Abstract
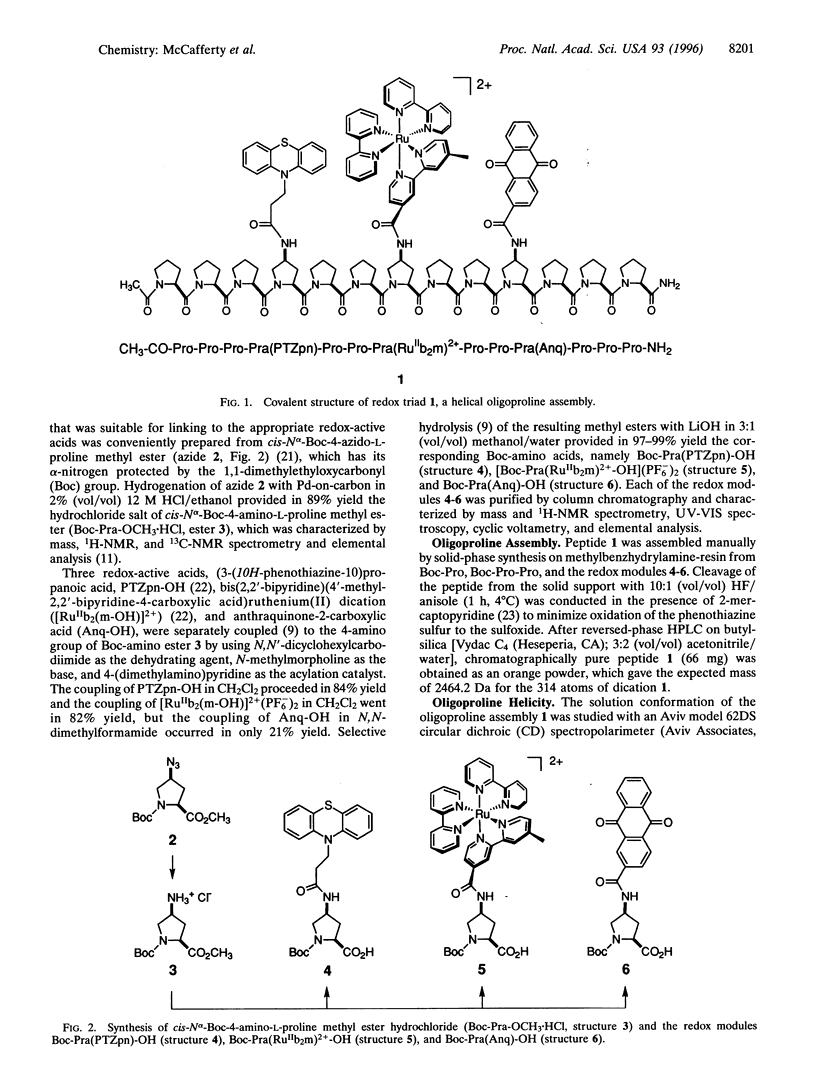
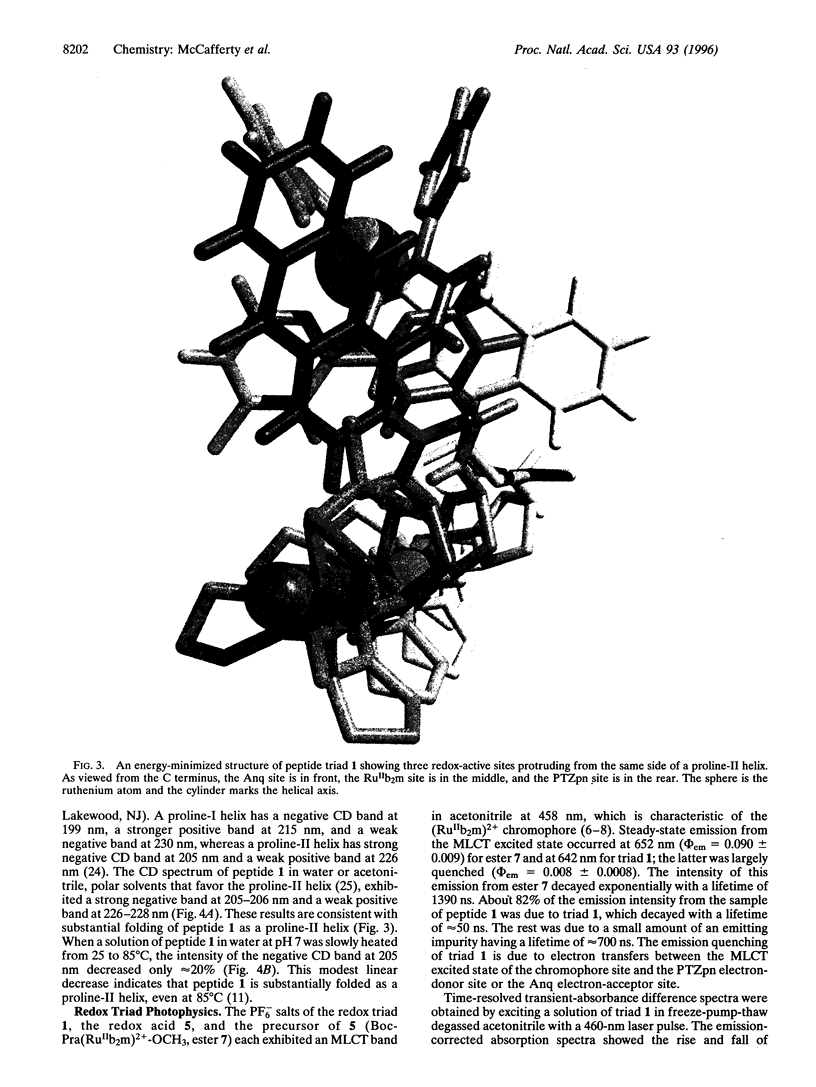
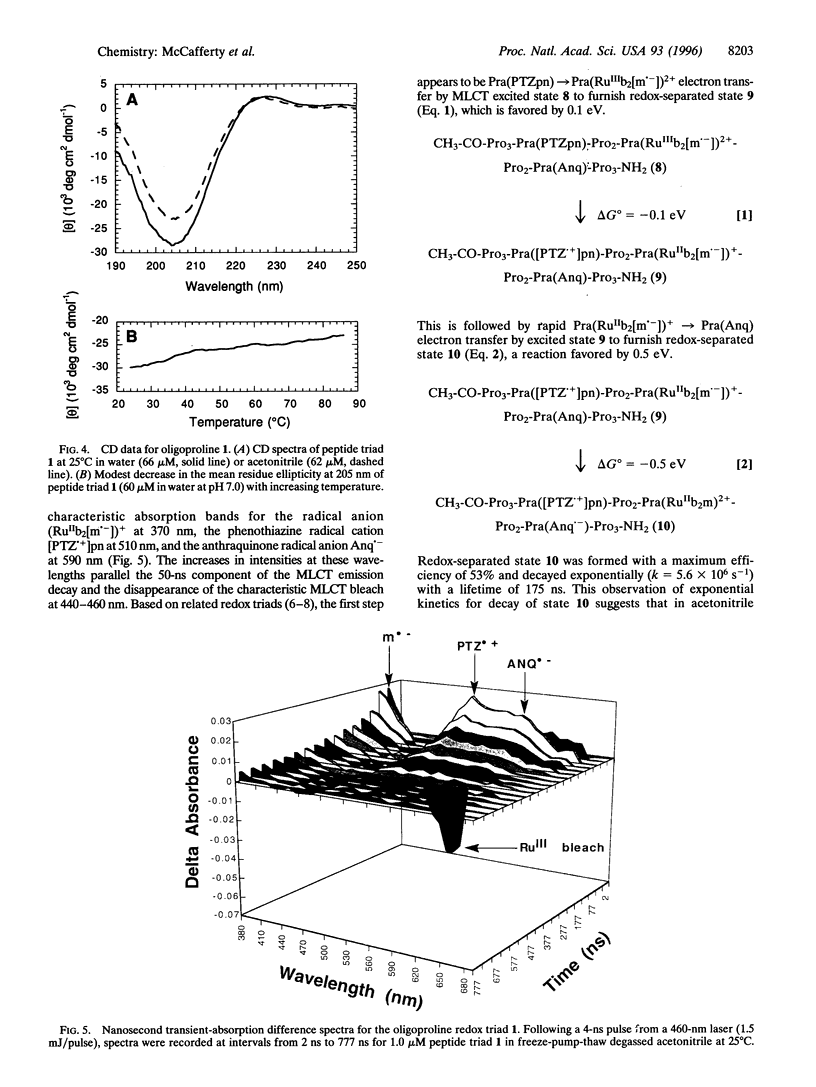
A general method is described for constructing a helical oligoproline assembly having a spatially ordered array of functional sites protruding from a proline-II helix. Three different redox-active carboxylic acids were coupled to the side chain of cis-4-amino-L-proline. These redox modules were incorporated through solid-phase peptide synthesis into a 13-residue helical oligoproline assembly bearing in linear array a phenothiazine electron donor, a tris(bipyridine)ruthenium(II) chromophore, and an anthraquinone electron acceptor. Upon transient 460-nm irradiation in acetonitrile, this peptide triad formed with 53% efficiency an excited state containing a phenothiazine radical cation and an anthraquinone radical anion. This light-induced redox-separated state had a lifetime of 175 ns and stored 1.65 eV of energy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D. J., Mokotoff M., Sheh L., Simmons J. E. Design, synthesis, and testing of antisickling agents. 2. Proline derivatives designed for the donor site. J Med Chem. 1983 Apr;26(4):549–554. doi: 10.1021/jm00358a017. [DOI] [PubMed] [Google Scholar]
- Adzhubei A. A., Sternberg M. J. Conservation of polyproline II helices in homologous proteins: implications for structure prediction by model building. Protein Sci. 1994 Dec;3(12):2395–2410. doi: 10.1002/pro.5560031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust D., Moore T. A. Mimicking photosynthesis. Science. 1989 Apr 7;244(4900):35–41. doi: 10.1126/science.244.4900.35. [DOI] [PubMed] [Google Scholar]
- Merritt E. A., Murphy M. E. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994 Nov 1;50(Pt 6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- Meyer S., Hugo R., Louw B., Grimbeek R. J. Screening for middle-ear disease in schools for hearing-impaired children. Int J Pediatr Otorhinolaryngol. 1989 May;17(2):163–170. doi: 10.1016/0165-5876(89)90091-8. [DOI] [PubMed] [Google Scholar]
- Peek B. M., Ross G. T., Edwards S. W., Meyer G. J., Meyer T. J., Erickson B. W. Synthesis of redox derivatives of lysine and related peptides containing phenothiazine or tris(2,2'-bipyridine)ruthenium(II). Int J Pept Protein Res. 1991 Aug;38(2):114–123. doi: 10.1111/j.1399-3011.1991.tb01418.x. [DOI] [PubMed] [Google Scholar]
- Strassmair H., Knof S., Engel J. Different binding of solvent to the peptide carbonyl group in different conformational environments induces the helix I is formed from helix II transition of poly L-proline. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1153–1154. [PubMed] [Google Scholar]
- Yamashiro D. Reduction of methionine sulfoxide in peptide synthesis by use of 2-mercaptopyridine in liquid hydrogen fluoride. Int J Pept Protein Res. 1982 Jul;20(1):63–65. doi: 10.1111/j.1399-3011.1982.tb02653.x. [DOI] [PubMed] [Google Scholar]