Abstract
Fibromyalgia syndrome is characterized by widespread pain that is exacerbated by cold and stress but relieved by warmth. We review the points along thermal and pain pathways where temperature may influence pain. We also present evidence addressing the possibility that brown adipose tissue activity is linked to the pain of fibromyalgia given that cold initiates thermogenesis in brown adipose tissue via adrenergic activity, while warmth suspends thermogenesis. Although females have a higher incidence of fibromyalgia as well as more resting thermogenesis, they are less able to recruit brown adipose tissue in response to chronic stress than males. In addition, conditions that are frequently comorbid with fibromyalgia compromise brown adipose activity making it less responsive to sympathetic stimulation. This results in lower body temperatures, lower metabolic rates, and lower circulating cortisol/corticosterone in response to stress - characteristics of fibromyalgia. In the periphery, sympathetic nerves to brown adipose also project to surrounding tissues, including tender points characterizing fibromyalgia. As a result, the musculoskeletal hyperalgesia associated with conditions like fibromyalgia may result from referred pain in the adjacent muscle and skin.
Keywords: Thermoregulation, Thermogenesis, Nociception, Adrenergic, Sympathetic, Catecholamine, Positron emission tomography, PET
Introduction
Fibromyalgia syndrome is a chronic condition characterized by musculoskeletal pain that persists for many years and is unresponsive to anti-inflammatory and analgesic compounds.1 In addition to decreased body temperature,2 several characteristics of fibromyalgia syndrome suggest altered thermoregulatory activity. First, the distribution of brown adipose tissue (BAT) resembles that of tender points, anatomical locations that have been used to diagnose fibromyalgia.1 This relationship may support referred pain in muscles similar to the referred pain of angina. Secondly, BAT activity at rest and the incidence of fibromyalgia are each relatively greater in females than males, whereas adaptive thermogenesis is greater in males than females.3,4 Thirdly, stress and cold each stimulate thermogenesis5 and aggravate symptoms of fibromyalgia,6 whereas warmth suspends thermogenesis and temporarily relieves the symptoms of fibromyalgia. Fourth, regulation of thermogenesis and pain share several areas in the brain where they may influence each other. Fifth, injections of a local anesthetic into stellate ganglia (sympathetic projections to subclavicular BAT) reduce pain in patients with fibromyalgia.7 Sixth, extended programs of exercise relieve symptoms of fibromyalgia, improve thermoregulation,8–10 decrease adrenergic activity, and inhibit recruitment of BAT. Based on these associations, we examine here the possible overlap between thermoregulation and the modulation of nociception that are consistent with the symptoms of fibromyalgia.
We include information from studies that address the overlap in circuitry of thermoregulatory and pain pathways with a focus on how these topics may relate to our current knowledge of the biological characteristics of fibromyalgia. The result is a literature review that is not intended to be comprehensive as excellent reviews of thermoregulation and of fibromylaglia already exist. Instead, this review highlights multiple areas that warrant additional study to delineate the nature of the relationship between fibromyalgia and thermoregulation.
Fibromyalgia syndrome
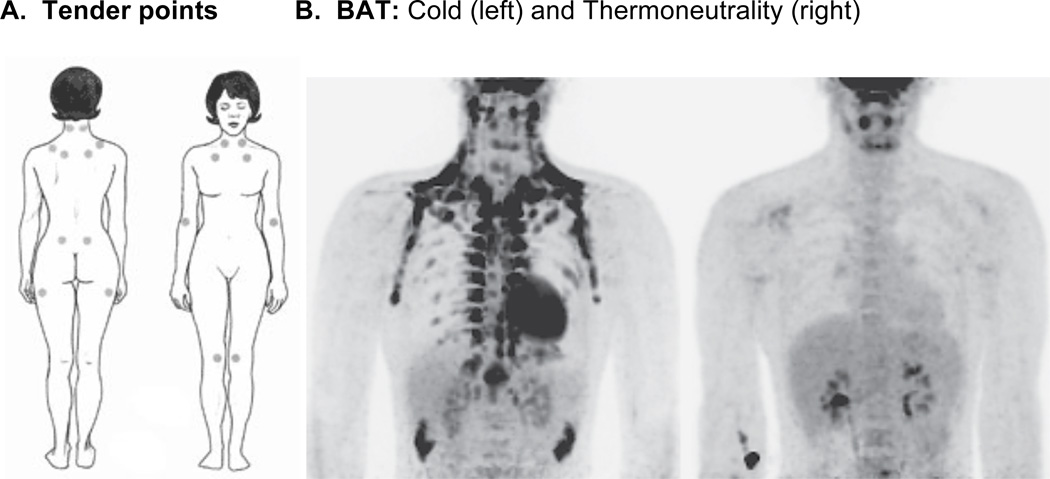
After exclusion of other painful disorders, the widespread pain of fibromyalgia is characterized by pain in spite of an absence of gross pathology at these or the surrounding large areas of hyperalgesia. While newer diagnostic criteria are proposed,10 the sensitivity and number of tender points out of 18 specific anatomical locations (Figure 1A) remain a useful investigative tool. These 18 points are distributed symmetrically on the trunk and proximal regions of limbs rather than areas that are usually more sensitive to tactile stimulation in healthy individuals, such as hands, feet, genitals, and mouth.11,12 Pain is not restricted to tender points; rather, the location of tender points was selected based upon their relative insensitivity to palpation in healthy normal controls. In contrast, only slight pressure at these sites often induces pain in patients with fibromyalgia. Hormones may be important as fibromyalgia is more common in women than in men, and pain sensitivity in healthy women varies over the menstrual cycle.13
Figure 1.
Comparison between the location of tender points and BAT depots in humans. Panel A shows the location of the 18 tender points, located symmetrically, used to characterize the pain of fibromyalgia (http://www.niams.nih.gov/Health_Info/Fibromyalgia/default.asp). Panel B shows the PET scan of an individual taken during exposure to cold (left panel of figure 1B), when BAT tissue is active and visible, compared to when exposed to thermoneutral conditions (right panel of figure 1B) (from Lichtenbelt, Vanhommerig, Smulders, Drossaerts, Kemerink, Bouvy, Schrauwen and Teule).89
Patients with fibromyalgia often report physical or emotional trauma prior to the onset of their condition,14 and stress exacerbates their symptoms. Patients are plagued by non-restorative sleep,15,16 fatigue,1 cold intolerance,17 and neuroendocrine abnormalities11 including abnormally high heart rate, low metabolic rate, low body temperature, and decreased temperature and vasoconstriction in skin over tender points.2 There is also a high prevalence of obesity,18,19 insulin resistance,20 and hyperlipidemia.20 Their pain appears sensitive to sympatholytic maneuvers and rekindles upon injection with norepinephrine.21 Except in a single study,22polymorphisms in catechol-O-methyl transferase have been linked to fibromyalgia,23–27 raising the possibility that faulty degradation of catecholamines increases the risk of developing fibromyalgia.
In healthy individuals, acute stress can either increase or decrease pain perception, depending on how stress affects various secreted proteins and their receptors. In patients with fibromyalgia syndrome, minor daily stresses, particularly cold stress, frequently exacerbate the symptoms of fibromyalgia. Conditioned pain modulation [CPM, previously called diffuse noxious inhibitory controls (DNIC)] can be measured in humans to assess pain sensitivity after immersion of their arms in hot (47°C)28 or cold (12°C)6 water. Healthy individuals perceive pain more in ascending trials when progressively greater areas of the arm are dipped into water, than when the whole arm is initially immersed and thereafter incrementally less surface area exposed. This suggests recruitment of inhibitory systems by immersion of the whole arm. That fibromyalgia patients fail to perceive this difference between the two types of exposure suggests a deficit of endogenous pain inhibitory activity.29,30 After the arm is withdrawn, pain induced by the cold water persists longer in fibromyalgia patients than in controls.
In cerebrospinal fluid from patients with fibromyalgia, there is an increased concentration of substance P,31,32 dynorphin,33 and nerve growth factor (NGF).34 In the serum, brain-derived neurotrophic factor (BDNF), the trophic factor that establishes mechanosensitivity of sensory nerves in the adult, is elevated.35 There is no obvious tissue damage in areas of the body that are reported as painful.36 Although peripheral defects have been identified,37 fibromyalgia is primarily attributed to hypersensitized pain pathways in the brain or spinal cord.38 Yet none of the proposed central or peripheral defects explains the unique distribution of mechanical hypersensitivity, i.e. in and near the trunk, characteristic of fibromyalgia pain.
While stress increases the severity of their symptoms,39–43 patients’ hypothalamic-pituitary-adrenal (HPA) responses to stress are attenuated.44 In contrast, their resting adrenergic activity remains high45 resulting in a diminished heart rate variability, especially at night.45 Corticotropin-releasing factor (CRF) together with enhanced sympathetic tone45 may underpin the characteristic sleep disturbances and elevated heart rate in these patients. Others report reduced epinephrine responses to hypoglycemia,44 yet an intravenous injection of interleukin-6 in patients with fibromyalgia dramatically increases norepinephrine more than in healthy controls.46
There is a high comorbidity between fibromyalgia and other painful or inflammatory disorders. For example, patients with fibromyalgia have a higher incidence of ulcerative colitis (19%),47 irritable bowel syndrome (about 70%),48–51 interstitial cystitis,52,53 vulvodynia,54 migraines (35.6%),55 Sjögren’s syndrome,56 and endometriosis57 than general female populations. The incidence of fibromyalgia in people with Crohn’s disease is reported to be as high as 49%47 or as low as 3%.58 These comorbidities may be due to similar etiologies, as previously proposed for migraine and fibromyalgia.59 Alternatively, they may result from viscerosomatic convergence on second order dorsal horn neurons in the spinal cord60 and thalamic neurons61,62 allowing somatic afferent activity to influence visceral sensations, and vice versa.63 This hypothesis, however, does not explain why patients with inflammatory disorders do not all develop fibromyalgia.
Exercise alleviates fibromyalgia, improving a variety of its symptoms. Even in healthy individuals aerobic exercise decreases blood pressure,64 lowers resting heart rate,65 increases heart rate variability,66 increases vagal tone,67 and decreases sympathetic tone.68 Individuals who regularly exercise have increased basal metabolic rates,69 lower white adipose tissue,70 greater baseline cortisol concentrations, and an increased response to stress-induced release of cortisol.71 Moreover, regular exercise improves sleep patterns,72 and lessens physical and mental fatigue.73 It improves circulation to muscles, skin and organs67,74 and lowers the risk of obesity or diabetes by improving the regulation of insulin and blood glucose.75 Exercise not only increases bowel motility in normal individuals,76 it improves symptoms associated with irritable bowel syndrome,77 a condition frequently diagnosed in patients with fibromyalgia.
Brown adipose tissue (BAT)
BAT is distributed in areas of the body that maintain body temperature in the face of cold exposure by a process referred to as nonexercise thermogenesis.78,79 In addition to its characteristic distribution, primarily in interscapular (rodents) or supraclavicular (humans) depots, it can also be found in the supra axial and perirenal areas; in striations of skeletal muscle; between the skin and underlying muscle; and on top of sympathetic ganglia (Figure 1B). BAT does not store fat and glucose but uses them to generate heat. Vascular convection carries this heat to adjacent vital organs, such as the thoracocervical regions of the spinal cord, heart, and other thoracic organs. BAT undergoes ‘recruitment’ (increased mass) in response to repeated cold, diets chronically high in calories, or repeated stress. Cross-adaptation between cold and stress (e.g., immobilization of rats) enhances thermogenesis,5 whereas increasing age in rats80 and corticosteroid administration in mice81,82 reduce thermogenic activity and thermal capacity of BAT.
Although non-shivering thermogenesis induced by cold and stress prevails in many nonhuman primates, it was previously thought inactive in human adults. However, residual BAT remains in most humans.83 When active, BAT takes up 18F-fluorodeoxyglucose and is visible in and interferes with positron emission tomography (PET), motivating practices that decrease thermogenesis during routine scans. Visualization of BAT on PET scans is decreased by a high fat, very-low-carbohydrate, protein-permitted diet for 5 hr before scans at a normal room temperature84 by suppressing glucose metabolism.85 Though found primarily in cervical-supraclavicular depots, BAT is widely distributed, but few PET scans taken at 22°C (72°F) reveal BAT when analyzed retrospectively (only 7.5% of women and 3.1% of men),86 likely due to fasting prior to scans. Other PET studies report higher percentages, ranging from 25% of patients with visible BAT87 to more than 80%88 when taken at room temperature. One autopsy series identified BAT in the necks of 84% of patients over the age of 20,83 indicating that this tissue is generally retained into adulthood. Consistent with this, BAT is readily apparent in PET scans in men exposed to 16°C (61°F) cold temperatures prior to their scan.89
Thermogenesis is initiated by enhanced sympathetic activity to BAT.90 Norepinephrine turnover is higher in cold-acclimated rats than in rats kept at thermoneutrality, reflecting a high sympathetic tone under cold conditions. The released norepinephrine acts at α2- and β3-adrenergic receptors, causing production of heat in areas surrounding vasculature leading to vital organs. While β-adrenergic receptors increase thermogenesis and adenosine 3′,5′-cyclic monophosphate (cAMP) production in humans, α2A-receptors decrease them,91 such that the balance between α2- and β3-receptor activity is key. Cold, stress, or overfeeding initiate sympathetic activity, increasing release of norepinephrine and β-adrenergic activity. Chronically, they increase lipolysis; raise thermogenic activity; induce uncoupling protein (UCP) expression; and recruit BAT. After cold adaptation in hamsters, although the noradrenergic system is tonically activated, the sensitivity of BAT to norepinephrine decreases due to down-regulation of adrenergic receptors,92–94 especially β3-receptors in rats,95 and Gs-protein,96 limiting adrenergic activity.
Thermogenic activity contributes to metabolic activity, temperature regulation, and regulation of body weight. During thermogenesis, adrenergic stimulation of lipolysis in BAT produces fatty acids that are channeled into the mitochondrial β-oxidation pathway. Lipid oxidation in turn leads to increased NADH and FADH2 production and electron transport chain-dependent proton pumping producing an increased proton gradient across mitochondrial inner membranes. Such a gradient is dissipated by ATPase or by uncoupling proteins (UCPs) that facilitate proton leak and heat production. Although several UCPs have been identified, only UCP1, found in BAT, takes part in thermogenesis. UCP2 is distributed in many tissues, including the brain and primary afferent fibers,97 areas involved in temperature regulation whereas UCP3 is localized in skeletal and cardiac muscle.
The likelihood of having substantial BAT activity at rest is threefold greater in women than in men, indicating a greater resting thermogenesis in females. The greater mass of BAT and greater percent of their body devoted to BAT suggest that women have a high thermogenic capacity as well as activity.86 Physiologic concentrations of female sex hormones promote, while testosterone inhibits, thermogenesis. While ovarectomy decreases UCP1 in BAT,98 estrogen increases BAT fat pads and oxygen consumption in rats.99 Estrogen and progesterone each increase β3-adrenergic receptors, while there are lower levels of inhibitory α2A-adrenergic receptors in female than in male rats.4 This higher ratio of β3- to α2-receptors may be key to the higher basal thermogenic capacity and resting expression of UCP1 in females.
Gender influences the recruitment of BAT in rats in response to chronic cold100 and chronic overfeeding.101 There is a greater thermogenic capacity and a lesser body weight gain in males than in females after chronic feeding, chronic cold or chronic stress. This suggests “induced” thermogenic activity is lower in females than in males. High concentrations of estrogen inhibit cold-induced thermogenesis in rats102 in spite of similar sympathetic activity (norepinephrine turnover) across sexes during cold acclimation.103 There is a preferential recruitment of β3-receptors by norepinephrine, resulting in higher levels of β3-adrenergic receptors in male than in female rats. In chronic situations, the high ratio of β3- to α2A-activity in males likely sustains their greater thermogenic capacity in rats3 and recruitment of BAT in mice.104
An important therapeutic intervention in patients with fibromyalgia is a sustained pattern of gradually increased daily exercise. Acutely, physical activity generates heat in skeletal muscle and inhibits thermogenesis in BAT. However, the effect of chronic exercise training on BAT is unclear. In mice, BAT activity and mass were unchanged by swim-training,105 whereas more frequent daily swims increased the mass of BAT in mice.106 In Wistar-Shizouka Takagi rats, intermittent training produced no change in BAT activity (treadmill),107 while more continuous training programs decreased BAT mass in Wistar rats (treadmill)108 and Sprague-Dawley rats (swimming).109 To resolve these conflicting results, de Castro and Hill110 speculated that exercise can be a stressor, and thereby increase sympathetic activity to BAT. Using voluntary running of rats, their results were consistent with those in humans indicating that as physical activity increases, the metabolic activity of BAT also increases, generally improving thermoregulation.9,111 At temperatures that normally induce recruitment in rats, chronic exercise eliminates the recruiting effect of cold.112–114 In summary, exercise prevents recruitment of BAT.
Association of BAT with fibromyalgia
Basal thermogenic activity and a predisposition to having fibromyalgia are greater in females than in males. Thermogenic activity is increased by the same conditions that exacerbate the symptoms of fibromyalgia, i.e. cold and mild daily stress. In contrast, thermogenic activity and symptoms of fibromyalgia are minimized by warmth and by gradually increasing daily exercise. Yet patients generally have sluggish rather than stimulated rates of metabolism. Lower than anticipated thermogenic responses to cold mimic the tendency for these patients to have relatively low basal concentrations of circulating cortisol and attenuated cortisol responses to stress in spite of excessive activation of the HPA axis.41 The following summarize thermoregulatory and nociceptive processing (Table 1) that may overlap, supporting abnormal responses in patients with fibromyalgia to stress and cold.
Table 1.
| Substrate | Thermoregulation | Nociception |
|---|---|---|
| Sympathetic nerve | Activates BAT Dilates blood vessels in muscle |
Constricts vessels in skin Sensitizes primary afferents to pain |
| Primary afferent C-fibers | Detects heat in BAT Inhibits thermogenesis Detects heat in muscle and skin |
Detects pain in muscle and skin |
| TRPV1 receptors | Thermodetectors | Sensitize nociceptors |
| Substance P | Dilates vesssels in periphery Initiates cooling centrally |
Sensitizes C-fibers directly Sensitizes indirectly (mast cells) |
| Nerve growth factor | Supports sympathetic fibers (BAT) | Supports primary afferent C-fibers Increases substance P, TRPV1, etc |
| Subcortical areas | ||
| Parabrachial nucleus: | Receives temperature input | Receives tactile & visceral input |
| DMH: | On-cells inhibit BAT activity | On-cells support hyperalgesia |
| Cortical areas | ||
| Insula: | Associated with skin temperature variation | Abnormal somesthesis in FMS Autonomic & visceral pain |
| VMPFC: | Crossroad for temperature Integrates interoceptive information Activated by self-referential processing e.g., angina activates |
Crossroad for pain Reduced gray matter in FMS |
| Amygdala: | Signatures of emotion/thermal regulation Stress responses (e.g. sweating) |
Reduced grey matter in FMS |
Abbreviations: BAT, Brown adipose tissue; DMH, dorsomedial hypothalamus; FMS, fibromyaglia syndrome; TRP, transient receptor potential cation channel; TRPV1, TR vanilloid receptor-1 or TR potential cation channel subfamily V member-1; VMPFC, ventromedial prefrontal cortex
Innervation of BAT and surrounding tissues
Although the pain of fibromyalgia is widespread, tender points have been traditionally tested in the diagnosis of fibromyalgia. Tender points have greater sensitivity to pain upon palpation in fibromyalgia patients compared to healthy controls. In humans, nerves projecting to BAT are located near regions surrounding tender points, primarily in the supraclavicular region, but also in supra axial, perirenal and subcutaneous areas. This anatomical overlap may provide collateral innervation of tissue adjacent to BAT, e.g., skin and muscle, by sympathetic and primary afferent nerves. Consistent with this, when BAT is activated by injections of adrenaline in rats, muscles surrounding interscapular BAT have greater blood flow than in muscles of the anterior limbs,115 indicating that adjacent tissues operate in synergy with BAT. Consistent with increased adrenergic tone to these areas, the temperature is lower in the skin above tender points,2 suggesting that vasoconstriction and local hypoxia in skin coincides with vasodilation in muscle, as shown schematically in figure 2. Consistent with a possible simultaneous activation of BAT and skeletal muscle, UCP1 mRNA is detected in progenitor cells in human skeletal muscle and its expression is increased in vivo by PPARγ agonist treatment.116 As a result, sympathetic activity that induces thermogenesis is poised to induce hyperalgesia in tissues surrounding BAT by referred pain. Angina is a good example of referred pain perceived in non-cardiac structures as well as the heart.117 The pain of angina overlaps unilaterally with some tender points associated with fibromyalgia consistent with their common innervation via stellate ganglia.
Figure 2.
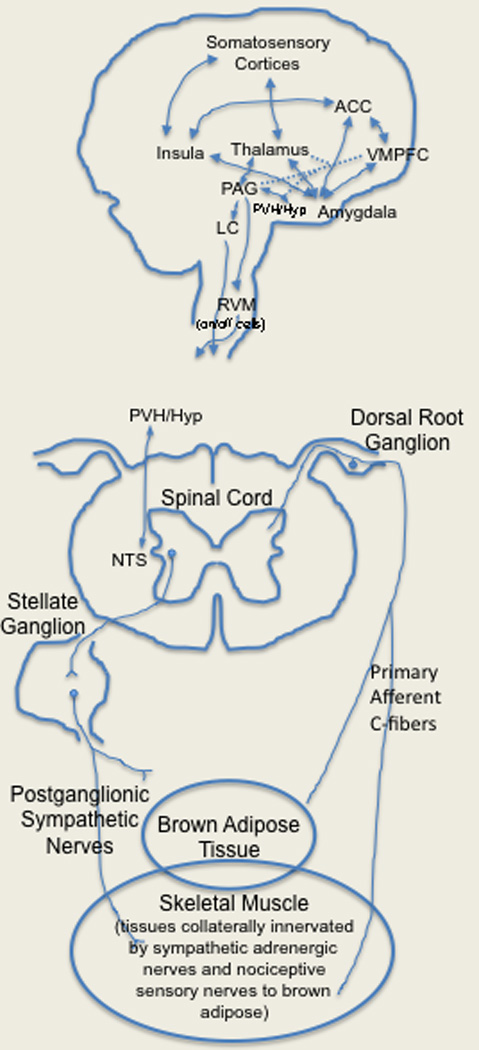
Activation of brown adipose tissue may support referred pain in a fashion similar to that during attacks of angina or during the processing of other internal signals (interoception). Schematic diagram of the possible circuitry linking brown adipose tissue to surrounding muscle and skin via collateral projections of sympathetic and primary afferent C-fibers. Visceral afferent information also projects upward in the neuraxis to the nucleus tractus solitarius (NTS) and paraventricular hypothalamus (PVH). In turn, the NTS projects to the PVH, central nucleus of the amygdala, PB, and the bed nucleus of the stria terminalis (BSST). The PVH has reciprocal connections with periaqueducal grey (PAG) and amygdala. The latter directly and indirectly modulates paralimbic cortices such as the ventromedial prefrontal cortex (VMPFC), anterior cingulate cortex (ACC) and insula thereby processing the emotional responses to pain and temperature proposed to be dysfunctional in fibromyalgia. In turn, the VMPFC has efferent modulatory projections to the PAG, hypothalamus (Hyp), and amygdala. Descending inhibitory control is exerted by the periaqueductal gray (PAG) and locus coeruleus (LC) via the rostroventral medulla (RVM) where ‘on’ and ‘off’ cells differentially modulate nociceptive signals entering at the spinal cord level.
Sympathetically-maintained pain
That sympathetically-maintained hyperalgesia118 contributes to pain in patients with fibromyalgia is evidenced by the ability of norepinephrine to cause pain in these patients.21 Consistent with this, local anesthetics applied to stellate sympathetic ganglia7 innervating BAT produces analgesia in patients with fibromyalgia. This suggests sensitization of adrenergic receptors or a failure of these sites to desensitize as they usually do in rats.92–94 Adrenergic receptors are upregulated and pronociceptive after sympathectomy, as shown by enhanced nociception produced by norepinephrine after removal of the superior cervical ganglion in rats.119 Hyperalgesia can also be mediated by α2-adrenergic receptors on postganglionic sympathetic nerves, such as after application of chloroform to rat paws,120 or by increased expression of α2-adrenergic receptors on primary afferent fibers,121 such as in rabbits after tissue injury.121,122 Capsaicin-sensitive thermal hyperalgesia in the rat depends on α1-adrenergic receptors on primary afferent C-fibers123 whereas mechanical hyperalgesia elicited by epinephrine injected intradermally in rats results from activation of G-protein-coupled β2-adrenergic receptors on primary afferent fibers.124 Large-diameter axotomized sensory neurons can be activated by sympathetic stimulation when nerve injury causes basket-like structures to sprout from sympathetic nerves within dorsal root ganglia of rats,125 a hyperalgesic condition relieved rather than caused by sympathectomy.126
Primary afferent fibers
After adrenergic fibers activate BAT, primary afferent C-fibers detect heat generated127 and feedback to inhibit BAT activity by releasing calcitonin gene-related peptide (CGRP) peripherally and centrally.128 Paradoxically, during chronic cold, primary afferent C-fiber activity is needed to recruit BAT by adaptive thermogenesis (i.e. increased UCP1 synthesis and enhanced mass) as desensitization of these afferents prevents recruitment.129 Sensitized primary afferent C-fibers projecting to BAT discharge at lower temperatures, enhancing feedback inhibition of thermogenesis (causing hypothermia) and simultaneously causing hyperalgesia in adjacent skin and muscle that is innervated by these sensitized collaterals. Based on the high pain sensitivity at tender points near areas of BAT together with abnormally low body temperature, the symptoms of fibromyalgia may result from sensitized primary afferent C-fibers innervating BAT and adjacent tissues128 causing referred pain. Patients with fibromyalgia may respond abnormally to cold and stress as conditions that are frequently comorbid with fibromyalgia inhibit adaptive thermogenesis.
Sensitization
Sensitization of primary afferent C-fibers may occur due to activation of capsaicin-sensitive, transient receptor potential vanilloid (TRPV1) receptors on nociceptors and on temperature-sensing afferents. TRPV1 antagonists induce hyperthermia in rat130 indicating these receptors remain tonically active in mammals to protect against hot temperatures causing tissue damage. This tone is provided by oxidized linoleic acid metabolites (OLAMs), such as 9- and 13-HODE acids. OLAMs activate TRPV1 sites on nociceptors causing hyperalgesia131,132 and elicit a sensation of warmth but decrease body temperature.133 Low doses of TRPV1 agonists enhance pain,133,134 but high doses desensitize TRPV1 sites. OLAMs are also ligands of PPARγ receptors whose activity is necessary for adaptive thermogenesis.135 By altering concentrations of 9- or 13-HODE, chronic defeat stress in rodents not only causes hyperthermia136 and increases UCP1 mRNA,137 it also increases thermal138,139 and mechanical hyperalgesia.140 Chronic inflammation also increases expression of TRPV1 receptors.141 which could account for both the lower body temperature and mechanical hyperalgesia in fibromyalgia patients as various inflammatory conditions are frequently comorbid with fibromyalgia. Capsaicin-induced hyperalgesia even correlates with overall pain scores in these patients.142
Substance P
Substance P, released from primary afferent C-fibers transmitting pain or temperature, causes vasodilation in skin to dissipate heat and induces hyperalgesia. Centrally, substance P sensitizes nociceptive pathways and initiates cooling behaviors.133,143 Although the high concentration of substance P in the cerebrospinal fluid of patients with fibromyalgia has been postulated to originate from nociceptive fibers, it may originate instead from temperature-sensitive fibers. Regardless of its origin, elevated substance P may account for lower body temperatures and hyperalgesia in patients with fibromyalgia. One model of fibromyalgia based on repeated exposures of rodents to cold144 depends on spinal substance P activity for hyperalgesia.145 If substance P decreases body temperature below normal, this may trigger thermogenic activity, enhancing sympathetic tone to BAT. Thus pain may result from the combined effect of substance P along nociceptive pathways together with sympathetically-mediated hyperalgesia in skin and muscle surrounding BAT, similar to the pain of angina.117
Nerve growth factor (NGF)
NGF, elevated in the cerebrospinal fluid of patients with fibromyalgia,34 produces thermal and mechanical hyperalgesia in rodents146,147 and humans when injected centrally148 or peripherally into muscle.149 Sympathetic nerves and primary afferent C-fibers each depend on NGF in target tissues for survival and specialization.150 There is competition between adrenergic- and primary afferent C-fibers for NGF in tissues where both exist. Normally NGF originates from target tissues and is transported retrogradely to dorsal root ganglia where it regulates protein synthesis (e.g. substance P and CGRP),150 including TRPV1 receptors.151 Destruction of one fiber type increases availability of NGF to remaining fibers. For example, postganglionic sympathectomy increases CGRP and substance P152 due to enhanced NGF along nociceptors in rat.153 Sympathectomy increases154 while sympathetic activity decreases NGF synthesis in rats and mice.155,156 NGF in mice causes aberrant growth of peripheral ganglia as well as abnormal sympathetic innervation of sensory ganglia157,158 together with hyperalgesia. In summary, NGF might contribute to the hyperalgesia of fibromyalgia by increasing substance P or TRPV1 receptors and/or by promoting sympathetic tone.31,32
Subcortical areas
Of nuclei involved in thermoregulation,159 the parabrachial, raphe pallidus (RP), and dorsomedial hypothalamus (DMH) are also involved in nociception.160,161 This convergence may allow thermoregulatory signals to influence nociception. Disinhibition of the DMH activates rostroventral medulla (RVM) ON-cells and suppresses RVM OFF-cell firing causing hyperalgesia in rats.162 Blocking serotonergic ON-cell activity prevents hyperalgesia, but does not interfere with thermogenesis, suggesting differentiation of autonomic and nociceptive transmission. Non-serotonergic ON-cells facilitate nociception but inhibit BAT activity whereas OFF-cells suppress nociceptive transmission163,164 and potentiate BAT activity in rat.165 Although pain and opioids each influence thermogenesis,165 it is not known whether thermogenesis influences pain such that ON-cells, activated by thermogenesis, potentiate nociception as well as inhibit thermogenic activity. Opioid receptors contribute to antinociception by acting as are pattern generators in RVM raphe nuclei,166 where mu opioid agonists decrease ON-cell activity and increase OFF-cell activity.167–170 In contrast, kappa opioid agonists increase nociception in rats by suppressing OFF-cells.171 Repeated cold exposure in mice, similar to that modeling fibromyalgia,144 increases the pronociceptive effect of kappa opioids172 and decreases the antinociceptive effect of mu opioid receptors, which are also down-regulated in fibromyalgia.173
Thermoregulatory and nociceptive pathways also converge at the parabrachial nucleus receiving somatosensory signals from spinothalamic and trigeminothalamic lamina I neurons in the rat.174,175 Rat lamina I neurons160 project specifically to parabrachial neurons responding to cooling176 and to noxious stimuli,177 as well as to polymodal nociceptive cells responding to noxious mechanical heat and cold stimuli.178 Whereas some neurons respond primarily to cool signals from skin,179 the majority of parabrachial neurons respond to both tactile and visceral and/or temperature input.180 Following chronic cold or stress, thermoregulatory and nociceptive signals activate common sites, as indicated by c-fos labeling in rats,181 increasing the role of parabrachial neurons in situations that recruit BAT. The parabrachial nucleus is a major relay for visceral inputs from the nucleus tractus solitarius to the forebrain.182 This raises the possibility that parabrachial neurons transmit the visceral nociceptive input of abdominal disorders (like irritable bowel syndrome and interstitial cystitis that are common in fibromyalgia) to widespread nociceptive areas.
Cortical Integrators
Cortical areas implicated in fibromyalgia include the insula, amygdala, ventromedial prefrontal cortex (VMPFC), and anterior cingulate cortex (ACC) (Figure 2). They are frequently referred to in aggregate as limbic/paralimbic cortex; they activate in response to many emotions or when affective processing is required. These regions are involved in the processing of thermal and nociceptive information, perhaps influencing their affective quality. At least some components of these networks appear dysfunctional in fibromyalgia.
The insula is the principal cortical convergence zone for the processing of internal signals that monitor the body’s physiological state (e.g., pH, visceral pressures and motility, heart rate, body temperature).183 The insula, also described as interoceptive cortex, modulates conditions within the body. For example, the human insula activates in response to visceral pain,184,185 and insular infarcts can cause severe hypothermia.186 Decreased metabolism of the insula occurs in healthy subjects who have detectable BAT and are exposed to environmental heat.187 Insular activity also correlates inversely with the thermal response during hot flashes induced by adjuvant endocrine therapies188 and plays a critical role in thermal responses to emotional processing. Human thermosensory cortex lies in the dorsal margin of the middle/posterior insula189 and activity in the posterior insula participates in processing abnormal somatosensory feedback in fibromyalgia,190 similar to its role in monkeys.191 Together, the anterior insula and amygdala play a prominent role in anxiety and stress,192–194 activating in response to aversive sensory stimuli.162,195–197
The ACC also participates in pain and temperature processing. Pressure on the thumb at levels that only activate somatosensory cortex in healthy controls also activate the ACC in patients with fibromyalgia.198 The VMPFC further connects the ACC, amygdala, and insula to integrate internal signals.199 Dysfunctional processing of such information is a component of fibromyalgia. For example, the VMPFC monitors internal milieu and is activated by introspection, by self-referential processing200 and by the default mode state.201 Angina arising from cardiac ischemia activates the VMPFC.202 Even self-monitoring one’s heart rate in the absence of angina activates the VMPFC.203 The VMPFC is the origin of galvanic skin responses, reflecting a component of somatic markers involved in decision-making.203 Lesions of the VMPFC impair decision-making, reflecting dysfunctional processing of somatic markers.204,205 The VMPFC plays a role in sickness-related mood changes reflecting a possible overlap between fibromyalgia and depressive symptoms.206 Fibromyalgia patients have reduced grey matter in the VMPFC and amygdala207 similar to that in patients with chronic pain.208 Such structural abnormalities can result from chronic abnormal metabolic activity perhaps through excitotoxic mechanisms.209 Fibromyalgia patients differ from controls in affective processing (acoustic startle eyeblink reflex) mediated by the amygdala.210 The VMPFC is also anatomically connected to the lateral and medial hypothalamus and periaqueductal grey,199 making it a prime crossroad for mediating not only arousal and mood, but also pain and thermal regulation. Emotional arousal is associated with facial thermal signatures indicating an overlap between emotion and thermal regulation. Skin temperature variation in humans is associated with changes in activity within the insula, ACC, and VMPFC.211
Together these studies demonstrate a convergence of pain and temperature information upon central brain systems including the insula, amygdala, VMPFC, and ACC. These limbic and paralimbic structures are associated with emotion and may color the affective components of pain and sensory processing. Fibromyalgia appears to involve many of these regions.
Conclusions
This review summarizes the literature describing commonalities between the regulation of pain and temperature that may contribute to the widespread pain of fibromyalgia. Both fibromyalgia and thermoregulation are exquisitely sensitive to stress. Acute cold and stress increase the generation of heat by increasing UCP1 activity in BAT while chronically high sympathetic tone increases the synthesis of UCP1, increasing BAT volume (adaptive thermogenesis) to guard against persistent stress. The high sympathetic tone in patients with fibromyalgia aggravates their pain and should initiate adaptive thermogenesis, yet their temperature is lower than healthy individuals. This indicates either insufficient heat production or enhanced heat loss, the latter being unlikely as sympathetic tone tends to curb heat loss. Multiple points along pain and thermoregulatory pathways exist where they may influence each other. In the periphery, warmth and pain are both detected by primary afferent C-fibers that release substance P into the spinal cord, causing hyperalgesia, cooling behaviors and inhibition of BAT activity. In the brain, pain and temperature are regulated by overlapping pathways in subcortical and cortical areas. It is doubtful that body temperature alone influences nociception. Rather, the challenge of maintaining normal body temperatures in the face of acute cold or of initiating adaptive thermogenesis in response to chronic stress may be compromised by conditions that are comorbid with fibromyalgia. For example, inflammatory conditions inhibit the response of BAT to sympathetic stimulation while conditions that sensitize primary afferent C-fibers may prematurely terminate thermogenic activity by enhanced feedback inhibition of BAT, consistent with the lower body temperatures in patients with fibromyalgia. Women may be more susceptible to fibromyalgia because they are inherently less able to initiate adaptive thermogenesis than men. Insufficient activation of BAT may lead to even greater sympathetic tone, compounding the possibility of referred pain via collateral nerves projecting to areas surrounding BAT. Exercise may relieve symptoms of fibromyalgia and improve thermoregulation by gradually decreasing adrenergic activity and providing an alternate source of body heat.
Significance.
This review summarizes multiple intersections between the regulation of nociception and temperature that may be relevant to fibromyalgia syndrome. The working hypotheses raised may contribute to the elucidation of the etiology of fibromyalgia.
Acknowledgements
The PET scan displayed was generously provided to us by Dr. Wouter D. van Marken Lichtenbelt. We extend special thanks to Ms. Allison Leuin, Drs. I. Jon Russell, Katalin J. Kovács, Tina Clarkson, Barbara Segal, and David Bernlohr for their input and helpful editorial comments on this manuscript. The authors declare no conflict of interest with any institution or company.
Support
AAL is supported by an NIH grant (AT056092) from the National Institutes on Arthritis and Musculoskeletal and Skin Diseases and by a grant from the University of Minnesota Graduate School. JVP is supported by the National Alliance for Research in Schizophrenia and Depression (NARSAD) and the Department of Veterans Affairs USA. JDP is supported by an NIH Institutional Research and Academic Career Development Award (IRACDA) grant (5K12GM074628) from the National Institute of General Medical Sciences.
Abbreviations
- ACC
anterior cingulate cortex
- BAT
brown adipose tissue
- BDNF
brain-derived neurotrophic factor
- CGRP
calcitonin gene-related peptide
- CRF
corticotropin-releasing factor
- DMH
dorsomedial hypothalamus
- FMS
fibromyalgia syndrome
- MPO
medial preoptic subregion
- 9-HODE
(9S,10E,12Z)-9-hydroperoxy-10,12-octadecadienoic
- 13-HODE
(13S,9Z,10E)-13-hydroperoxy-9,11-octadecadienoic
- NGF
nerve growth factor
- NT3
neurotrophin-3
- NTS
nucleus tractus solitarius
- PVN
paraventricular nucleus
- PET
positron emission tomography
- POA
preoptic area
- RP
raphe pallidus
- TRP
transient receptor potential cation channel
- TRPV1
TR vanilloid receptor-1 or TR potential cation channel subfamily V member-1
- UCP
uncoupling protein
- VMPFC
ventromedial prefrontal cortex
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford) 2000;39:917–921. doi: 10.1093/rheumatology/39.8.917. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez E, Monjo M, Rodriguez-Cuenca S, et al. Sexual dimorphism in the adrenergic control of rat brown adipose tissue response to overfeeding. Pflugers Arch. 2001;442:396–403. doi: 10.1007/s004240100556. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Cuenca S, Pujol E, Justo R, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;277:42958–42963. doi: 10.1074/jbc.M207229200. [DOI] [PubMed] [Google Scholar]
- 5.Kuroshima A, Habara Y, Uehara A, et al. Cross adaption between stress and cold in rats. Pflugers Arch. 1984;402:402–408. doi: 10.1007/BF00583941. [DOI] [PubMed] [Google Scholar]
- 6.Julien N, Goffaux P, Arsenault P, et al. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Bengtsson A, Bengtsson M. Regional sympathetic blockade in primary fibromyalgia. Pain. 1988;33:161–167. doi: 10.1016/0304-3959(88)90086-3. [DOI] [PubMed] [Google Scholar]
- 8.Pandolf KB. Effects of physical training and cardiorespiratory physical fitness on exercise-heat tolerance: recent observations. Med Sci Sports. 1979;11:60–65. [PubMed] [Google Scholar]
- 9.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia Criteria and Severity Scales for Clinical and Epidemiological Studies: A Modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 11.Bennett RM. Emerging concepts in the neurobiology of chronic pain: evidence of abnormal sensory processing in fibromyalgia. Mayo Clin Proc. 1999;74:385–398. doi: 10.4065/74.4.385. [DOI] [PubMed] [Google Scholar]
- 12.Russell IJ. Advances in fibromyalgia: possible role for central neurochemicals. Am J Med Sci. 1998;315:377–384. doi: 10.1097/00000441-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hapidou EG, Rollman GB. Menstrual cycle modulation of tender points. Pain. 1998;77:151–161. doi: 10.1016/S0304-3959(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 14.Buskila D, Neumann L. Musculoskeletal injury as a trigger for fibromyalgia/posttraumatic fibromyalgia. Curr Rheumatol Rep. 2000;2:104–108. doi: 10.1007/s11926-000-0049-z. [DOI] [PubMed] [Google Scholar]
- 15.Affleck G, Urrows S, Tennen H, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 16.Moldofsky H, Scarisbrick P, England R, et al. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Skuse D, Albanese A, Stanhope R, et al. A new stress-related syndrome of growth failure and hyperphagia in children, associated with reversibility of growth-hormone insufficiency. Lancet. 1996;348:353–358. doi: 10.1016/s0140-6736(96)01358-x. [DOI] [PubMed] [Google Scholar]
- 18.Elert J, Kendall SA, Larsson B, et al. Chronic pain and difficulty in relaxing postural muscles in patients with fibromyalgia and chronic whiplash associated disorders. J Rheumatol. 2001;28:1361–1368. [PubMed] [Google Scholar]
- 19.Okifuji A, Donaldson GW, Barck L, et al. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J Pain. 2010;11:1329–1337. doi: 10.1016/j.jpain.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessein PH, Shipton EA, Joffe BI, et al. High frequency of insulin resistence and hyperlipidemia in fibromyalgia. Ann Rheum Dis. 1999;58:137. [Google Scholar]
- 21.Martinez-Lavin M, Vidal M, Barbosa RE, et al. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study [ISRCTN70707830] BMC Musculoskelet Disord. 2002;3:2. doi: 10.1186/1471-2474-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagen K, Pettersen E, Stovner LJ, et al. No association between chronic musculoskeletal complaints and Val158Met polymorphism in the Catechol-O-methyltransferase gene. The HUNT study. BMC Musculoskelet Disord. 2006;7:40. doi: 10.1186/1471-2474-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen H, Neumann L, Glazer Y, et al. The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val(158) met and fibromyalgia. Clin Exp Rheumatol. 2009;27:S51–56. [PubMed] [Google Scholar]
- 24.Finan PH, Zautra AJ, Davis MC, et al. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152:300–307. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Fructuoso FR, Lao-Villadoniga JI, Beyer K, et al. Relación entre genotipos del gen catecol-o-metiltransferasa y la gravedad de la fibromialgia. Reumatol Clin. 2006;2:168–172. doi: 10.1016/S1699-258X(06)73042-X. [DOI] [PubMed] [Google Scholar]
- 26.Gursoy S, Erdal E, Herken H, et al. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–107. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Jauand M, Sitges C, Rodriguez V, et al. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain. 2013;17:16–27. doi: 10.1002/j.1532-2149.2012.00153.x. [DOI] [PubMed] [Google Scholar]
- 28.Marchand S, Arsenault P. Spatial summation for pain perception: interaction of inhibitory and excitatory mechanisms. Pain. 2002;95:201–206. doi: 10.1016/S0304-3959(01)00399-2. [DOI] [PubMed] [Google Scholar]
- 29.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 30.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593–1601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- 32.Vaeroy H, Helle R, Forre O, et al. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain. 1988;32:21–26. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 33.Vaeroy H, Nyberg F, Terenius L. No evidence for endorphin deficiency in fibromyalgia following investigation of cerebrospinal fluid (CSF) dynorphin A and Met-enkephalin-Arg6-Phe7. Pain. 1991;46:139–143. doi: 10.1016/0304-3959(91)90068-9. [DOI] [PubMed] [Google Scholar]
- 34.Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564–1569. [PubMed] [Google Scholar]
- 35.Laske C, Stransky E, Eschweiler GW, et al. Increased BDNF serum concentration in fibromyalgia with or without depression or antidepressants. J Psychiatr Res. 2007;41:600–605. doi: 10.1016/j.jpsychires.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Yunus MB, Kalyan-Raman UP, Masi AT, et al. Electron microscopic studies of muscle biopsy in primary fibromyalgia syndrome: a controlled and blinded study. J Rheumatol. 1989;16:97–101. [PubMed] [Google Scholar]
- 37.Vierck CJ., Jr Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–263. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Staud R, Rodriguez ME. Mechanisms of disease: pain in fibromyalgia syndrome. Nat Clin Pract Rheumatol. 2006;2:90–98. doi: 10.1038/ncprheum0091. [DOI] [PubMed] [Google Scholar]
- 39.Clauw DJ, Chrousos GP. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. 1997;4:134–153. doi: 10.1159/000097332. [DOI] [PubMed] [Google Scholar]
- 40.Crofford LJ. Neuroendocrine abnormalities in fibromyalgia and related disorders. Am J Med Sci. 1998;315:359–366. doi: 10.1097/00000441-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Neeck G, Crofford LJ. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin North Am. 2000;26:989–1002. doi: 10.1016/s0889-857x(05)70180-0. [DOI] [PubMed] [Google Scholar]
- 42.Riedel W, Schlapp U, Leck S, et al. Blunted ACTH and cortisol responses to systemic injection of corticotropin-releasing hormone (CRH) in fibromyalgia: role of somatostatin and CRH-binding protein. Ann N Y Acad Sci. 2002;966:483–490. doi: 10.1111/j.1749-6632.2002.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 43.Weigent DA, Bradley LA, Blalock JE, et al. Current concepts in the pathophysiology of abnormal pain perception in fibromyalgia. Am J Med Sci. 1998;315:405–412. doi: 10.1097/00000441-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Adler GK, Kinsley BT, Hurwitz S, et al. Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med. 1999;106:534–543. doi: 10.1016/s0002-9343(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Lavin M, Hermosillo AG, Rosas M, et al. Circadian studies of autonomic nervous balance in patients with fibromyalgia: a heart rate variability analysis. Arthritis Rheum. 1998;41:1966–1971. doi: 10.1002/1529-0131(199811)41:11<1966::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 46.Torpy DJ, Papanicolaou DA, Lotsikas AJ, et al. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia. Arthritis Rheum. 2000;43:872–880. doi: 10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 47.Buskila D, Odes LR, Neumann L, et al. Fibromyalgia in inflammatory bowel disease. J Rheumatol. 1999;26:1167–1171. [PubMed] [Google Scholar]
- 48.Barton A, Pal B, Whorwell PJ, et al. Increased prevalence of sicca complex and fibromyalgia in patients with irritable bowel syndrome. Am J Gastroenterol. 1999;94:1898–1901. doi: 10.1111/j.1572-0241.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 49.Sivri A, Cindas A, Dincer F, et al. Bowel dysfunction and irritable bowel syndrome in fibromyalgia patients. Clin Rheumatol. 1996;15:283–286. doi: 10.1007/BF02229708. [DOI] [PubMed] [Google Scholar]
- 50.Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541–3546. doi: 10.1111/j.1572-0241.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- 51.Veale D, Kavanagh G, Fielding JF, et al. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. Br J Rheumatol. 1991;30:220–222. doi: 10.1093/rheumatology/30.3.220. [DOI] [PubMed] [Google Scholar]
- 52.Alagiri M, Chottiner S, Ratner V, et al. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–57. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- 53.Clauw DJ, Schmidt M, Radulovic D, et al. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125–131. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- 54.Arnold LD, Bachmann GA, Rosen R, et al. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107:617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peres MF, Young WB, Kaup AO, et al. Fibromyalgia is common in patients with transformed migraine. Neurology. 2001;57:1326–1328. doi: 10.1212/wnl.57.7.1326. [DOI] [PubMed] [Google Scholar]
- 56.Kang JH, Lin HC. Comorbidities in patients with primary Sjogren’s syndrome: a registry-based case-control study. J Rheumatol. 2010;37:1188–1194. doi: 10.3899/jrheum.090942. [DOI] [PubMed] [Google Scholar]
- 57.Nothnick WB. Novel targets for the treatment of endometriosis. Expert Opin Ther Targets. 2004;8:459–471. doi: 10.1517/14728222.8.5.459. [DOI] [PubMed] [Google Scholar]
- 58.Palm O, Moum B, Jahnsen J, et al. Fibromyalgia and chronic widespread pain in patients with inflammatory bowel disease: a cross sectional population survey. J Rheumatol. 2001;28:590–594. [PubMed] [Google Scholar]
- 59.Valenca MM, Medeiros FL, Martins HA, et al. Neuroendocrine dysfunction in fibromyalgia and migraine. Curr Pain Headache Rep. 2009;13:358–364. doi: 10.1007/s11916-009-0058-1. [DOI] [PubMed] [Google Scholar]
- 60.Gebhart GF. Visceral pain-peripheral sensitisation. Gut. 2000;47(Suppl 4):iv54–iv55. doi: 10.1136/gut.47.suppl_4.iv54. discussion iv58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willis WD, Al-Chaer ED, Quast MJ, et al. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci U S A. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang HQ, Al-Chaer ED, Willis WD. Effect of tactile inputs on thalamic responses to noxious colorectal distension in rat. J Neurophysiol. 2002;88:1185–1196. doi: 10.1152/jn.2002.88.3.1185. [DOI] [PubMed] [Google Scholar]
- 63.Gebhart GF, Ness TJ. Central mechanisms of visceral pain. Can J Physiol Pharmacol. 1991;69:627–634. doi: 10.1139/y91-093. [DOI] [PubMed] [Google Scholar]
- 64.Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 65.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 66.De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J. 1993;125:726–731. doi: 10.1016/0002-8703(93)90164-5. [DOI] [PubMed] [Google Scholar]
- 67.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 68.Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- 69.Poehlman ET. A review: exercise and its influence on resting energy metabolism in man. Med Sci Sports Exerc. 1989;21:515–525. [PubMed] [Google Scholar]
- 70.Tremblay A, Despres JP, Bouchard C. The effects of exercise-training on energy balance and adipose tissue morphology and metabolism. Sports Med. 1985;2:223–233. doi: 10.2165/00007256-198502030-00005. [DOI] [PubMed] [Google Scholar]
- 71.Sinyor D, Schwartz SG, Peronnet F, et al. Aerobic fitness level and reactivity to psychosocial stress: physiological, biochemical, and subjective measures. Psychosom Med. 1983;45:205–217. doi: 10.1097/00006842-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 73.Puetz TW, Flowers SS, O’Connor PJ. A randomized controlled trial of the effect of aerobic exercise training on feelings of energy and fatigue in sedentary young adults with persistent fatigue. Psychother Psychosom. 2008;77:167–174. doi: 10.1159/000116610. [DOI] [PubMed] [Google Scholar]
- 74.Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–251. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- 75.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 76.Oettle GJ. Effect of moderate exercise on bowel habit. Gut. 1991;32:941–944. doi: 10.1136/gut.32.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daley AJ, Grimmett C, Roberts L, et al. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: a randomised controlled trial. Int J Sports Med. 2008;29:778–782. doi: 10.1055/s-2008-1038600. [DOI] [PubMed] [Google Scholar]
- 78.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 79.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 80.Rothwell NJ, Stock MJ. Effects of Age on Diet-Induced Thermogenesis and Brown Adipose-Tissue Metabolism in the Rat. International Journal of Obesity. 1983;7:583–589. [PubMed] [Google Scholar]
- 81.Aronson SM, Teodoru CV, Adler M, et al. Influence of cortisone upon brown fat of hamsters and mice. Proc Soc Exp Biol Med. 1954;85:214–218. doi: 10.3181/00379727-85-20834. [DOI] [PubMed] [Google Scholar]
- 82.Galpin KS, Henderson RG, James WP, et al. GDP binding to brown-adipose-tissue mitochondria of mice treated chronically with corticosterone. Biochem J. 1983;214:265–268. doi: 10.1042/bj2140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- 84.Williams G, Kolodny GM. Method for decreasing uptake of 18F–FDG by hypermetabolic brown adipose tissue on PET. AJR Am J Roentgenol. 2008;190:1406–1409. doi: 10.2214/AJR.07.3205. [DOI] [PubMed] [Google Scholar]
- 85.Frayn KN. The glucose-fatty acid cycle: a physiological perspective. Biochem Soc Trans. 2003;31:1115–1119. doi: 10.1042/bst0311115. [DOI] [PubMed] [Google Scholar]
- 86.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dobert N, Menzel C, Hamscho N, et al. Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Q J Nucl Med Mol Imaging. 2004;48:33–38. [PubMed] [Google Scholar]
- 88.Rousseau C, Bourbouloux E, Campion L, et al. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. 2006;33:785–791. doi: 10.1007/s00259-006-0066-x. [DOI] [PubMed] [Google Scholar]
- 89.Lichtenbelt WDV, Vanhommerig JW, Smulders NM, et al. Cold-Activated Brown Adipose Tissue in Healthy Men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 90.Rothwell NJ, Stock MJ. Effects of denervating brown adipose tissue on the responses to cold, hyperphagia and noradrenaline treatment in the rat. J Physiol. 1984;355:457–463. doi: 10.1113/jphysiol.1984.sp015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lafontan M, Barbe P, Galitzky J, et al. Adrenergic regulation of adipocyte metabolism. Hum Reprod. 1997;12(Suppl 1):6–20. doi: 10.1093/humrep/12.suppl_1.6. [DOI] [PubMed] [Google Scholar]
- 92.Nedergaard J. Catecholamine sensitivity in brown fat cells from cold-acclimated hamsters and rats. Am J Physiol. 1982;242:C250–257. doi: 10.1152/ajpcell.1982.242.3.C250. [DOI] [PubMed] [Google Scholar]
- 93.Unelius L, Bronnikov G, Mohell N, et al. Physiological desensitization of beta 3-adrenergic responses in brown fat cells: involvement of a postreceptor process. Am J Physiol. 1993;265:C1340–C1348. doi: 10.1152/ajpcell.1993.265.5.C1340. [DOI] [PubMed] [Google Scholar]
- 94.Unelius L, Mohell N, Nedergaard J. Cold acclimation induces desensitization to adenosine in brown fat cells without changing receptor binding. Am J Physiol. 1990;258:C818–C826. doi: 10.1152/ajpcell.1990.258.5.C818. [DOI] [PubMed] [Google Scholar]
- 95.Malo A, Puerta M. Oestradiol and progesterone change beta3-adrenergic receptor affinity and density in brown adipocytes. Eur J Endocrinol. 2001;145:87–91. doi: 10.1530/eje.0.1450087. [DOI] [PubMed] [Google Scholar]
- 96.Svoboda P, Unelius L, Cannon B, et al. Attenuation of Gs alpha coupling efficiency in brown-adipose-tissue plasma membranes from cold-acclimated hamsters. Biochem J. 1993;295(Pt 3):655–661. doi: 10.1042/bj2950655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horvath B, Spies C, Warden CH, et al. Uncoupling protein 2 in primary pain and temperature afferents of the spinal cord. Brain Research. 2002;955:260–263. doi: 10.1016/s0006-8993(02)03364-4. [DOI] [PubMed] [Google Scholar]
- 98.Pedersen SB, Bruun JM, Kristensen K, et al. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun. 2001;288:191–197. doi: 10.1006/bbrc.2001.5763. [DOI] [PubMed] [Google Scholar]
- 99.Kemnitz JW, Glick Z, Bray GA. Ovarian hormones influence brown adipose tissue. Pharmacol Biochem Behav. 1983;18:563–566. doi: 10.1016/0091-3057(83)90281-2. [DOI] [PubMed] [Google Scholar]
- 100.Quevedo S, Roca P, Pico C, et al. Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue. Pflugers Arch. 1998;436:689–695. doi: 10.1007/s004240050690. [DOI] [PubMed] [Google Scholar]
- 101.Roca P, Rodriguez AM, Oliver P, et al. Brown adipose tissue response to cafeteria diet-feeding involves induction of the UCP2 gene and is impaired in female rats as compared to males. Pflugers Arch. 1999;438:628–634. doi: 10.1007/s004249900107. [DOI] [PubMed] [Google Scholar]
- 102.Puerta ML, Nava MP, Abelenda M, et al. Inactivation of brown adipose tissue thermogenesis by oestradiol treatment in cold-acclimated rats. Pflugers Arch. 1990;416:659–662. doi: 10.1007/BF00370611. [DOI] [PubMed] [Google Scholar]
- 103.Nava MP, Fernandez A, Abelenda M, et al. Dissociation between brown adipose tissue thermogenesis and sympathetic activity in rats with high plasma levels of oestradiol. Pflugers Arch. 1994;426:40–43. doi: 10.1007/BF00374668. [DOI] [PubMed] [Google Scholar]
- 104.Monjo M, Rodriguez AM, Palou A, et al. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- 105.Bell RR, McGill TJ, Digby PW, et al. Effects of dietary protein and exercise on brown adipose tissue and energy balance in experimental animals. J Nutr. 1984;114:1900–1908. doi: 10.1093/jn/114.10.1900. [DOI] [PubMed] [Google Scholar]
- 106.Ueno N, Oh-ishi S, Kizaki T, et al. Effects of swimming training on brown-adipose-tissue activity in obese ob/ob mice: GDP binding and UCP m-RNA expression. Res Commun Mol Pathol Pharmacol. 1997;95:92–104. [PubMed] [Google Scholar]
- 107.Scarpace PJ, Yenice S, Tumer N. Influence of exercise training and age on uncoupling protein mRNA expression in brown adipose tissue. Pharmacol Biochem Behav. 1994;49:1057–1059. doi: 10.1016/0091-3057(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 108.Yamashita H, Yamamoto M, Sato Y, et al. Effect of running training on uncoupling protein mRNA expression in rat brown adipose tissue. Int J Biometeorol. 1993;37:61–64. doi: 10.1007/BF01212769. [DOI] [PubMed] [Google Scholar]
- 109.Sullo A, Brizzi G, Maffulli N. Triiodothyronine deiodinating activity in brown adipose tissue after short cold stimulation test in trained and untrained rats. Physiol Res. 2004;53:69–76. [PubMed] [Google Scholar]
- 110.de Castro JM, Hill JO. Exercise and brain catecholamine relationships with brown adipose tissue and whole-body oxygen consumption in rats. Physiol Behav. 1988;43:9–12. doi: 10.1016/0031-9384(88)90090-x. [DOI] [PubMed] [Google Scholar]
- 111.Nadel ER, Pandolf KB, Roberts MF, et al. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- 112.Arnold J, Richard D. Exercise during intermittent cold exposure prevents acclimation to cold rats. J Physiol. 1987;390:45–54. doi: 10.1113/jphysiol.1987.sp016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harri M, Dannenberg T, Oksanen-Rossi R, et al. Related and unrelated changes in response to exercise and cold in rats: a reevaluation. J Appl Physiol. 1984;57:1489–1497. doi: 10.1152/jappl.1984.57.5.1489. [DOI] [PubMed] [Google Scholar]
- 114.Larueachagiotis C, Rieth N, Goubern M, et al. Exercise-Training Reduces Bat Thermogenesis in Rats. Physiology & Behavior. 1995;57:1013–1017. doi: 10.1016/0031-9384(94)00005-p. [DOI] [PubMed] [Google Scholar]
- 115.Sbarbati A, Cavallini I, Marzola P, et al. Contrast-enhanced MRI of brown adipose tissue after pharmacological stimulation. Magn Reson Med. 2006;55:715–718. doi: 10.1002/mrm.20851. [DOI] [PubMed] [Google Scholar]
- 116.Crisan M, Casteilla L, Lehr L, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells. 2008;26:2425–2433. doi: 10.1634/stemcells.2008-0325. [DOI] [PubMed] [Google Scholar]
- 117.Foreman RD. Neurophysiology of Heart pain. In: Ter Horst TJ, editor. The Nervous system and the Heart. Totowa, NJ: Humana Press; 2000. pp. 343–363. [Google Scholar]
- 118.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 119.Bossut DF, Perl ER. Effects of nerve injury on sympathetic excitation of A delta mechanical nociceptors. J Neurophysiol. 1995;73:1721–1723. doi: 10.1152/jn.1995.73.4.1721. [DOI] [PubMed] [Google Scholar]
- 120.Levine JD, Taiwo YO, Collins SD, et al. Noradrenaline hyperalgesia is mediated through interaction with sympathetic postganglionic neurone terminals rather than activation of primary afferent nociceptors. Nature. 1986;323:158–160. doi: 10.1038/323158a0. [DOI] [PubMed] [Google Scholar]
- 121.Perl ER. Causalgia, pathological pain, and adrenergic receptors. Proc Natl Acad Sci U S A. 1999;96:7664–7667. doi: 10.1073/pnas.96.14.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 123.Ren Y, Zou X, Fang L, et al. Involvement of peripheral purinoceptors in sympathetic modulation of capsaicin-induced sensitization of primary afferent fibers. J Neurophysiol. 2006;96:2207–2216. doi: 10.1152/jn.00502.2006. [DOI] [PubMed] [Google Scholar]
- 124.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 125.McLachlan EM, Janig W, Devor M, et al. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 126.Kalmari J, Niissalo S, Konttinen YT, et al. Modulation of visceral nociceptive responses of rat spinal dorsal horn neurons by sympathectomy. Neuroreport. 2001;12:797–801. doi: 10.1097/00001756-200103260-00036. [DOI] [PubMed] [Google Scholar]
- 127.Shinozaki K, Shimizu Y, Shiina T, et al. A neurophysiological evidence of capsaicin-sensitive nerve components innervating interscapular brown adipose tissue. Auton Neurosci. 2005;119:16–24. doi: 10.1016/j.autneu.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Osaka T, Kobayashi A, Namba Y, et al. Temperature- and capsaicin-sensitive nerve fibers in brown adipose tissue attenuate thermogenesis in the rat. Pflugers Arch. 1998;437:36–42. doi: 10.1007/s004240050743. [DOI] [PubMed] [Google Scholar]
- 129.Cui J, Zaror-Behrens G, Himms-Hagen J. Capsaicin desensitization induces atrophy of brown adipose tissue in rats. Am J Physiol. 1990;259:R324–R332. doi: 10.1152/ajpregu.1990.259.2.R324. [DOI] [PubMed] [Google Scholar]
- 130.Gavva NR, Bannon AW, Surapaneni S, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patwardhan AM, Akopian AN, Ruparel NB, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patwardhan AM, Scotland PE, Akopian AN, et al. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther. 1984;26:389–416. doi: 10.1016/0163-7258(84)90041-x. [DOI] [PubMed] [Google Scholar]
- 134.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 135.Park JY, Kawada T, Han IS, et al. Capsaicin inhibits the production of tumor necrosis factor alpha by LPS-stimulated murine macrophages, RAW 264.7: a PPARgamma ligand-like action as a novel mechanism. FEBS Lett. 2004;572:266–270. doi: 10.1016/j.febslet.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 136.Keeney AJ, Hogg S, Marsden CA. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol Behav. 2001;74:177–184. doi: 10.1016/s0031-9384(01)00541-8. [DOI] [PubMed] [Google Scholar]
- 137.Chuang JC, Cui H, Mason BL, et al. Chronic social defeat stress disrupts regulation of lipid synthesis. J Lipid Res. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.King CD, Devine DP, Vierck CJ, et al. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain Res. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- 139.Marcinkiewcz CA, Green MK, Devine DP, et al. Social defeat stress potentiates thermal sensitivity in operant models of pain processing. Brain Res. 2009;1251:112–120. doi: 10.1016/j.brainres.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rivat C, Becker C, Blugeot A, et al. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150:358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 141.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 142.Morris V, Cruwys S, Kidd B. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neurosci Lett. 1998;250:205–207. doi: 10.1016/s0304-3940(98)00443-1. [DOI] [PubMed] [Google Scholar]
- 143.Dib B. Thermoregulatory behaviour induced by intrathecal injection of substance P in the rat. Eur J Pharmacol. 1987;133:147–153. doi: 10.1016/0014-2999(87)90145-2. [DOI] [PubMed] [Google Scholar]
- 144.Nishiyori M, Ueda H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain. 2008;4:52. doi: 10.1186/1744-8069-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuraishi Y, Satoh M. Participation of spinal cord substance P in hyperalgesia induced by repeated cold stress. Regul Pept. 1993;46:405–406. doi: 10.1016/0167-0115(93)90101-d. [DOI] [PubMed] [Google Scholar]
- 146.Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 147.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 148.Petty BG, Cornblath DR, Adornato BT, et al. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol. 1994;36:244–246. doi: 10.1002/ana.410360221. [DOI] [PubMed] [Google Scholar]
- 149.Svensson P, Cairns BE, Wang K, et al. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 150.Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7:13–17. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- 151.Ro JY, Lee JS, Zhang YP. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ekstrom J, Ekman R. Sympathectomy-induced increases in calcitonin gene-related peptide (CGRP)-, substance P- and vasoactive intestinal peptide (VIP)-levels in parotid and submandibular glands of the rat. Arch Oral Biol. 2005;50:909–917. doi: 10.1016/j.archoralbio.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 153.Ekstrom J, Reinhold AC. Increases in nerve growth factor immunoreactivity in the submandibular gland, but not in the parotid gland, of the rat following sympathetic denervation. Arch Oral Biol. 2004;49:3–9. doi: 10.1016/s0003-9969(03)00181-x. [DOI] [PubMed] [Google Scholar]
- 154.Supowit SC, Ethridge RT, Zhao H, et al. Calcitonin gene-related peptide and substance P contribute to reduced blood pressure in sympathectomized rats. Am J Physiol Heart Circ Physiol. 2005;289:H1169–H1175. doi: 10.1152/ajpheart.00973.2004. [DOI] [PubMed] [Google Scholar]
- 155.Nisoli E, Tonello C, Benarese M, et al. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/endo.137.2.8593794. [DOI] [PubMed] [Google Scholar]
- 156.Qin F, Vulapalli RS, Stevens SY, et al. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Am J Physiol Heart Circ Physiol. 2002;282:H363–H371. doi: 10.1152/ajpheart.00319.2001. [DOI] [PubMed] [Google Scholar]
- 157.Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davis BM, Albers KM, Seroogy KB, et al. Overexpression of nerve growth factor in transgenic mice induces novel sympathetic projections to primary sensory neurons. J Comp Neurol. 1994;349:464–474. doi: 10.1002/cne.903490310. [DOI] [PubMed] [Google Scholar]
- 159.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 161.Heinricher MM, Tavares I, Leith JL, et al. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- 164.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 165.Nason MW, Mason P., Jr Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–R698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 167.Fields HL, Vanegas H, Hentall ID, et al. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- 168.Gao K, Chen DO, Genzen JR, et al. Activation of serotonergic neurons in the raphe magnus is not necessary for morphine analgesia. J Neurosci. 1998;18:1860–1868. doi: 10.1523/JNEUROSCI.18-05-01860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 170.Heinricher MM, Morgan MM, Tortorici V, et al. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 171.Meng ID, Johansen JP, Harasawa I, et al. Kappa opioids inhibit physiologically identified medullary pain modulating neurons and reduce morphine antinociception. J Neurophysiol. 2005;93:1138–1144. doi: 10.1152/jn.00320.2004. [DOI] [PubMed] [Google Scholar]
- 172.Omiya Y, Goto K, Ishige A, et al. Changes in analgesia-producing mechanism of repeated cold stress loading in mice. Pharmacol Biochem Behav. 2000;65:261–266. doi: 10.1016/s0091-3057(99)00215-4. [DOI] [PubMed] [Google Scholar]
- 173.Harris RE, Clauw DJ, Scott DJ, et al. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bester H, Chapman V, Besson JM, et al. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- 175.Hylden JL, Anton F, Nahin RL. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience. 1989;28:27–37. doi: 10.1016/0306-4522(89)90229-7. [DOI] [PubMed] [Google Scholar]
- 176.Menendez L, Bester H, Besson JM, et al. Parabrachial area: electrophysiological evidence for an involvement in cold nociception. J Neurophysiol. 1996;75:2099–2116. doi: 10.1152/jn.1996.75.5.2099. [DOI] [PubMed] [Google Scholar]
- 177.Bester H, Menendez L, Besson JM, et al. Spino (trigemino) parabrachiohypothalamic pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1995;73:568–585. doi: 10.1152/jn.1995.73.2.568. [DOI] [PubMed] [Google Scholar]
- 178.Craig AD, Dostrovsky JO. Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol. 2001;86:856–870. doi: 10.1152/jn.2001.86.2.856. [DOI] [PubMed] [Google Scholar]
- 179.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bernard JF, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol. 1994;71:1646–1660. doi: 10.1152/jn.1994.71.5.1646. [DOI] [PubMed] [Google Scholar]
- 181.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 182.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 183.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 184.Cohen SJ, Hobson AR, Aziz Q. Processing of visceral sensory signals in the brain. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. San Diego, CA: Academic Press; 2012. pp. 689–702. [Google Scholar]
- 185.Smith JK, Humes DJ, Head KE, et al. fMRI and MEG analysis of visceral pain in healthy volunteers. Neurogastroenterol Motil. 2011;23 doi: 10.1111/j.1365-2982.2011.01712.x. 648-e260. [DOI] [PubMed] [Google Scholar]
- 186.Helmchen CD, Dopke M, Trillenberg P, et al. [accessed 03/11/2013];Abnormal thermoregulation following left fronto-insular stroke. :511. [Web Page]. Available at: ( http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=134748&XNSPRACHE_ID=1&XNKONGRESS_ID=143&XNMASKEN_ID=900.
- 187.Miao Q, Zhao XL, Zhang QY, et al. Stability in brain glucose metabolism following brown adipose tissue inactivation in chinese adults. AJNR Am J Neuroradiol. 2012;33:1464–1469. doi: 10.3174/ajnr.A3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Joffe H, Deckersbach T, Lin NU, et al. Metabolic activity in the insular cortex and hypothalamus predicts hot flashes: an FDG-PET study. J Clin Endocrinol Metab. 2012;97:3207–3215. doi: 10.1210/jc.2012-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Craig AD, Chen K, Bandy D, et al. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 190.Harris RE, Sundgren PC, Pang Y, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 191.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 192.Britton JC, Phan KL, Taylor SF, et al. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 193.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 194.Pawlak R, Magarinos AM, Melchor J, et al. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 195.Zald DH, Lee JT, Fluegel KW, et al. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121(Pt 6):1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- 196.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002;16:746–753. doi: 10.1006/nimg.2002.1115. [DOI] [PubMed] [Google Scholar]
- 198.Pujol J, Lopez-Sola M, Ortiz H, et al. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS One. 2009;4:e5224. doi: 10.1371/journal.pone.0005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 200.Gusnard DA, Akbudak E, Shulman GL, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 202.Rosen SD, Paulesu E, Frith CD, et al. Central nervous pathways mediating angina pectoris. Lancet. 1994;344:147–150. doi: 10.1016/s0140-6736(94)92755-3. [DOI] [PubMed] [Google Scholar]
- 203.Critchley HD, Elliott R, Mathias CJ, et al. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Damasio ARNeuropsychology. Towards a neuropathology of emotion and mood. Nature. 1997;386:769–770. doi: 10.1038/386769a0. [DOI] [PubMed] [Google Scholar]
- 205.Bechara A, Damasio H, Tranel D, et al. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 206.Harrison NA, Brydon L, Walker C, et al. Inflammation Causes Mood Changes Through Alterations in Subgenual Cingulate Activity and Mesolimbic Connectivity. Biological Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Burgmer M, Gaubitz M, Konrad C, et al. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009;71:566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- 208.Rodriguez-Raecke R, Niemeier A, Ihle K, et al. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Putcha D, Brickhouse M, O’Keefe K, et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bartley EJ, Rhudy JL, Williams AE. Experimental assessment of affective processing in fibromyalgia. J Pain. 2009;10:1151–1160. doi: 10.1016/j.jpain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 211.Egan GF, Johnson J, Farrell M, et al. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci U S A. 2005;102:5262–5267. doi: 10.1073/pnas.0409753102. [DOI] [PMC free article] [PubMed] [Google Scholar]