigators found that children with William’s syndrome, a genetic condition marked by overfriendliness, had reduced amygdala reactivity to threats and decreased FFA and amygdala interactions, showed no pro-white racial bias, but had intact gender role associations, supporting a role of the amygdala and threat detection in race, but not gender, attitudes31. These results suggest that variability in amygdala reactivity across individuals and groups reflects individual differences that emerge from many sources, including temperament differences and exposure to cultural attitudes. In other words, variability in amygdala activity may reflect the mind of the perceiver rather than differences in the perceptual characteristics of race stimuli.
Although the amygdala is the brain region most consistently implicated in fMRI studies of race, the expression of race attitudes undoubtedly reflects a network of regions. Notably, even though amygdala activation consistently correlates with implicit race preference measures, damage to the amygdala only impairs performance on physiological measures of implicit preference, leaving performance on the IAT intact32. These results, and others, suggest the neural representation of implicit and explicit race attitudes likely entails a range of overlapping and interacting brain systems.
The ACC
Another brain region that has often been reported in neuro-imaging studies of race is the dorsal ACC. Neuroscientists generally observe activation of the dorsal ACC when individuals experience conflict between prepotent and intentional response tendencies, including those elicited using cognitive control tasks such as the Stroop and Ericksen flanker tasks33,34. It has been proposed that the ACC is involved in monitoring for response competition and, once a conflict is detected, serves to engage executive control35. In the race context, conflict between automatic, prepotent feelings and conscious intentions to respond fairly may explain the involvement of the ACC. Equality norms in American society dictate that behaving in a racially biased manner is unacceptable and many individual Americans share that aspiration. Ironically, although contemporary cultural norms stress equality and fairness, the culture is also saturated with negative associations of black Americans. Thus, for many individuals, conflict persists between egalitarian goals and automatic negative attitudes and stereotypes36,37.
A number of studies report evidence of ACC activation in response to black and white faces, and a few suggest that BOLD response patterns reflect conflict between prepotent, automatic race attitudes and explicit, intentional race beliefs about equality. Such a specific claim is possible because of convergence between neural activity and behavior. Evidence that increased ACC activation in white participants to black faces correlates with their pro-white IAT scores indicates that the stronger the automatic negative response to black, the greater the conflict as reflected in the ACC11. In addition, when white American participants view black and white faces either subliminally or supraliminally, differential BOLD responses in the amygdala are stronger during subliminal presentation9. Supraliminal presentation results in greater activation of the ACC, DLPFC and ventral lateral PFC11. Furthermore, the reduction in differential BOLD responses in the amygdala during the supraliminal condition correlates with increased activation in the ACC and DLPFC. Again, increases in ACC activation during supraliminal presentation of black faces has been attributed to the detection of a conflict between egalitarian conscious beliefs, activated by conscious perception of other race faces, and pro-white implicit attitudes.
Although these fMRI findings imply a role for the ACC in the detection of conflicting equalitarian conscious beliefs and implicit pro-white attitudes, they do not, with the exception of the IAT, examine variability in any psychological measure that indicates increased conflict. An event-related brain component measured using electroencephalography, error-related negativity (ERN), reflects ACC activation in a number of cognitive conflict tasks38. To address the dissociation between explicit and implicit attitudes, researchers assessed the magnitude of the ERN in participants that varied in their desire and motivations to be free of prejudice39. Previous research has shown that some individuals may be more internally motivated to control racial attitudes as a chronic tendency, but others need external motivation, such as equality norms, to implement cognitive control efforts40,41. Individuals that were internally motivated by their own standards to be nonprejudiced showed larger ERN responses during stereotype incongruent errors than individuals that required external motivation to be nonprejudiced, indicating that increased internal motivation to respond without prejudice may amplify cognitive conflict mechanisms39,42. These findings and others suggest the detection of conflict, as expressed in BOLD responses in the ACC and ERN, is a first step in engaging regulatory mechanisms to control the expression of implicit race attitudes, even when individuals are not explicitly instructed to do so42–44.
The DLPFC
The majority of the race studies that report activation of the ACC also report activation of the DLPFC. The DLPFC is a region that has been shown across a range of cognitive tasks to be involved in top-down executive control45. For instance, in working memory, it was proposed that the DLPFC is involved in the top-down control of sensory and motor representations necessary for performance on the task45. Affective neuroscientists believe that the DLPFC is important in the cognitive regulation of emotion, modulating responses in the amygdala and striatum, most likely indirectly through its connectivity with the ventral medial PFC46. Thus, the ACC is involved in performance monitoring, whereas the DLPFC is involved in implementing control47. Dysregulation in either region can lead to reductions in self-control, either through a failure to note a potential error or a failure to implement task- or goal-appropriate responses. Studies of race processing posit that the DLPFC works in concert with the ACC, with the ACC detecting a conflict between conscious intentions and implicit attitudes and the DLPFC engaging a regulatory mechanism to control unwanted, implicit racial associations48.
Evidence for a role of the DLPFC in the regulation of implicit attitudes comes from a series of studies in which the effect of interracial contact on cognitive control was measured11. White American participants with more negative implicit race attitudes performed worse on a Stroop test after interracial interactions than after same-race interactions49,50. Stroop impairment, the researchers argued, may have resulted from depletion of cognitive resources with efforts to control negative racial attitudes in the interracial context. Notably, the magnitude of BOLD activity in the ACC and DLPFC when viewing black and white faces correlated with pro-white IAT scores, but only DLPFC activation mediated the relationship between implicit race preference and Stroop interference11. These findings suggest that a DLPFC regulatory mechanism may be engaged during interracial interactions to inhibit implicit race attitudes based on internal beliefs or awareness of societal attitudes.
The FFA
What the reviewed studies have in common is that they investigated responses to ingroup and outgroup faces rather than to alternative, verbal representations of racial groups19. Many studies on face processing demonstrate the importance of a region of the fusiform gyrus, the FFA. Research on face recognition, such as distinguishing between faces and non-faces, and between familiar faces and unfamiliar or new faces, implicates the FFA51,52. This distinction is apparent in patients with damage to the fusiform gyrus who typically have difficultly differentiating between faces, but can readily distinguish faces from objects52,53. The ability to distinguish among faces is influenced by race, suggesting a possible role for the FFA. Research shows that individuals are faster and more accurate at recognizing faces of ingroup members than outgroup members, often referred to as the other-race, cross-race or same-race effect54,55.
Following from the cross-race recognition findings, researchers recorded BOLD activity from black and white participants while they viewed and attempted to remember pictures of unfamiliar black and white faces and objects (for example, radios)56. Behaviorally, they replicate the same-race advantage. Moreover, participants exhibit greater FFA activation when viewing same-race faces compared with other-race faces. This activation difference correlates with the memory advantage for same-race faces (see refs. 14,57 for similar findings in the N170, but also see refs. 58,59), suggesting that out-group or unfamiliar faces may not be ‘faces’ with the same intensity as ingroup or familiar faces60. Researchers examining the cross-race effect argue that participants process outgroup members primarily at the category level (race group) at the expense of encoding individuating information because of differences in category expertise or motivated ingroup attention61–63. Lower FFA activity may reflect a failure to encode outgroup members at the individual level and promote an outgroup homogeneity effect, whereby outgroup members are perceived as more similar to each other than ingroup members. This perceptual homogeneity may then contribute to poor memory for racial outgroup members and negative implicit evaluations.
A recent investigation using multivoxel pattern analysis to determine whether BOLD activation patterns can predict race from face stimuli found successful prediction in the FFA, but only in participants high in implicit pro-white race bias60. The finding that higher bias decreases the similarity of FFA representations suggests that stronger race bias may be associated with larger differences in the perceptual experience of black and white faces.
Brain systems of detecting race and controlling attitudes
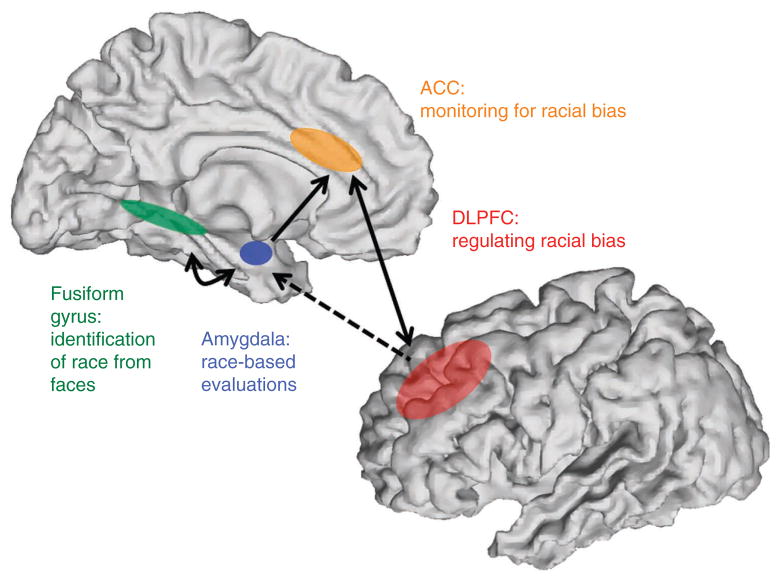
In the social psychological literature on race, a hierarchical control model has been proposed in which higher order personal and societal motivations influence other, lower order aspects of person construal, such as the application of category-based evaluations derived from race64. This psychological model implies at least two stages in the processing of race stimuli. The first requires the detection, categorization and automatic evaluation of race. In the neuroimaging research using face stimuli described above, this initial stage likely involves the amygdala and FFA, both of which show responses to subliminally presented ingroup and outgroup race faces12 (Fig. 1). The amygdala appears to be involved in the detection of race stimuli and its evaluation as expressed with the IAT and physiological responses18. The FFA also seems to rapidly process race information, but shows a greater response to ingroup race faces. Although there is evidence of connectivity between the amygdala and FFA in the processing of fear faces, such that increased activation of the FFA to fear versus neutral faces is abolished with amygdala damage65, it is unknown how this connectivity may influence responses to faces that vary by race.
Figure 1.
The brain regions most often reported in studies of race. The amygdala has been linked to automatic race evaluations and the FFA is involved in the rapid identification of other race individuals. The ACC is thought to detect conflict between implicit race attitudes and conscious intentions to be nonbiased. When such conflicts are detected, the DLPFC may regulate negative evaluations.
The second proposed stage of race processing involves higher order personal and societal motivations that exert some control over lower order processes. In the fMRI research of race, this stage likely involves the ACC and DLPFC, which may not respond without awareness9 (but see ref. 14). Consistent with its role in cognitive tasks, the ACC may detect conflict between prepotent, evaluative race preference and personal and/or societal goals to be nonprejudiced. Individual variability in these higher order goals appears to be reflected in indicators of ACC activity39. When a conflict exists, the ACC engages top-down regulatory mechanisms that involve the DLPFC. Consistent with studies of emotion regulation, the DLPFC is likely involved in modulating amygdala activity, resulting in diminished BOLD responses12. This emotion regulation circuitry, in which the DLPFC exerts some control over the amygdala, perhaps through its connectivity with more ventral PFC regions66, results in diminished emotional or evaluative responses in affect tasks. However research has yet to link decreased evaluative responses to race stimuli to this circuitry. Unlike studies of emotion regulation, this control circuitry may engage automatically when personal or societal egalitarian goals conflict with implicit evaluative attitudes, but there is no reason to think that this circuitry could not be intentionally engaged.
Our initial discussion of fMRI race literature involves a look at isolated regions of interest and this discussion reflects the current status of this literature. However, with advancement in fMRI methods, researchers are now able to move beyond modular discussions and to begin to understand the coherence among regions using network analytic techniques (for example, psychophysio-logical interaction analysis, structural equation modeling, multivariate autoregressive modeling, Granger-causality and dynamic causal modeling). These techniques have the advantage of moving beyond functional segregation to understand how these areas integrate and the strength of that integration across different aspects of race processing and prejudicial behaviors. For example, these techniques may increase our understanding of what regions are critical for producing and reducing racially biased responding and whether these networks differ across domains. In the future, researchers should incorporate these techniques to build a more sophisticated understanding of these networks across perception, evaluation and decision tasks.
The malleability of the circuitry of race
In the early days of research using indirect measures of race attitudes, especially those that relied on automatic responses, researchers assumed that the outcome of racially negative attitudes was fixed. After all, conscious attitudes are markedly different in their valence, showing far more positive attitudes, and the subjective experience of taking a test of implicit attitudes does not evoke confidence in changing them. The initial assessment was a grim future for intergroup relations67–69. However, emerging research demonstrates that race-based preferences, even those that are automatic, seem to be malleable and dependent on both situational and dispositional factors70,71. Consistent with this malleability of the expression of race attitudes, a number of studies have found that alterations of stimuli, context and task demands can change race-related BOLD responses.
For example, in the initial investigation of the role of the amygdala in the expression of indirect race preferences, a second study used pictures of familiar and admired black and white individuals11. This change in stimuli diminished differential amygdala activation to outgroup race faces and its relation to measures of implicit race preference. Similarly, changing task demands to promote focusing on the individual rather than the social group diminished differential amygdala BOLD responses to outgroup versus ingroup faces13. These findings imply that exposure to counterstereotypic/familiar faces or introducing individuating goals shapes amygdala involvement in responding to race.
Changing the context of the interracial interaction also alters race-related BOLD responses linked to conflict and regulation. Although ACC activation is usually greater to outgroup race faces11, when participants play a cyberball game with partners of different races, social exclusion by ingroup race partners results in greater ACC activation than social exclusion by outgroup race partners16, presumably reflecting greater conflict and social pain with social exclusion from members of your own race group. The situation can also increase or decrease internal goals for reducing race-based discrimination. For example, presenting individuals with music primes associated with race categories (rap, heavy metal or no music) reveals that priming with rap music increases DLPFC and amygdala activation to black versus white faces in white American participants internally motivated to be non-prejudiced17. These findings suggest that both internal goals and other stimuli in the environment can alter the neurocircuitry of race.
What has been interpreted to be race-based processing may also reflect a more general response in intergroup contexts. In two recent studies, researchers arbitrarily assigned mixed-race participants to one group or another. Subjects viewed pictures of members of their own arbitrarily assigned group or the other group during fMRI. The studies report heightened activity in the FFA to faces of arbitrarily assigned ingroup members compared with outgroup members, regardless of race72,73. These results suggest that expertise with ingroup race exemplars per se may not lead to altered FFA responses74, but rather that the salient social group identity in the situation, which may or may not be race, could dictate lowered attention to outgroup members at the expense of encoding individuating features.
It is also important to note that differences in BOLD responses as a function of race may reflect a number of psychological processes, including basic visual perception differences to reflections of personally held beliefs and/or culturally acquired semantic associations that may result in behavioral expressions of prejudice. In the absence of data linking brain activation with discriminative behavior or measures of racial prejudice that are reliably predictive of discriminative behavior, such as the IAT, it is difficult to interpret BOLD differences linked to race. Future scholars interested in the neuroscience of race should aim to flush out the contributions of the different brain regions and networks implicated in race processing to real world discrimination using more diverse techniques, such as network analyses, studies in patients with brain lesions, physiological manipulations (for example, transcranial magnetic stimulation or psychopharmacology), and/or existing psychological manipulations that may engage or disrupt processing (for example, racial bias malleability techniques or emotion regulation strategies).
Emerging trends and future directions
The research to date provides a framework for understanding how race is processed by the brain, and how neural circuits that are implicated in a range of affective and cognitive functions may also be involved in the identification and evaluation of race groups and the expression of behavior. As mentioned above, this research is limited by the range of analytic and neuroscience techniques that have been used. Future research will be enhanced by expanding the range of approaches used to understand the representation of race in the brain. By integrating research on race with the larger human neuroscience literature, we can take advantage of our knowledge of brain function to explore domains relevant to our understanding of race.
Race-based preferences in decision-making
Understanding how people process and evaluate social groups is invaluable in that it provides insight into the mechanisms of prejudice and prejudice reduction. Ultimately, however, researchers seek to push this frontier further by bridging the gap between laboratory brain science and real-world behaviors and judgments in socially consequential scenarios. Researchers are increasingly interested in the psychological and neural correlates of social decisions. One relatively new avenue for investigation uses emerging advances in decision science to study how race influences social decision-making.
For example, neuroeconomics provides a framework for understanding human decisions by combining classic behavioral economic tasks, quantitative tools and human neuroscience techniques75. The neural circuits typically implicated in neuroeconomic studies of decision-making overlap with those observed in studies of race. For instance, the amygdala is involved when emotion affects actions and values through its connectivity with the striatum76 and ventral medial PFC77. The amygdala is linked to judgments of trust78, as well as to implicit race preference. This overlap inspired a recent investigation of implicit preferences on decisions in a behavioral economics trust game79. During this game, measures of implicit race preference, as assessed with the IAT, correlate with decisions to trust, such that those with a stronger pro-white preference choose to invest more money with white economic partners. An examination of BOLD responses during this task revealed that activation of amygdala to black versus white economic partners correlates with implicit preference. Moreover, the decision bias, that is, the differential investments with black versus white partners, correlates with activation in the striatum9. These findings are consistent with a model in which the amygdala codes evaluative information that is integrated with action values via its connectivity with the striatum80.
One potentially important tool that is commonly used in decision science is the quantification of behavior. Much like perceptual psychophysicists, neuroeconomists and decision scientists increasingly use mathematical models to both precisely characterize individual differences in decision variables, as well as to differentiate components of updating value representations75. Given that race influences both decisions and affective learning about others81, incorporating the quantitative tools of decision science may provide a more nuanced understanding of the effect of racial group on behavior and brain function.
Of course, economic decisions are only a subset of the real-world decisions that race may influence. To the extent that we can integrate insights from neuroeconomics and decision science into social decision making, it is important to extend these tools to other domains in which race may be involved. For example, there is strong evidence that race attitudes influence legal decisions, often unintentionally82. A recent investigation examined whether BOLD responses to black versus white faces correlates with legal decisions to award black victims damages for discrimination. Increased activation of the DLPFC and parietal cortex correlate with damages awarded, implying a larger network of regions involved in producing discriminative behaviors83. There is also a growing literature examining electrophysiological correlates of racially biased decisions to shoot in potential crime scenes2,84, exploring how race influences this exceptionally consequential domain.
In addition to economic and legal decisions, unintended race bias influences hiring decisions and medical decisions85–88. Knowing how to extend insights from laboratory science to problems outside of the laboratory is always a challenge and is not unique to research on race. However, it is important to start with a clear and detailed understanding of the range of means by which race can influence decisions both generally and in specific social domains. By combining affective, social and economic neuroscience approaches and insights with decision tasks reflecting socially relevant consequences, we will obtain a better understanding of how our implicit attitudes may, or may not, seep into the choices we make.
Controlling and changing race preferences
If our implicit race preferences can influence social choices in a manner that is inconsistent with our values and intentions, than an obvious goal is to discover the means by which we can control or eliminate their effect. Given the overlap in circuitry involved in studies of race and emotion regulation, we can take cues from research on regulation to develop interventions that might alter race attitudes. A common technique in studies of emotion regulation is to reappraise or re-interpret an emotionally salient event in an effort to alter its emotional effect. This can be done by changing one’s perspective or goals when encountering a stimulus or, more explicitly, by instructing participants to interpret the stimulus in a way that alters their emotional reaction89.
Social psychologists already utilize techniques reminiscent of reappraisal that aim to decrease negative evaluations of outgroup members90–92. Recent neuroeconomic research suggests that a perspective-shifting instruction designed to encourage reappraisal alters the emotional effect of choice outcomes and changes decisions93, which appears to utilize an emotion regulation circuitry94. It is possible that strategically instructing participants to encourage the reappraisal of an outgroup member may help to reduce the effect of unwanted implicit attitudes on social decisions, such as on legal decisions.
However, a limitation of the typical reappraisal manipulation is that its effect can be transient. Emotion regulation strategies typically used in cognitive behavioral therapy, which is a compilation of techniques including effortful practice, demonstrate a more lasting effect on unwanted emotional responses, primarily in clinical populations. In some ways, diversity training protocols shown to affect implicit attitudes may be analogous95. One important limitation of these techniques is that they require effortful practice over time to be successful. As such, they may not always be practical or feasible.
In the broader emotion regulation literature, all of these techniques and others (for example, extinction) seek to control the emotional response through prefrontal inhibition of the amygdala, but they leave the original affective representation relatively unaltered. Behavioral research has shown that, although increasing cognitive control through manipulations decreases race preference, the original negative associations with the racial group remain intact96. A more effective and longer lasting technique would be to change the original negative association, thereby eliminating the need for cognitive control. Emerging neuroscience research suggests that one way to do this may be to target reconsolidation. Studies on reconsolidation have shown that every time a memory is retrieved it undergoes a re-storage or reconsolidation process97. During reconsolidation, much like initial consolidation, the memory is fragile and prone to disruption or interference. Recent research reveals that by precisely timing the presentation of interfering information to occur during the reconsolidation period, the original memory or association may be persistently changed, with one consequence being an altered emotional response98. To date, the use of these techniques has mostly been limited to simple laboratory associations and memories. In the future, as we start to understand these processes better, it may be possible to extend this technique and others to reduce or eliminate the negative associations underlying unwanted implicit race attitudes and stereotypes.
Conclusions
A few decades ago, it was unthinkable that looking at the brain to understand representations of social groups such as black versus white was even possible, let alone that such explorations could yield useful knowledge. Evidence from neuroscience has been vital in clarifying the nature of how intergroup cognition unfolds. Moreover, the neuroscience of race has been useful in pointing the way toward the type of new behavioral evidence needed to answer questions of not only what happens when intergroup cognition is at stake, but whether and how change is possible in real human interactions.
How to use this knowledge from brain and behavior to further extend basic knowledge and to drive applications is the obvious next generation of questions that we must pose. If good people who intend well act in a manner inconsistent with their own standards of egalitarianism because of the racial groups to which ‘the other’ belongs, then the question of change takes on new and urgent meaning. This urgency requires that we attend to the evidence about how our minds work when we confront racial and other group differences. Thus far, we have obtained modest evidence about these processes as they operate in our brains, unbeknownst to our conscious selves. The question of what we will do with these insights awaits an answer.
Acknowledgments
We would like to thank L. Atlas for help with Figure 1, A. Meilich for help with Table 1 and J. Reitzes for feedback on various stages of this manuscript. We would like to acknowledge support by grants from the Macarthur Foundation Law and Neuroscience Network and the National Institute of Mental Health (MIT080756).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.U.S. Census Bureau. Overview of race and Hispanic origin: 2010. 2010 < http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf>.
- 2.Ito TA, Bartholow BD. The neural correlates of race. Trends Cogn Neurosci. 2009;13:524–531. doi: 10.1016/j.tics.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 6.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 7.Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AK, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 9.Stanley DA, et al. Race and reputation: perceived racial group trustworthiness influences the neural correlates of trust decisions. Phil Trans R Soc Lond B. 2012;367:744–753. doi: 10.1098/rstb.2011.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart AJ, et al. Differential response in the human amygdala to racial outgroup versus ingroup face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Richeson JA, et al. An fMRI investigation of the impact of interracial contact on executive function. Nat Neurosci. 2003;6:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham WA, et al. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler ME, Fiske ST. Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychol Sci. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 14.Ronquillo J, et al. The effects of skin tone on race-related amygdala activity: an fMRI investigation. Soc Cogn Affect Neurosci. 2007;2:39–44. doi: 10.1093/scan/nsl043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richeson JA, Todd AR, Trawalter S, Baird AA. Eye-gaze direction modulates race-related amygdala activity. Group Process Intergroup Relat. 2008;11:233–246. [Google Scholar]
- 16.Krill AL, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Front Evol Neurosci. 2009;1:1. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes CE, Cox CL, Schmader T, Ryan L. Negative stereotype activation alters interaction between neural correlates of arousal, inhibition and cognitive control. Soc Cogn Affect Neurosci. 2011 Sep 27; doi: 10.1093/scan/nsr052. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelps EA, et al. Performance on indirect measures of race evaluation predicts amygdala activation. J Cogn Neurosci. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- 20.McConahay JB, Hardee BB, Batts V. Has racism declined in America? It depends on who is asking and what is asked. J Conflict Resolut. 1981;25:563–579. [Google Scholar]
- 21.Dovidio JF, Gaertner SL. Aversive racism. In: Zanna MP, editor. Advances in Experimental Social Psychology. Vol. 36. Academic Press; 2004. pp. 1–52. [Google Scholar]
- 22.Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- 23.Ames DL, Jenkins AC, Banaji MR, Mitchell JP. Harvesting implicit group attitudes and beliefs from a demonstration web site. Group Dyn. 2002;6:101–115. [Google Scholar]
- 24.Cuddy AJC, et al. Stereotype content model across cultures: towards universal similarities and some differences. Br J Soc Psychol. 2009;48:1–33. doi: 10.1348/014466608X314935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiske ST, Bergsieker HB, Russell AM, Williams L. Images of black Americans. Du Bois Rev Soc Sci Race. 2009;6:83–101. doi: 10.1017/S1742058X0909002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caprariello PA, Cuddy AJC, Fiske ST. Social structure shapes cultural stereotypes and emotions: a causal test of the stereotype content model. Group Process Intergroup Relat. 2009;12:147–155. doi: 10.1177/1368430208101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwald AG, Poehlman TA, Uhlmann E, Banaji MR. Understanding and using the implicit association test. III Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- 28.Bechara A, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 29.LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. [PubMed] [Google Scholar]
- 31.Santos A, Meyer-Lindenberg A, Deruelle C. Absence of racial, but not gender, stereotyping in Williams syndrome children. Curr Biol. 2010;20:R307–R308. doi: 10.1016/j.cub.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Phelps EA, Cannistraci CJ, Cunningham WA. Intact performance on an indirect measure of race bias following amygdala damage. Neuropsychologia. 2003;41:203–208. doi: 10.1016/s0028-3932(02)00150-1. [DOI] [PubMed] [Google Scholar]
- 33.Barch DM. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- 34.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 35.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 36.Blair IV, Banaji MR. Automatic and controlled processes in stereotype priming. J Pers Soc Psychol. 1996;70:1142–1163. [Google Scholar]
- 37.Fazio RH, Jackson JR, Dunton BC, Williams CJ. Variability in automatic activation as an unobtrusive measure of racial attitudes: a bona fide pipeline? J Pers Soc Psychol. 1995;69:1013–1027. doi: 10.1037//0022-3514.69.6.1013. [DOI] [PubMed] [Google Scholar]
- 38.Dehaene S, Posner M, Tucker D. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- 39.Amodio DM, et al. Neural signals for the detection of unintentional race bias. Psychol Sci. 2004;15:88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- 40.Dunton BC, Fazio RH. An individual difference measure of motivation to control prejudiced reactions. Pers Soc Psychol Bull. 1997;23:316–326. [Google Scholar]
- 41.Plant EA, Devine PG. Internal and external motivation to respond without prejudice. J Pers Soc Psychol. 1998;75:811–832. doi: 10.1177/0146167205275304. [DOI] [PubMed] [Google Scholar]
- 42.Amodio DM, Kubota JT, Harmon-Jones E, Devine PG. Alternative mechanisms for regulating racial responses according to internal versus external cues. Soc Cogn Affect Neurosci. 2006;1:26–36. doi: 10.1093/scan/nsl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beer JS, et al. The quadruple process model approach to examining the neural underpinnings of prejudice. Neuroimage. 2008;43:775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 45.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Neurosci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 46.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 48.Stanley D, Phelps EA, Banaji MR. The neural basis of implicit attitudes. Perspect Psychol Sci. 2008;17:164–170. [Google Scholar]
- 49.Richeson JA, Trawalter S, Shelton JN. African Americans’ implicit racial attitudes and the depletion of executive function after interracial interactions. Soc Cogn. 2005;23:336–352. [Google Scholar]
- 50.Richeson JA, Shelton JN. When prejudice does not pay: effects of interracial contact on executive function. Psychol Sci. 2003;14:287–290. doi: 10.1111/1467-9280.03437. [DOI] [PubMed] [Google Scholar]
- 51.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for the perception of faces. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform face areas discriminate novel from visually familiar face. Neuroimage. 2003;19:877–883. doi: 10.1016/s1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 53.Tranel D, Damasio AR. Knowledge without awareness: an autonomic index of facial recognition by prosopagnosics. Science. 1985;228:1453–1454. doi: 10.1126/science.4012303. [DOI] [PubMed] [Google Scholar]
- 54.Malpass RS, Kravitz J. Recognition for faces of own and other race. J Pers Soc Psychol. 1969;13:330–334. doi: 10.1037/h0028434. [DOI] [PubMed] [Google Scholar]
- 55.Brigham JC, Malpass RS. The role of experience and contact in the recognition of faces of own- and other-race persons. J Soc Issues. 1985;41:139–155. [Google Scholar]
- 56.Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential fusiform responses to same- and other-race faces. Nat Neurosci. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- 57.Ito TA, Urland GR. The influence of processing objectives on the perception of faces: an ERP study of race and gender perception. Cogn Affect Behav Neurosci. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- 58.Caldara R, et al. Face versus non-face object perception and the “other-race” effect: a spatio-temporal event-related potential study. Clin Neurophysiol. 2003;114:515–528. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- 59.Ito TA, Thompson E, Cacioppo JT. Tracking the time course of social perception: the effects of racial cues on event-related brain potentials. Pers Soc Psychol Bull. 2004;30:1267–1280. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- 60.Brosch T, Bar-David E, Phelps EA. Implicit race bias decreases the similarity of neural representations of black and white faces. Psychol Sci. doi: 10.1177/0956797612451465. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ostrom TM, Carpenter SL, Sedikides C, Li F. Differential processing of in-group and out-group information. J Pers Soc Psychol. 1993;64:21–34. [Google Scholar]
- 62.Sangrigoli S, Pallier C, Argenti AM, Ventureyra VAG, de Schonen S. Reversibility of the other-race effect in face recognition during childhood. Psychol Sci. 2005;16:440–444. doi: 10.1111/j.0956-7976.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- 63.Young SG, Hugenberg K. Individuation motivation and face experience can operate jointly to produce the own-race bias. Soc Psychol Personal Sci. 2012;3:80–87. [Google Scholar]
- 64.Bodenhausen GV, Macrae CN, Milne AB. Disregarding social stereotypes: implications for memory, judgment and behavior. In: Golding JM, MacLeod CM, editors. Intentional Forgetting: Interdisciplinary Approaches. Mahwah: 1998. pp. 349–368. [Google Scholar]
- 65.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bargh JA. The cognitive monster: the case against the controllability of automatic stereotype effects. In: Chaiken S, Trope Y, editors. Dual-Process Theories in Social Psychology. Guilford Press; 1999. pp. 361–382. [Google Scholar]
- 68.Devine PG, Monteith MJ. Automaticity and control in stereotyping. In: Chaiken S, Trope Y, editors. Dual-Process Theories in Social Psychology. Guilford Press; 1999. pp. 339–360. [Google Scholar]
- 69.Fiske S. Examining the role of intent: toward understanding its role in stereotyping and prejudice. In: Uleman J, Bargh J, editors. Unintended Thought: The Limits of Awareness, Intention, and Control. Guilford; 1989. pp. 253–283. [Google Scholar]
- 70.Blair IV. The malleability of automatic stereotypes and prejudice. Pers Soc Psychol Rev. 2002;6:242–261. [Google Scholar]
- 71.Dasgupta N. Mechanisms underlying the malleability of implicit prejudice and stereotypes: the role of automaticity and cognitive control. In: Nelson T, editor. Handbook of Stereotyping, Prejudice, and Discrimination. Psychol. Press; 2009. pp. 267–284. [Google Scholar]
- 72.Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias: a functional magnetic resonance imaging investigation. Psychol Sci. 2008;19:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 73.Van Bavel JJ, Cunningham WA. Self-categorization with a novel mixed-race group moderates automatic social and racial biases. Pers Soc Psychol Bull. 2009;35:321–335. doi: 10.1177/0146167208327743. [DOI] [PubMed] [Google Scholar]
- 74.Phelps EA. Faces and races in the brain. Nat Neurosci. 2001;4:775–776. doi: 10.1038/90467. [DOI] [PubMed] [Google Scholar]
- 75.Glimcher PW, Fehr E, Rangel A, Camerer C, Poldrack RA. Neuroeconomics. Academic Press; 2009. [Google Scholar]
- 76.LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- 77.De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Todorov A, Duchaine B. Reading trustworthiness in faces without recognizing faces. Cogn Neuropsychol. 2008;25:395–410. doi: 10.1080/02643290802044996. [DOI] [PubMed] [Google Scholar]
- 79.Stanley DA, Sokol-Hessner P, Banaji MR, Phelps EA. Implicit race attitudes predict trustworthiness judgments and economic trust decisions. Proc Natl Acad Sci USA. 2011;108:7710–7715. doi: 10.1073/pnas.1014345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delgado MR, Jou RL, LeDoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olsson A, Ebert JP, Banaji MR, Phelps EA. The role of social groups in the persistence of learned fear. Science. 2005;309:785–787. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- 82.Lane KA, Kang J, Banaji MR. Implicit social cognition and law. Annu Rev Law Soc Sci. 2007;3:427–451. [Google Scholar]
- 83.Korn HA, Johnson MA, Chun MM. Neurolaw: differential brain activity for black and white faces predicts damage awards in hypothetical employment discrimination cases. Soc Neuro. 2012;7:398–409. doi: 10.1080/17470919.2011.631739. [DOI] [PubMed] [Google Scholar]
- 84.Correll J, Urland GR, Ito TA. Event-related potentials and the decision to shoot: the role of threat perception and cognitive control. J Exp Soc Psychol. 2006;42:120–128. [Google Scholar]
- 85.Green AR, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22:1231–1238. doi: 10.1007/s11606-007-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabin J, Nosek BA, Greenwald AG, Rivara FP. Physicians’ implicit and explicit attitudes about race by MD race, ethnicity, and gender. J Health Care Poor Underserved. 2009;20:896–913. doi: 10.1353/hpu.0.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Snipes SA, et al. Is race medically relevant? A qualitative study of physicians’ attitudes about the role of race in treatment decision-making. BMC Health Serv Res. 2011;11:183. doi: 10.1186/1472-6963-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rooth DO. Automatic associations and discrimination in hiring: real world evidence. Labour Econ. 2010;17:523–534. [Google Scholar]
- 89.Gross JJ. The emerging field of emotion regulation: An integrative review. Rev Gen Psychol. 1998;2:271–299. [Google Scholar]
- 90.Stewart BD, Payne BK. Bringing automatic stereotyping under control: implementation intentions as efficient means of thought control. Pers Soc Psychol Bull. 2008;34:1332–1345. doi: 10.1177/0146167208321269. [DOI] [PubMed] [Google Scholar]
- 91.Gollwitzer PM, Brandstaetter V. Implementation intentions and effective goal pursuit. J Pers Soc Psychol. 1997;73:186–199. doi: 10.1037//0022-3514.81.5.946. [DOI] [PubMed] [Google Scholar]
- 92.Galinsky AD, Moskowitz GB. Perspective-taking: decreasing stereotype expression, stereotype accessibility, and in-group favoritism. J Pers Soc Psychol. 2000;78:708–724. doi: 10.1037//0022-3514.78.4.708. [DOI] [PubMed] [Google Scholar]
- 93.Sokol-Hessner P, et al. Thinking like a trader selectively reduces individuals’ loss aversion. Proc Natl Acad Sci USA. 2009;106:5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sokol-Hessner P, Camerer C, Phelps EA. Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Soc Cogn Affect Neurosci. 2012 Feb 15; doi: 10.1093/scan/nss002. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudman LA, Ashmore RD, Gary ML. “Unlearning” automatic biases: the malleability of implicit prejudice and stereotypes. J Pers Soc Psychol. 2001;81:856–868. doi: 10.1037//0022-3514.81.5.856. [DOI] [PubMed] [Google Scholar]
- 96.Payne BK. Conceptualizing control in social cognition: how executive functioning modulates the expression of automatic stereotyping. J Pers Soc Psychol. 2005;89:488–503. doi: 10.1037/0022-3514.89.4.488. [DOI] [PubMed] [Google Scholar]
- 97.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 98.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. Neuroimage. 2010;51:1468–1475. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 100.Mathur VA, Harada T, Chiao JY. Racial identification modulates default network activity for same and other races. Hum Brain Mapp. 2011 May 26; doi: 10.1002/hbm.21330. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]