Abstract
To characterize adherence to post-exposure prophylaxis after non-forcible sexual exposure to HIV, we conducted a review of the literature and meta-analysis. Articles were considered if they contained primary adherence data following non-forcible sexual exposure. Random-effects meta-analysis was used to create pooled point estimates for adherence. Of 1,257 abstracts identified through our search algorithm, 17 were eligible for inclusion in this review, representing 3,634 patients enrolled in 3 randomized controlled trials (RCTs), 9 prospective and 5 retrospective observational studies. Pooled adherence, primarily assessed by self-report, was 77% (95% confidence interval (CI): 68 to 87%) in prospective observational studies, 81% (95% CI: 65 to 96%) in retrospective studies, 78% (95% CI: 65 to 91%) in RCTs, and 78% (95% CI: 72 to 85%) overall. Overall adherence was moderately high, with high variability between studies. Assessment of adherence could be enhanced by the use of objective measurements.
Keywords: post-exposure prophylaxis, meta-analysis, HIV, medication adherence
INTRODUCTION
Three recent randomized controlled trials (RCT) have offered promising evidence of the efficacy of once-daily combined tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) for the prevention of human immunodeficiency virus (HIV) seroconversion among seronegative but at-risk individual(1–3). However, efficacy of pre-exposure prophylaxis (PrEP) appears to be closely correlated with adherence to therapy(1–4). Adherence data, especially outside of the clinical trial setting, are sparse, and a body of research is just beginning to emerge(5–8). Given that PEP has been used for more than 15 years(9), if individuals who accessed PEP would benefit from PrEP, characterization of adherence patterns to PEP could potentially be useful for informing PrEP programs. This characterization is particularly important at this point in time as PEP strategies move from the clinical trial setting to programmatic implementation.
PEP was first shown to be effective for reduction of risk of seroconversion after occupational exposure to HIV(10). There has been no placebo-controlled trial confirming the efficacy of PEP. Randomization to PEP or placebo would not be ethical, due to evidence supporting the effectiveness of PEP from animal and observational human studies, and would be logistically challenging because of the large sample size that would be required to show a difference in seroconversion, since the probability of becoming HIV infected per any given exposure is relatively low. Estimates of the degree to which individuals must be adherent to PEP in order to maximize protection are not available, due to the lack of randomized controlled trials and limited power of the existing observational studies. Despite the lack of evidence from randomized controlled trials, PEP is now recommended for non-occupational exposure to HIV, including sexual exposure(11,12). Exposure to HIV is considered a medical emergency, and PEP is recommended to be initiated within 72 hours of exposure, the sooner within this time interval, the better. Recommended regimens typically consist of a 28-day course of antiretroviral therapy (ART) with 2 or 3 drugs, frequently depending on the HIV status of the source, experience of the source taking antiretroviral medications, and if the exposure is very high risk.
A number of factors are thought to be involved in poor adherence to PEP, including stigma, side effects, cost, and lack of perceived risk(13,14). A number of studies have reported acceptability, tolerability, and effectiveness of PEP in various non-occupational settings and populations(15–18). In this report, we reviewed the literature for studies that reported adherence outcomes with non-occupational, non-forcible sexual exposure to HIV, because this patient population may be the most similar to those who would access PrEP, to characterize adherence patterns in different PEP regimens, interventions, and risk groups, and to extrapolate lessons learned from experience with PEP for future PrEP programs.
METHODS
Search strategy
Three electronic databases were searched through December 1, 2012: MEDLINE, EMBASE, and PsycINFO. Permutations of the following search terms were used: “post-exposure prophylaxis” or “postexposure prophylaxis”, “HIV”, “adherence” or “adhere*”, and “non-occupational” or “nonoccupational”. Titles and/or abstracts of all retrieved citations were reviewed. Full articles were retrieved for studies that passed this initial review. Reference lists of all retrieved articles were reviewed for additional articles. In addition, we performed a search of the grey literature as a sensitivity analysis to assess publication bias using the search terms “post-exposure prophylaxis and adherence” and “post-exposure prophylaxis and HIV” in the Google Internet search engine as well as a search of OpenSIGLE (System for Information on Grey Literature in Europe).
Inclusion and exclusion criteria
We chose to limit the review to non-occupational, non-forcible sexual exposure in order to assess adherence patterns in populations most similar to those that may be interested in or ideally suited for PrEP. We specifically did not include occupational exposure or forcible sexual exposure because these populations are accessing PEP for reasons that are dissimilar to reasons for accessing PrEP. Inclusion criteria included articles in English that reported primary data on PEP and included data on adherence among individuals who had non-forcible sexual exposure to HIV. In studies with mixed cohorts with excluded groups (for example, occupational exposure, injection drug use, or forcible sexual exposure), we included the study if adherence outcomes were reported separately by risk group or if the majority (>75%) of the participants in the cohort had non-occupational, non-forcible sexual exposure.
Data Extraction
Data were extracted independently on a standardized data collection form by two separate reviewers with >90% agreement. Adjudication for inconsistencies was done through discussion. Patients were determined to be “adherent” if they reported that they took the full 28 days of medication and were included in the numerator of the adherence estimate. Participants who did not return to collect their full medication course after being prescribed a starter kit were considered non-adherent. Loss to follow-up was defined as not returning at the primary study assessment (typically 28 days) for assessment of adherence. Adherence estimates were calculated after excluding participants who were reported lost to follow-up after collecting all of their medication. The estimates also excluded those who discontinued PEP for medical reasons (i.e. being found to be HIV-infected at baseline after initiating PEP, HIV-negative status of the sexual partner, or if the physician recommended discontinuation of PEP for any reason). In both cases it is not possible to know if these participants had been, or would have been, adherent to the full 28-day regimen. Information was also extracted regarding refusal of PEP, HIV seroconversion, drug regimen(s) used in the study, study design (RCT, retrospective or prospective observational study), interventions conducted in the study (behavioral or comparison of PEP regimens), adherence measure (self-report or objective measure such as pill count), and risk group (MSM, heterosexual transmission, or sex work).
Quality of Included Studies
To assess the risk of bias of adherence estimates within studies that were included, we extracted data related to adherence measure, study design, refusals, reporting side effects or medical discontinuation, loss to follow-up at 28 days, if the study specified inclusion and exclusion criteria. In addition, we adapted the GRADE scoring system to quantitatively assess study quality and risk of bias (Supplementary Table 1)(19). Specifically, we focused on criteria that would be most meaningful for risk of bias with descriptive summary statistics, as opposed to efficacy or effectiveness. Studies were scored higher for more rigorous study design. Interventional prospective study designs, regardless of randomization, were scored the highest, followed by non-interventional prospective, and then retrospective designs. Points were then deducted for high rates of loss to follow-up, lack of a pill count adherence measure or did not report how adherence was measured, lack of definition of study population, and generalizability to the overall population of interest.
Data Analysis
Point estimates and 95% confidence intervals (CI) for the proportion of adherent participants were calculated. Meta-analysis was used to generate an overall pooled point estimate and 95% CI for adherence as well as separate pooled estimates for observational studies and RCTs. A DerSimonian-Laird random effects model was used to pool estimates for the overall estimate(20). A random effects model was chosen to account for heterogeneity of studies by including a parameter for inter-study variation. Random effects meta-regression was used to assess the relationship between adherence and covariates, with the purpose of explaining heterogeneity between the studies in terms of study-level covariates: 2 versus 3-drug regimen; study design (RCT vs. observational study, prospective vs. retrospective, and risk reduction intervention vs. no risk reduction intervention); year; and risk group. Three sensitivity analyses were conducted: excluding the two studies that did not report full 28-day adherence(14,21), including all participants who were lost to follow-up assuming they were all non-adherent, and including any adherence estimates identified in the grey literature. Publication bias was assessed with Egger’s test(22) and Begg’s test(23), and an adjusted adherence estimate was attained by the Trim and Fill method(24). Briefly, Egger’s and Begg’s tests provide formal statistical tests of funnel plots for the detection of publication bias, Egger’s via regression analysis of funnel plot asymmetry and Begg’s by an adjust rank correlation between adjusted effect size and meta-analysis weight. The Trim and Fill method estimates missing studies and provides an adjusted estimate for the effect missing studies may have had on the pooled estimate. Analyses were conducted in Stata 12.0 (StataCorp, College Station, TX).
RESULTS
Studies screened and reviewed
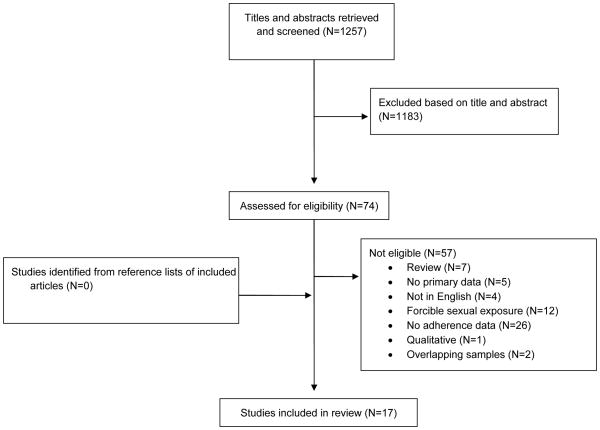
Of 1,257 titles and/or abstracts initially reviewed, 74 were further assessed for inclusion in the study and 17 were included in the final review (Figure 1). Common reasons for exclusion at the second stage included no adherence data (n=26) and forcible sexual exposure as the most prevalent exposure group (n=12). Three studies reported an overlapping cohort; the study with the most complete data in terms of reported adherence and duration of follow-up was included(25), and thus two studies were excluded.(26,27) The final 17 articles comprised 3,634 participants enrolled in 3 RCTs (n=602) and 14 observational studies (n=2,966), including 10 prospective observational studies (n=1,622) and 7 retrospective observational studies (n=1,344). Interventions conducted within RCTs included behavioral interventions (n=2) and comparison of PEP regimens (n=1). Table I lists characteristics of included articles. One adherence estimate was identified in the grey literature, consisting of 643 participants in Australia(28).
Figure 1.
Flow diagram for selection of included studies
Table I.
Characteristics of included articles
| Author | Year | Country | Study Design | Na | Risk Group | Proportion Refusing | Reported Inclusion/Exclusion Criteria | Adherence Measure | Drug Regimen | Reported Side Effects | Loss to Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minas(44) | 2012 | Australia | Prospective | 160 | Mixed | NR | N | NR | NR | N | NR |
| Bentz(14) | 2010 | France | RCT | 39 | Mixed | NR | Y | Self report | 3-drug | Y | 27.8% at day 15 |
| Rey(30) | 2008 | France | Retrospective | 629 | Mixed | 3.0% | N | Retention in treatment | NR | Y | 26.3% after starter kit; 41.9% at day |
| Lacombe(13) | 2006 | France | Retrospective | 137 | Mixed | NR | Y | NR | 3-drug | Y | NR at day 28 |
| Sonder(25) | 2010 | Netherlands | Prospective | 259 | MSM | NR | N | NR | 3-drug | Y | 5.9% at day 28 |
| Tissot(29) | 2010 | Switzerland | Retrospective | 483 | Mixed | 2.2% | Y | NR | 3-drug | Y | 19.5% at day 28 |
| McCarty(45) | 2011 | UK | Retrospective | 50 | Mixed | NR | N | NR | 2-drug | Y | 30.6% at day 28 |
| Day(46) | 2006 | UK | Retrospective | 45 | MSM | NR | Y | NR | NR | Y | 31.1% at day 28 |
| Diaz-Brito(34) | 2011 | Spain | RCT | 161 | Mixed | NR | Y | Self report | 3-drug | Y | 15.7% at day 28 |
| Landovitz(47) | 2012 | US | Prospective | 35 | MSM | NR | Y | Pill count/Self report | 2-drug | Y | NR at day 28 |
| Mayer(15) | 2012 | US | Prospective | 84 | MSM | NR | Y | Pill count | 3-drug | Y | 15% at day 28 |
| Roland(33) | 2011 | US | RCT | 402 | Mixed | NR | Y | Self report | 3-drug | Y | 10.3% at 28 days |
| Shoptaw(48) | 2008 | US | Prospective | 84 | Mixed | NR | Y | Pill count/Self report | 2-drug | Y | 14.3% at day 28 |
| Mayer(16) | 2008 | US | Prospective | 231 | MSM | NR | Y | Pill count | 2-drug | Y | 37.7% at day 28 |
| Kahn(49) | 2001 | US | Prospective | 346 | MSM | NR | Y | Self report | 2-drug | Y | 9.2% at day 28 |
| Schechter(17) | 2004 | Brazil | Prospective | 97 | MSM | 43% | Y | Pill count/Self report | 2-drug | Y | 2.0% at day 28 |
| Izulla(21) | 2012 | Kenya | Prospective | 326 | FSW | NR | Y | Self report | 2-drug | N | 44.5% at 10 days |
Abbreviations: RCT (randomized controlled trial); MSM (men who have sex with men); FSW (female sex worker); Y (Yes); N (No); NR (not reported)
N includes all patients who initiated PEP, excluding those who tested HIV-positive at baseline or whose source tested HIV-negative and those who collected a full 28-day course of PEP but did not return for follow-up at 28 days
Study characteristics
Eight of the studies were conducted in Europe, with six others from the United States and one each from Australia, Brazil and Kenya. Seven studies included exclusively or almost exclusively men who have sex with men (MSM), nine studies were mixed cohorts of people who reported heterosexual or homosexual exposure, and one study included only female sex workers (FSW). One study included injection drug users (IDUs); however, only 1.3% of participants in the study sought PEP after needle-sharing exposure with the remainder seeking PEP for non-forcible sexual exposure, and thus this study was included in the meta-analysis.
Of studies reporting their adherence measure, 2 studies measured adherence with pill counts, 5 with self-report, 3 with both pill count and self-report and 1 as retention in treatment, defined as returning for scheduled PEP follow-up visits without discontinuation of medication. The remainder did not specify what their measure of adherence was. Fourteen studies reported the PEP regimens used. Of these, 8 used 2-drug regimens and 6 reported a 3-drug regimen. Drug regimens varied within studies, with some studies reporting more than one regimen. Regimens included zidovudine/lamivudine (n=6), zidovudine/lamivudine plus nelfinavir, atazanavir, or ritonavir (n=4), tenofovir/emtricitabine (n=3), tenofovir/emtricitabine plus raltegravir (n=1) and tenofovir plus lamivudine (n=1).
Outcomes
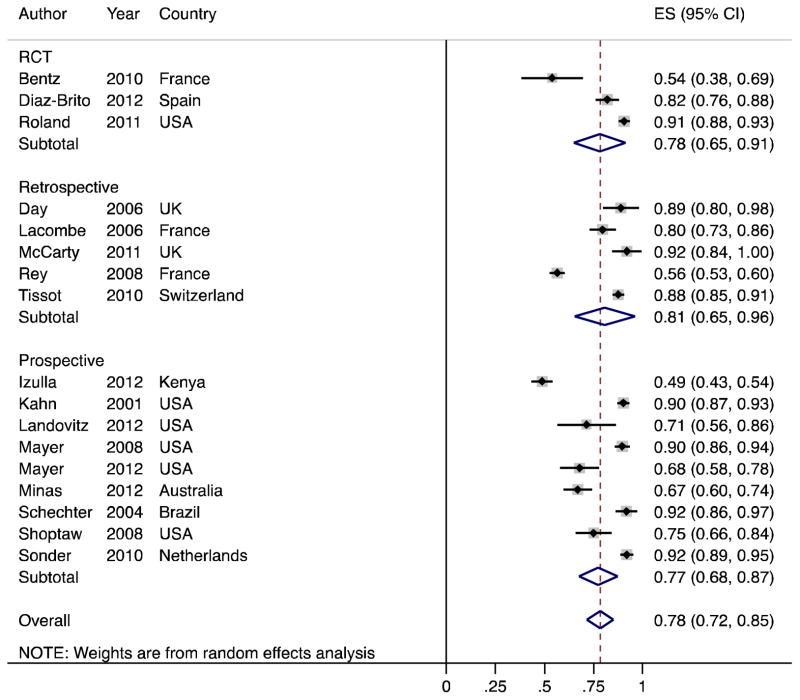
In the 17 studies, the pooled overall adherence to PEP was 78% (95% CI 72 to 85%, Figure 2). Adherence ranged from 49% (95% CI 43 to 54%) to 92% (95% CI 89 to 95%). In prospective observational studies, pooled adherence was 77% (95% CI 68 to 87%), retrospective observational studies 81% (95% CI 65 to 96%), and RCTs 78% (95% CI 65 to 91%). As expected, heterogeneity was high (τ2=492). Excluding two studies that did not report full 28-day adherence yielded a pooled adherence estimate of 82% (95% CI 76 to 88%)(14,21). Assuming that all participants who were lost to follow-up, pooled adherence was 67% (95% CI 59 to 74%). Including one estimate from the grey literature, pooled adherence was 77% (95% CI 70 to 84%).
Figure 2.
Forest plot of pooled proportions of adherent participants by observational studies (N=14) and randomized controlled trials (RCT, N=3). Effect size (ES) indicates the proportion of participants who completed the entire 28-day course of PEP.
Table II lists the results of bivariate meta-regression models. In a multivariable model (Table III), adherence among RCTs compared to observational studies was not significantly different (coefficient 0.41, 95% CI −0.11 to 0.94, P=0.09). There was no difference in adherence between 2- and 3-drug regimens (3-drug versus 2-drug: coefficient −0.17, 95% CI −0.53 to 0.19, P=0.23) or between studies that relied only on self-report versus those that included pill count as a measure of adherence (coefficient 0.20, 95% CI −0.24 to 0.63, P=0.25). Because most studies did not report their data by risk group, we could only compare studies that enrolled predominantly MSM versus mixed cohorts (heterosexual and homosexual exposure). There was no significant difference between MSM-only and mixed cohorts (MSM only versus mixed: coefficient 0.05, 95% CI −0.47 to 0.57, P=0.79).
Table II.
Bivariate meta-regressions predicting adherence
| Predictor | k | N (%)a | Coefficient (95% Confidence Interval) | P |
|---|---|---|---|---|
| RCT (vs. observational) | 17 | 3/17 (17.7%) | −0.014 (−0.22 to 0.19) | 0.88 |
| Prospective (vs. retrospective) | 17 | 12/17 (70.6%) | −0.04 (−0.20 to 0.13) | 0.66 |
| Risk reduction risk reduction strategy) | 17 | 3/17 (17.7%) | −0.04 (−0.25 to 0.16) | 0.66 |
| 3-drug regimen (vs. 2-drug) | 14 | 6/14 (42.9%) | −0.03 (−0.21 to 0.14) | 0.69 |
| MSM (vs. mixed cohort) | 17 | 7/17 (41.2%) | 0.11 (−0.03 to 0.26) | 0.11 |
| Objective measure of adherence (vs. self- report only) | 11 | 5/11 (45.5%) | 0.09 (−0.14 to 0.31) | 0.40 |
| Year of publication | 17 | 2001: 1/17 (5.9%) 2004: 1/17 (5.9%) 2006: 2/17 (11.8%) 2008: 3/17 (17.7%) 2010: 3/17 (17.7%) 2011: 2/17 (11.8%) 2012: 5/17 (29.4%) |
−0.02 (−0.04 to 0.01) | 0.12 |
Denominator is the number of studies reporting this characteristic
Table III.
Multivariable meta-regression model predicting adherence (k=10 studies with data for each predictor)
| Predictor | Coefficient (95% Confidence Interval) | P |
|---|---|---|
| RCT (vs. observational) | 0.41 (−0.11 to 0.94) | 0.09 |
| Risk reduction intervention (vs. no risk reduction strategy) | −0.13 (−0.59 to 0.33) | 0.44 |
| 3-drug regimen (vs. 2-drug) | −0.17 (−0.53 to 0.19) | 0.23 |
| MSM (vs. mixed cohort) | 0.05 (−0.47 to 0.58) | 0.79 |
| Objective measure of adherence (vs. self-report only) | 0.20 (−0.24 to 0.63) | 0.25 |
| Year of publication | −0.03 (−0.07 to 0.02) | 0.19 |
Loss to follow-up varied widely. Among studies that reported loss to follow-up at 28 days, loss to follow-up ranged from 2% to 38% (Table I). Most studies only assessed only patients who were prescribed PEP and did not report on refusals. Of those that did, proportion of refusals ranged from 2.2% to 42.6%(17,29,30). While were no significant small study effects as measured by Egger’s test (P=0.15), Begg’s test suggested there may be small sample bias (P=0.02). A trim-and-fill estimate of adherence adjusting for the effect of missing studies was 77% (95% CI 71 to 84%).
DISCUSSION
This review found a pooled overall adherence of 78% amongst studies of PEP among individuals with non-forcible sexual exposure to HIV; it also demonstrated widely variable rates of adherence between studies. In a sensitivity analysis assuming all participants who were lost to follow-up were non-adherent, pooled adherence dropped to 67%. This adherence estimate constitutes a lower bound for the range of changes in the overall adherence estimate due to missing data. It is unlikely that all of the participants who were lost to follow-up were non- adherent, so that true overall adherence will be higher than 67%. No single study has been powered to establish the effectiveness of PEP, and the number of HIV seroconversions in the studies was very low. It is therefore difficult to comment on what level of adherence is necessary for effectiveness, although it is expected that better adherence will result in better protection.
This review demonstrated a substantially higher proportion of patients adherent to their prescribed regimens compared to a meta-analysis of adherence to PEP after sexual assault, which found pooled adherence to be 40%(31). Psychological factors associated with sexual assault, such as fear, rape stigma, fear of being blamed for the rape, and anxiety related to the trauma, could negatively impacts survivors’ adherence levels(32). Individuals experiencing sexual assault may also have discontinued PEP following court-ordered HIV testing of the perpetrator. These factors are less likely to be experienced by PEP seekers who have had consensual exposure to HIV, which could explain the difference in adherence rates between these two analyses.
Many factors may impact the heterogeneity of the overall pooled adherence estimates, and to address this we conducted several meta-regressions to investigate potential sources of heterogeneity. Study design may affect adherence. Adjusting for other factors, adherence was 40% higher in RCTs compared to non-RCTs, although this difference was not significant. RCTs may have higher adherence because participants are followed closely by study staff and may be counseled to adhere, or may be more motivated because they volunteered for a study.
In this meta-analysis, interventions included risk reduction strategies following high-risk sexual exposure to HIV, counseling to improve adherence, and comparison of tolerability and discontinuation with two different PEP regimens(14,33,34). There was no difference in adherence between studies that implemented risk reduction strategies and those that did not. One study found that enhanced risk reduction counseling, including 5 sessions versus 2 that incorporated detailing risk exposure, strategies to reduce risky behavior, and development of a risk reduction plan, which was further assessed in the enhanced risk reduction group, resulted in reduction of risky behavior in individuals with higher baseline risk, but did not find a difference in adherence to PEP between standard and enhanced counseling groups(33). A separate study found that adherence to PEP was higher among participants randomized to additional counseling sessions(14). We were unable to analyze adherence by arm, by meta-analysis given the diverse nature of the interventions and the small number of interventional studies in this review. The fact that risk reduction interventions did not significantly affect PEP adherence in our meta-regression analysis does not necessarily suggest that such an effect does not exist; the pooled analysis may simply lack the power to detect such an effect. Moreover, our synthesis also does not indicate that PEP should not be accompanied by risk reduction interventions, because these interventions are primarily intended to achieve sexual behavior change, which we do not analyze here.
Varying measures of adherence can influence estimates in each of the studies. While plasma drug levels may be an accurate measure, they may not be a practical form of adherence measurement on a large scale, though costs for assays are declining. Some studies may over report adherence, particularly when relying entirely on self-report. Evidence from the FEMPrEP trial suggests that self-reported adherence does not correlate well with drug measurement via plasma drug levels, indicating that there may be social desirability bias resulting in overestimates of adherence(4). None of the PEP studies included in this review used plasma drug levels to corroborate self-reported adherence, and only five studies included an objective measure, in all cases pill counts. While there was no difference in adherence in studies that included a pill count measurement compared to those that did not, it is not clear how well either self-report or pill count correlate with plasma drug level with PEP, and thus how accurate the measurements of adherence in this review are. It will be important to continue to explore standardized, accurate measures of adherence to chemoprophylaxis as well as ART in future studies.
Choice of PEP regimen can greatly influence adherence. Some regimens are associated with lower tolerability and higher rates of adverse events such as nausea, and 3-drug regimens may be more complicated for patients than 2-drug regimens(35). There is limited evidence on adherence outcomes in patients receiving different PEP regimens. In this meta-analysis, we did not find a difference in adherence among studies that used predominantly a 2-drug regimen versus a 3-drug regimen. However, not all studies detailed their drug regimens, so we did not have complete data for this analysis, nor were we able to compare specific drug regimens. No studies directly compared 2- and 3-drug regimens, and separate studies may not be comparable. Newer regimens that include TDF-FTC may have higher adherence than zidovudine/lamivudine-based regimens(15,16). As we were unable to assess the effects of different drug regimens on adherence, we included calendar year as a proxy for drug regimen in our meta-regression analysis, as regimens change systematically over time. This proxy variable was neither a strong nor significant predictor of adherence. Since the majority of studies in this review assessed adherence to zidovudine-based regimens, it is hoped that adherence to TDF-FTC-based regimens will be even higher.
PrEP acceptability and utilization studies have indicated that individuals who have previously used PEP are more likely to report that they would use PrEP(36–38). PrEP may have advantages over PEP for individuals who have recurrent longer-term high-risk exposures, but these differences may result in very different initiation and adherence patterns(39). PEP requires individuals to identify instances of high-risk exposure, and to initiate therapy promptly, whereas PrEP must be initiated prior to occurrence or risky behavior, and thus requires acknowledgement of impending risky behavior. Sustained effectiveness for PrEP would require consistently adequate adherence, whereas PEP is a time-limited prescription. Careful studies will need to be done as PrEP is expanded on a larger scale to monitor adherence, but PEP results suggest that high levels of PrEP adherence may be sustained for at least short periods of time. Whether these results are applicable to PrEP over longer durations will require careful study.
This analysis may suffer from several limitations. The accuracy of the pooled adherence estimate is limited by the accuracy of the estimates in each study. Due to the small number of studies included in the review, the meta-regressions will be underpowered to detect a difference in adherence, and thus we may have failed to detect significant predictors of adherence. Publication bias could affect the results of this study. Although we only included studies that were published in English in our analysis, of the 4 non-English studies which were initially identified, all would have been excluded based on abstract review for other reasons: one enrolled primarily victims of sexual assault(40), in one only half of the exposures were sexual; and two did not include data on adherence(41,42). Begg’s test suggested there may have been small sample bias. However, we used rigorous methods and clear inclusion criteria in selecting our studies, and neither a trim-and-fill adjusted estimate nor a sensitivity analysis including grey literature substantially changed the results. Finally, the results of our review may not be generalizable to populations and settings for which data were sparse or not available, such as generalized HIV epidemics. Adherence to ART has been shown to be higher in developing countries compared to developed countries: one meta-analysis found a pooled estimate of adherence to ART in African studies of 77%, compared to an estimate of 55% in North America(43), while another found adherence to PEP after sexual assault was higher adherence in developing (53%) than developed countries (33%)(31). The only study included in our meta-analysis from a generalized epidemic setting included only female sex workers, a high-risk population may not be representative of the general population that may access PEP in Kenya or in other generalized epidemics(21).
This study demonstrated a higher overall adherence to PEP than previously been reported for exposure to HIV after sexual assault. Overall pooled adherence was around 80%, although estimates varied widely between studies. Although PrEP is a significantly more long-term intervention than PEP, and thus will pose additional challenges to adherence, the high adherence to PEP seen after non-forcible sexual exposure to HIV outside of a clinical trial setting is encouraging for future PrEP adherence, and indicates that it is possible to achieve high levels of adherence to ART for prevention. Continued assessment of adherence and counseling to improve adherence is important, and continuing to invest in better ways to collect adherence data and refine existing measures of adherence will be important for accurate measures of adherence to treatment as prevention interventions.
Supplementary Material
Acknowledgments
Funding: CEO is supported by a NIAID T32 NRSA grant (T32AI007535; PI: Seage).
Role of the funding source: The sponsors did not have a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Meeting Presentation: Presented in part at the International Association of Providers of AIDS Care 8th International Conference on HIV Treatment and Prevention Adherence, June 2-4, 2013, Miami, FL
Conflicts of interest: None to declare.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2012 Aug 2;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minnis AM, Gandham S, Richardson BA, Guddera V, Chen BA, Salata R, et al. Adherence and Acceptability in MTN 001: A Randomized Cross-Over Trial of Daily Oral and Topical Tenofovir for HIV Prevention in Women. AIDS Behav. 2012 Oct 14; doi: 10.1007/s10461-012-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elst EM, Mbogua J, Operario D, Mutua G, Kuo C, Mugo P, et al. High Acceptability of HIV Pre-exposure Prophylaxis but Challenges in Adherence and Use: Qualitative Insights from a Phase I Trial of Intermittent and Daily PrEP in At-Risk Populations in Kenya. AIDS Behav. 2012 Oct 19; doi: 10.1007/s10461-012-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and Adherence to Intermittent Pre-Exposure Prophylaxis (PrEP) for HIV-1 in African Men Who Have Sex with Men and Female Sex Workers. In: Maartens G, editor. PLoS ONE. 4. Vol. 7. 2012. Apr 12, p. e33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012 Mar 6;59:463–8. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DK, Grohskopf LA, Black RJ, Auerbach JD, Veronese F, Struble KA, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States. MMWR Recomm Rep. 2005 Jan 21;54(RR02):1–20. [PubMed] [Google Scholar]
- 10.Cardo DM, Culver DH, Ciesielski CA, Srivastava PU, Marcus R, Abiteboul D, et al. A Case–Control Study of HIV Seroconversion in Health Care Workers after Percutaneous Exposure. N Engl J Med. 1997 Nov 20;337:1485–90. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 11.Management of possible sexual, injection-drug use, or other nonoccupational exposure to HIV including considerations related to antiretroviral therapy public health service statement. MMWR Recomm Rep. 1998 Sep 25;47(RR17):1–14. [PubMed] [Google Scholar]
- 12.Lurie P, Miller S, Hecht F, Chesney M, Lo B. Postexposure Prophylaxis After Nonoccupational HIV Exposure. JAMA. 2000 Feb 15;280(20):1769–73. doi: 10.1001/jama.280.20.1769. [DOI] [PubMed] [Google Scholar]
- 13.Lacombe K, Daguenel-Nguyen A, Lebeau V, Fonguernie L, Girard PM, Meyohas MC. Determinants of adherence to non-occupational post HIV exposure prophylaxis. AIDS. 2005 Dec 27;20(2):291–4. doi: 10.1097/01.aids.0000199832.10478.83. [DOI] [PubMed] [Google Scholar]
- 14.Bentz L, Enel P, Dunais B, Durant J, Poizot-Martin I, Tourette-Turgis C, et al. Evaluating counseling outcome on adherence to prophylaxis and follow-up after sexual HIV-risk exposure: a randomized controlled trial. AIDS Care. 2010 Dec;22(12):1509–16. doi: 10.1080/09540121.2010.484457. [DOI] [PubMed] [Google Scholar]
- 15.Mayer KH, Mimiaga MJ, Gelman M, Grasso C. Raltegravir, tenofovir DF, and emtricitabine for postexposure prophylaxis to preven the sexual transmission of HIV: safety, tolerability, and adherence. J Acquir Immune Defic Syndr. 2012 Feb 21;59(4):354–9. doi: 10.1097/QAI.0b013e31824a03b8. [DOI] [PubMed] [Google Scholar]
- 16.Mayer KH, Mimiaga MJ, Cohen D, Grasso C, Bill R, VanDerwarker R, et al. Tenofovir DF Plus Lamivudine or Emtricitabine for Nonoccupational Postexposure Prophylaxis (NPEP) in a Boston Community Health Center. J Acquir Immune Defic Syndr. 2008 Apr 1;47(4):494–9. doi: 10.1097/QAI.0b013e318162afcb. [DOI] [PubMed] [Google Scholar]
- 17.Schechter M, do Lago RF, Mendelson AB, Moreira RI, Moulton LH, Harrison LH, et al. Behavioral Impact, Acceptability, and HIV Incidence Among Homosexual Men With Access to Postexposure Chemoprophylaxis for HIV. J Acquir Immune Defic Syndr. 2004 Mar 8;35:519–25. doi: 10.1097/00126334-200404150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Siika AM, Nyandiko WM, Mwangi A, Waxman M, Sidle JE, Kimaiyo SN, et al. The structure and outcomes of a HIV postexposure prophylaxis program in a high HIV prevalence setup in western Kenya. J Acquir Immune Defic Syndr. 2009 Apr 16;51(47–53):1–7. doi: 10.1097/QAI.0b013e318198a96a. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology Elsevier Inc. 2011 Apr 1;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-Analysis in Clinical Trials*. Controlled Clinical Trials. 1986 Jan 1;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Izulla P, McKibben PS, Munyao J, Karanja S, Koima W, Parmeres J, et al. HIV post-exposure prophylaxis in an urban population of female sex workers in Nairobi, Kenya. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012 Oct;:1. doi: 10.1097/QAI.0b013e318278ba1b. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 May 13;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994 Dec 15;50(4):1088–101. [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting forPublication Bias in Meta-Analysis. Biometrics. 2000 Jun 1;56(1):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Sonder GJB, Prins JM, Regez RM, Brinkman K, Mulder J-W, Veenstra J, et al. Comparison of Two HIV Postexposure Prophylaxis Regimens Among Men Who Have Sex With Men in Amsterdam: Adverse Effects Do Not Influence Compliance. Sexually Transmitted Diseases. 2010 Nov;37(11):681–6. doi: 10.1097/OLQ.0b013e3181e2f999. [DOI] [PubMed] [Google Scholar]
- 26.Sonder GJB, van den Hoek A, Regez RM, Brinkman K, Prins JM, Mulder J-W, et al. Trends in HIV Postexposure Prophylaxis Prescription and Compliance After Sexual Exposure in Amsterdam, 2000???2004. Sexually Transmitted Diseases. 2006 Sep;:PAP. doi: 10.1097/01.olq.0000237838.43716.ee. [DOI] [PubMed] [Google Scholar]
- 27.Heuker J, Sonder GJB, Stolte I, Geskus R, van den Hoek A. High HIV incidence among MSM prescribed postexposure prophylaxis, 2000–2009. AIDS. 2012 Feb;26(4):505–12. doi: 10.1097/QAD.0b013e32834f32d8. [DOI] [PubMed] [Google Scholar]
- 28.Zheng W, Smith D, Kippax S. Epidemiologically targeted non-occupational post exposure prophylaxis (NPEP) in Australia, 1998-2002. 14th Annual Conference of the Australasian Society for HIV Medicine. [Google Scholar]
- 29.Tissot F, Erard V, Dang T, Cavassini M. Nonoccupational HIV post-exposure prophylaxis: a 10-year retrospective analysis. HIV Med. 2010 Sep 3;11(9):584–92. doi: 10.1111/j.1468-1293.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- 30.Rey D, Bendiane MK, Bouhnik A-D, Almeda J, Moatti JP, Carrieri MP. Physicians’ and patients’ adherence to antiretroviral prophylaxis after sexual exposure to HIV: results from South-Eastern France. AIDS Care. 2008 May;20(5):537–41. doi: 10.1080/09540120701867198. [DOI] [PubMed] [Google Scholar]
- 31.Chacko L, Ford N, Sbaiti M, Siddiqui R. Adherence to HIV post-exposure prophylaxis in victims of sexual assault: a systematic review and meta-analysis. Sexually Transmitted Infections. 2012 Jul 13;88(5):335–41. doi: 10.1136/sextrans-2011-050371. [DOI] [PubMed] [Google Scholar]
- 32.Abrahams N, Jewkes R. Barriers to post exposure prophylaxis (PEP) completion after rape: a South African qualitative study. Culture, Health & Sexuality. 2010 Jun;12(5):471–84. doi: 10.1080/13691050903556316. [DOI] [PubMed] [Google Scholar]
- 33.Roland ME, Neilands TB, Krone MR, Coates TJ, Franses K, Chesney MA, et al. A Randomized Noninferiority Trial of Standard Versus Enhanced Risk Reduction and Adherence Counseling for Individuals Receiving Post-Exposure Prophylaxis Following Sexual Exposures to HIV. Clinical Infectious Diseases. 2011 Jun 7;53(1):76–83. doi: 10.1093/cid/cir333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz Brito V, León A, Knobel H, Peraire J, Domingo P, Clotet B, et al. Post-exposure prophylaxis for HIV infection: a clinical trial comparing lopinavir/ritonavir versus atazanavir each with zidovudine/lamivudine. Antivir Ther. 2011;17(2):337–46. doi: 10.3851/IMP1955. [DOI] [PubMed] [Google Scholar]
- 35.Luque A, Hulse S, Wang D, Shahzad U, Tanzman E, Antenozzi S, et al. Assessment of Adverse Events Associated With Antiretroviral Regimens for Postexposure Prophylaxis for Occupational and Nonoccupational Exposures to Prevent Transmission of Human Immunodeficiency Virus. Infect Control Hosp Epidemiol. 2007 Jun;28(6):695–701. doi: 10.1086/518349. [DOI] [PubMed] [Google Scholar]
- 36.Aghaizu A, Mercey D, Copas A, Johnson AM, Hart G, Nardone A. Who would use PrEP? Factors associated with intention to use among MSM in London: a community survey. Sexually Transmitted Infections. 2012 Sep 26; doi: 10.1136/sextrans-2012-050648. [DOI] [PubMed] [Google Scholar]
- 37.Mimiaga MJ, Case P, Johnson CV, Safren SA, Mayer KH. Preexposure Antiretroviral Prophylaxis Attitudes in High-Risk Boston Area Men Who Report Having Sex With Men: Limited Knowledge and Experience but Potential for Increased Utilization After Education. J Acquir Immune Defic Syndr. 2009 Jan 1;50(1):77–83. doi: 10.1097/QAI.0b013e31818d5a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, White JM, et al. Limited Awareness and Low Immediate Uptake of Pre-Exposure Prophylaxis among Men Who Have Sex with Men Using an Internet Social Networking Site. In: Vermund SH, editor. PLoS ONE. 3. Vol. 7. 2012. Mar 28, p. e33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu AY, Grant RM, Buchbinder SP. Preexposure Prophylaxis for HIV. JAMA. 2006 Aug 16;296(7):863–5. doi: 10.1001/jama.296.7.863. [DOI] [PubMed] [Google Scholar]
- 40.Vives N, Almeda J, Contreras CA, Garcia F, Campins M, Casabona J, et al. Use of non-occupational HIV post-exposure prophylaxis in Spain (2001–2005) Enferm Infect Microbiol Clin. 2008 Nov 1;26(9):546–51. doi: 10.1157/13128270. [DOI] [PubMed] [Google Scholar]
- 41.Wensing AM, Schneider MM, Schurink CA, Geerlings SE, Boucher CA. Post-exposure prophylaxis following exposure to HIV: adaptation to the situation may be indicated. Ned Tijdschr Geneeskd. 2005 Jul 2;149(27):1485–9. [PubMed] [Google Scholar]
- 42.Levy I. Post exposure prophylaxis (PEP) to prevent HIV after sexual exposure in Israel. Harefuah. 2005 Apr 1;144(4):252–4. [PubMed] [Google Scholar]
- 43.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to Antiretroviral Therapy in Sub-Saharan Africa and North America. 2006 Jul 31;296(6):679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 44.Minas B, Laing S, Jordan H, Mak DB. Improved awareness and appropriate use of non-occupational post-exposure prophylaxis (nPEP) for HIV prevention following a multimodal communication strategy. BMC Public Health. 2012 Oct 25;12(1):1–1. doi: 10.1186/1471-2458-12-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarty EJ, Quah S, Maw R, Dinsmore WW, Emerson CR. Post-exposure prophylaxis following sexual exposure to HIV: a seven-year retrospective analysis in a regional centre. International Journal of STD & AIDS. 2011 Jul 5;22(7):407–8. doi: 10.1258/ijsa.2009.009463. [DOI] [PubMed] [Google Scholar]
- 46.Day S, Mears A, Bond K, Kulasegaram R. Post-exposure HIV prophylaxis following sexual exposure: a retrospective audit against recent draft BASHH guidance. Sexually Transmitted Infections. 2006 Jun 1;82(3):236–7. doi: 10.1136/sti.2005.017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landovitz RJ, Fletcher JB, Inzhakova G, Lake JE, Shoptaw S, Reback CJ. A Novel Combination HIV Prevention Strategy: Post-Exposure Prophylaxis with Contingency Management for Substance Abuse Treatment Among Methamphetamine-Using Men Who Have Sex with Men. AIDS Patient Care and STDs. 2012 Jun;26(6):320–8. doi: 10.1089/apc.2011.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoptaw S, Rotheram-Fuller E, Landovitz RJ, Wang J, Moe A, Kanouse DE, et al. Non-occupational post exposure prophylaxis as a biobehavioral HIV-prevention intervention. AIDS Care. 2008 Mar;20(3):376–81. doi: 10.1080/09540120701660353. [DOI] [PubMed] [Google Scholar]
- 49.Kahn JO, Martin JN, Roland ME, Bamberger JD, Chesney MA, Chambers D, et al. Feasibility of Postexposure Prophylaxis (PEP) against Human Immunodeficiency Virus Infection after Sexual or Injection Drug Use Exposure: The San Francisco PEP Study. Journal of Infectious Diseases. 2001 Feb 6;183:707–14. doi: 10.1086/318829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.