Introduction
Cerebral ischemia spans a temporal continuum from hyperacute presentation and extends into acute, subacute and chronic phases. Imaging provides detailed information to the clinician, which must be evaluated in light of the patient’s symptomatic presentation and clinical examination. Imaging results in the context of the patient’s examination are valuable in confirming diagnosis, ruling out pathology, evaluating the degree of disease progression, helping in selection of optimal treatment and adjusting treatment based on patient response. It also provides invaluable information on patients who may carry the burden of cerebrovascular pathology but are clinically asymptomatic. For these patients, knowledge of imaging finding will drive a return to the clinical exam and most certainly affect treatment decision-making. The wide availability of imaging in the current era allows for the possibility of serial evaluation of patients throughout their disease course. This is of particular value in the monitoring of cerebral ischemic disorders, which inherently follow a dynamic course. Irrespective of clinical practice settings, the evolution and refinement of imaging techniques now permit treatment decisions to be made in real time.
‘Reason for Consultation’
One may propose that the ‘reason for consultation’ is perhaps one of the most important guiding aspects in obtaining supportive imaging. Knowing how the specific clinical question can be asked and appropriately answered by a specific imaging modality is fundamental to selecting the appropriate imaging test. Whether the question is related to evaluation of the ischemic core, penumbral tissue or areas at-risk, a specific vascular lesion or pathology which may culminate in cerebral ischemia, understanding the advantages and limitations of neuroimaging techniques increases the yield of imaging data gathered. Also, much like a subspecialist consultation, obtaining ancillary imaging in an outpatient clinical setting requires providing a framework for the subspecialty imaging expert who is analyzing and coordinating the studies. Providing a reason for consultation, whether it may be assessing intracranial arterial stenosis in a specific vascular distribution, evaluating a pattern of cerebral ischemia to better understand disease mechanism, or shedding light on ischemic disease progression in order to modify disease management, is imperative in order to provide the evaluating imaging expert with a focus for their study interpretation. This promotes focus on a specific region of interest in the interpretation and will also lead to potential adjustment of the imaging protocol if needed to best address the question of interest. Improved patient care, more cost-effective measures and reduced need for unnecessary repeat imaging will ultimately result that augments the clinical examination findings and aids in patient management.
Pathophysiology of Cerebral Ischemia
The pathophysiology of cerebral ischemia extends beyond the direct effects of anatomical changes in the arterial system leading to brain tissue. Unlike focal ischemia of ischemic stroke, cerebral hypoperfusion and cardiac arrest may both lead to global ischemic injury unrestricted to a specific vascular territory. The pathophysiology of ischemia is similar at the tissue and cellular level, involving metabolic dysfunction and cell death due to hypoxia. The regulation of tissue perfusion in the brain is modulated differently from any other organ in the body given that nearly half of cerebrovascular resistance relies on the large arteries at the circle of Willis, in addition to both intra- as well as extracranial vasculature 1,2. These arteries and their end arterioles play a primary role in oxygen delivery to the brain parenchyma through their regulation of cerebral blood flow (CBF). Many studies in animals and humans have investigated the threshold below which a reduction in CBF manifests neurological symptoms and those which correlate to pathologically irreversible neuronal damage 3–5. Across studies, depending on study design, neurological symptoms and ischemia have been reported to range in values from below nearly 20ml/100ml/minute to between 8–12ml/100ml/min where tissue oxygenation was no longer sufficient to support the cellular machinery 5–8. While conventionally, cerebral ischemia has been thought to result as a direct consequence of a reduction in CBF, Ostergaard and colleagues have recently discussed the concept of capillary transit time heterogeneity and its contribution to the brain’s efficacy in extracting oxygen at a given CBF 9. Regional CBF changes can be demonstrated using both computed tomography (CT) and magnetic resonance imaging (MRI) imaging (Figure 1). A number of fatal outcomes result from the final aftermath of tissue oxygen deprivation which on a cellular level include cell body shrinkage, chromatin condensation, nuclear fragmentation as well as changes to the membrane phospholipid structure 10–13. One of the members of the phospholipid structure, specifically, phosphatidylethanolamine has been proposed to serve a regulatory role in the blebbing and formation of apoptotic bodies as well as in mediation of cellular necrosis as its externalization may serve in part to contribute to cytoskeletal organization 12. In combination, an overwhelming failure of cellular energy resources ensues and results in subsequent cell death.
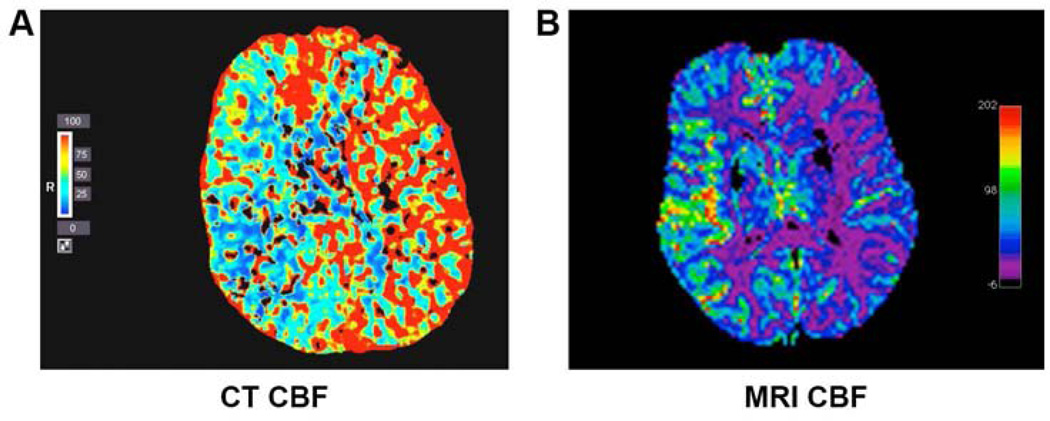
Figure 1. Demonstration of CBF using MRI and CT.
Regional changes in CBF are shown throughout cerebral parenchyma (A) CT of patient presenting with acute right MCA stroke with blue color demonstrating most severely decreased CBF in ischemic hemisphere. (B) MRI of patient presenting with acute left MCA stroke with purple color demonstrating most severely decreased CBF in ischemic hemisphere.
Imaging of Ischemia
The outcomes of cerebral ischemia are not isolated to the acute changes that occur on a tissue, cellular and molecular level, but also encompasses the resultant subacute and chronic lesion evolution. A number of imaging modalities may be utilized to reflect the pathophysiological changes and outcome of cerebral ischemia at the tissue level. The iterative refinement and versatility of these recent imaging techniques has expanded our understanding of cerebral ischemia as a disorder from multiple perspectives. It has enabled the use of multiple modalities in the imaging of vascular lesions, blood flow and many facets of cerebral perfusion, as well as parenchyma changes demonstrating ischemic lesions, stages of evolving ischemia, consequences of ischemic injury and tissue repair that have been invaluable in advancing therapeutic management of stroke patients. Given that each modality offers specific strengths and limitations, understanding the strengths and weaknesses of these techniques is imperative in choosing the appropriate method of investigation to discern critical mechanisms and to facilitate decision-making for our patients.
Imaging Modalities in the Assessment of Ischemia
Non-contrast CT was initially one of the only options in assessing cerebral ischemia, however in recent years, the development of MRI and the application of multimodal techniques with either CT or MRI have provided insight into disease pathogenesis, mechanism and evolution. Multimodal CT typically includes non-contrast CT, CT angiography of the both head and neck (CTA) as well as CT perfusion (CTP). Similarly, multimodal MRI also includes a number of sequences, such as diffusion-weighted imaging (DWI), perfusion weighted imaging (PWI), fluid attenuated inversion recovery (FLAIR), gradient recalled echo (GRE) in addition to MR angiography of head and neck (MRA). Figure 2 highlights the use of multimodal imaging in acute MCA stroke.
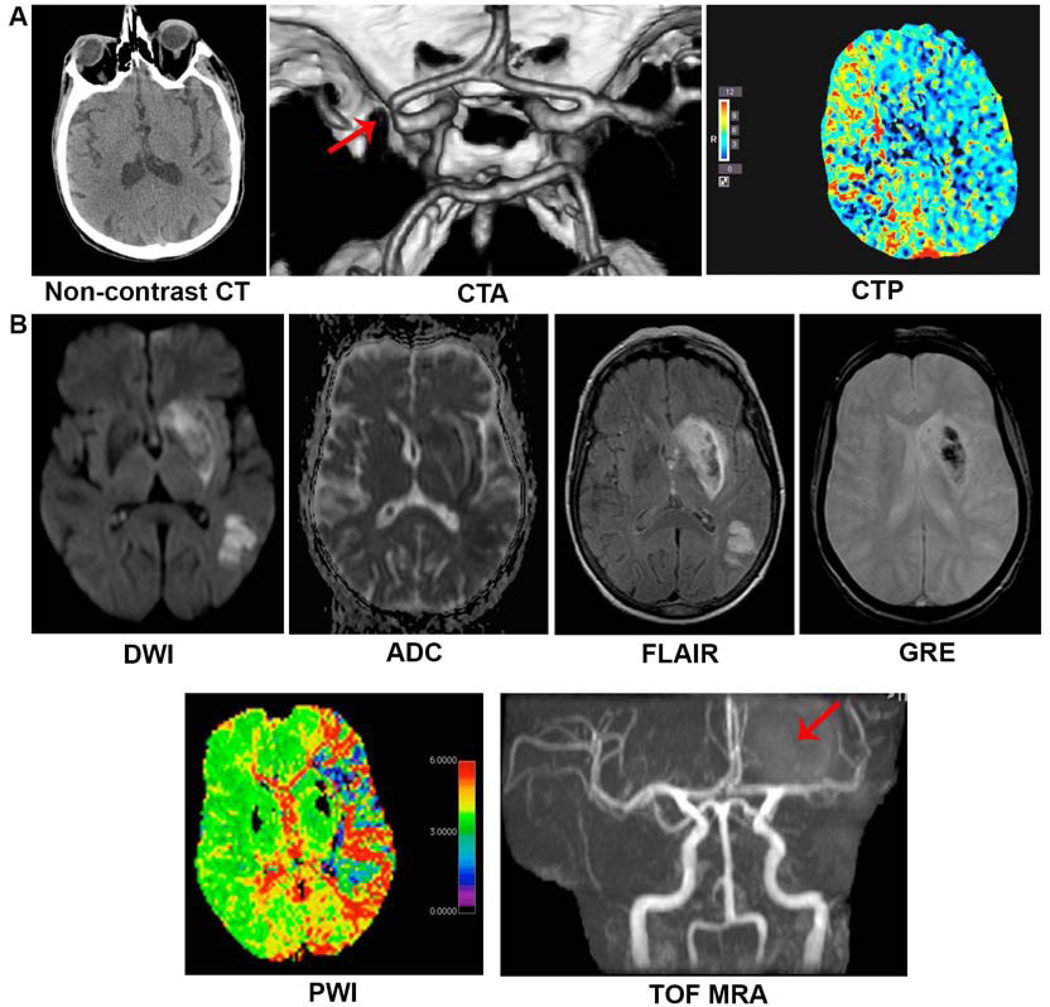
Figure 2. Multimodal CT and MRI.
The informative nature of multimodal imaging using both CT and MRI is shown. (A) Multimodal CT of an 88-year-old gentleman with history of heart failure status post pacemaker placement, diabetes, hypertension, hyperlipidemia and hypothyroidism presenting acutely with left-sided hemiplegia. Initial non-contrast CT with no obvious hypodensity to suggest parenchymal ischemia while CTA demonstrates clear right MCA signal cutoff and CTP shows decreased perfusion to the right hemisphere with areas most severely affected represented in red. (B) Multimodal MRI of an 82-year-old woman with history of hypothyroidism later found to have cardiac arrhythmia presenting acutely with right-sided hemiplegia. Ischemic lesion is represented by DWI hyperintensity corresponding with ADC hypointensity. FLAIR also demonstrates hyperintense signal providing information with regards to the age of the lesion, likely several hours from onset of ischemia. GRE sequence hypointensity represents hemorrhagic transformation with surrounding edema. PWI clearly demonstrates extent of decreased perfusion throughout the left hemisphere which extends beyond the burden of the ischemic lesion demonstrated on DWI/ADC/FLAIR, most severely represented by red color. TOF MRA demonstrates abrupt cutoff of the distal left M1 segment with decreased number of left M2 branches.
Customization of imaging protocols in patients being evaluated for cerebral ischemia, is typically tailored by institution. Multimodal imaging offers a comprehensive view of the patient’s condition including integrity of brain parenchyma, extent of vascular involvement to a dynamic view of tissue viability based on status of cerebral perfusion. The choice between CT versus MRI can be tailored to the patient and to their symptoms at presentation. CT becomes the preferred imaging modality in patients who are unable to undergo MRI as in the case of those who have pacemaker devices or implanted metallic objects and for patients too unstable to sustain placement in a supine position for an extended period of time. However, in patients who are able to undergo MRI testing, this imaging technique while relatively more time consuming, offers the unique advantage of providing exquisite detail of brain parenchyma with early sensitivity, which far surpasses CT approaches. Minutes from the onset of neurologic symptoms, and presumably ischemic insult, MRI will display new ischemic lesions as hyperintensities on DWI and corresponding ADC hypointensity. These lesions, which reflect parenchymal cytotoxic edema, acutely are thought to approximate the ischemic core. It is important to note that with resolution of ischemia, it is possible for DWI lesions to become reversible 14,15. The pattern of distribution of DWI lesions may provide invaluable insight into disease mechanism whether thromboembolic in nature, related to a specific vascular territory occlusion or due to hypoperfusion and borderzone ischemia 16. Mismatch between DWI and FLAIR positivity may reveal temporal facets or the duration of ischemia17.
CT has limitations in the detection of very early ischemic injury in the acute setting and is quiet restricted in ability to accurate visualize posterior fossa pathology 18. However, with the wide availability of CT technology, in the community and some institutions alike, CT scanning continues to prevail as the first line imaging modality. CTA and CTP, when combined with CT, yield additional insight on early ischemia. In conjunction with a patient presentation and clinical exam which is consistent with a vascular event, non-contrast CT may at least be helpful in ruling out cerebral hemorrhage or an existing, non-vascular reason for presentation such as mass related to infection, malignancy or other etiology. In the cases of suspected cerebral ischemia where non-contrast CT did not show abnormality, repeat, serial imaging with the evolution of the patient’s symptoms is also critical.
The importance of serial imaging and approaching cerebral ischemia as a dynamic condition is best demonstrated by the concept of “fogging”. Using CT, this term had long been described referring to the temporary loss of visibility of ischemic lesion approximately 2 weeks after the onset of stroke 19,20. The area of infarction develops a similar signal intensity as the surrounding normal tissue likely secondary to inflammatory cell infiltration of the lesion. In 2004, O’Brien and colleagues performed an independent, blinded review of serial MRI images for up to 7 weeks from patient presenting with symptoms of cortical ischemic stroke and assessed for the possible presence of fogging using T2 weighted MRI 21. While on CT imaging fogging translated into the non-visibility of the lesion at the 2-week interval, on MRI the investigators found “fogging” in 50% of patients (15 patients) ranging temporally from 6 to 36 days following ischemic event, with a median of ten days. In these patients with moderately to large infarcts, a reduction in severity of infarct was suggested to lead to an underestimation of the final outcome of stroke and based on this advised perhaps obtaining final imaging 7 weeks after ischemic event 21.
Angiographic Assessment of Vascular Lesions
Indirect features of parenchymal imaging with CT and MRI can be helpful in gleaning information about vascular pathology. For example, a large vessel occlusion in the middle cerebral artery (MCA) has been described as a ‘hyperdense’ MCA sign (Figure 3) on non-contrast CT and signifies vascular occlusion. Parallel findings have also been described in the setting of internal carotid and posterior cerebral artery occlusion 22,23. The MRI correlate to this phenomenon of parenchymal imaging shedding light on vascular occlusion can be demonstrated on the GRE sequence of MRI when hypointensities in the vessel correlate with clot. More direct evidence of vascular disease can be obtained from the angiographic components of multimodal CT and MRI which both can provide vital and detailed information with regards to vascular pathological changes.
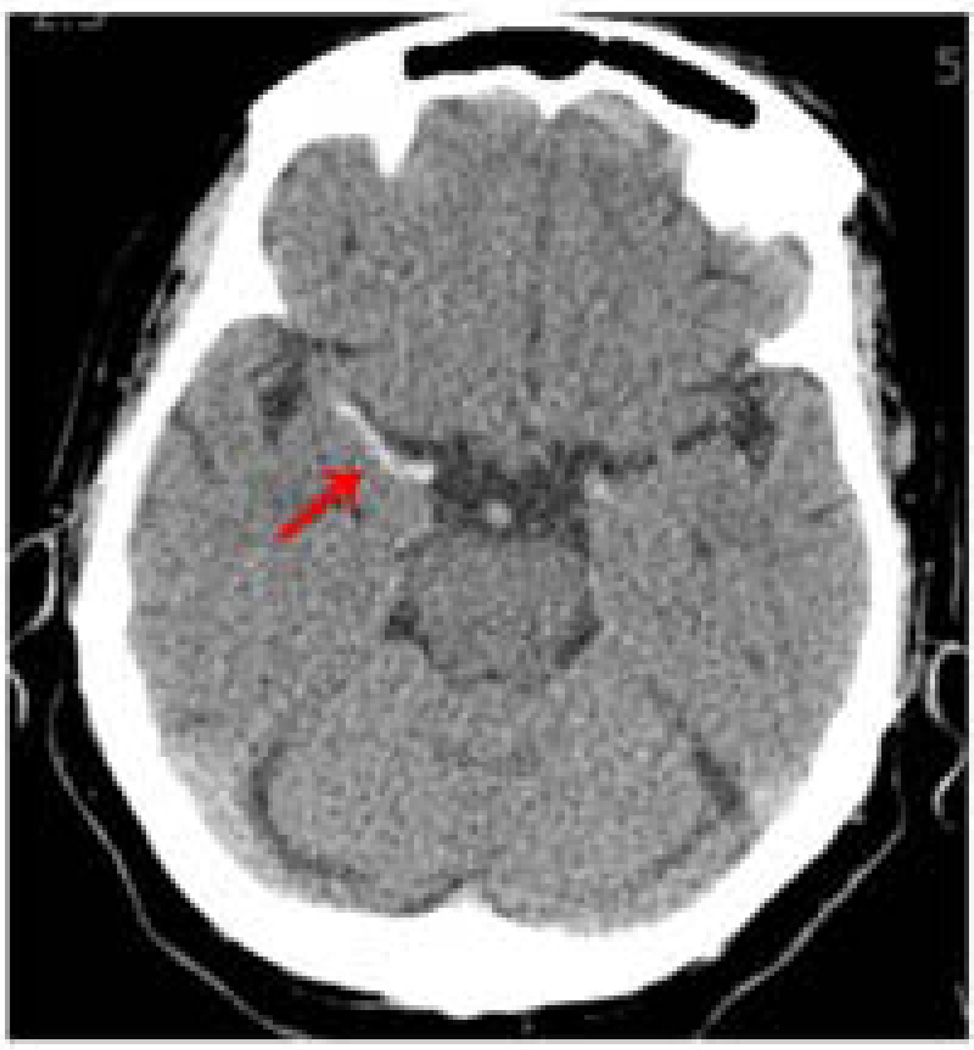
Figure 3. Hyperdense MCA Sign.
Hyperdensity (arrow) of right MCA artery shown on non-contrast CT signifies vascular occlusion of the artery.
CTA offers multiple advantages as it is easily and efficiently performed following non-contrast CT in an estimated ten-minute time window 24. In the hyperacute presentation of a presumed ischemic event, CTA provides benefit in excluding patients without vascular lesions from acute interventions. For use across the temporal continuum of disease, there are a number of both advantages and potential limitations of CTA technology. This method is advantageous for its remarkable speed able to capture the anatomical parameters of the neurovascular tree, including both intra- and extracranial vasculature in approximately 15 seconds, which in itself decreases the likelihood of motion artifact and also lessens the need for large amounts of contrast 24. Unlike flow dependent imaging techniques, specifically MRA and ultrasound, CTA has the distinctive advantage of being able to provide anatomically accurate information regarding vascular pathology including information regarding degree of calcification, residual lumen dimensions as well as length of stenosis 24. For patients who are critically ill requiring support machinery, those who are not able to tolerate lying in a supine position for an extended period of time, or for those for whom MRI in contraindicated, CTA is particularly advantageous. From an outpatient referral standpoint, patients with a suspected vascular lesion who are able to tolerate CT contrast, CTA is a particularly important in quantifying vascular lesion burden and can easily be used to monitor intra- or extracranial lesion progression over time in a serial fashion. The limitations of CTA technology include the inability to lend information regarding direction or velocity or direction of flow. Such physiological and dynamic information is readily provided by both MRA and ultrasound. Another disadvantage is related to CT beam hardening in which heavily calcified vascular plaques can alter the precision of image reconstructions 24. The CTA portion of multimodal imaging, unlike MRA (time-of-flight), does necessitate the administration of iodinated contrast material and this in itself may be disadvantageous in some patient populations whether in the inpatient or outpatient settings 24.
The variations in MR angiography techniques are numerous. MRA protocols can be tailored and may range from two-dimensional to three-dimensional time-of-flight (TOF) or alternatively, contrast may be used to enhance the imaging of vascular pathology. TOF MRA is a gradient recall echo sequence that relies on radio frequency pulsing of tissue. In terms of 2-dimensional versus 3-dimensional versions of this technology, the latter offers the advantage of a higher resolution albeit limited by vascular saturation artifacts. The former is limited by signal loss in areas where flow turbulence is a component of pathology. Another factor that may limit interpretation of TOF MRA is inadequate suppression of background that adds to the artifactual data gathered. Relying on radiofrequency pulsation for artifact suppression, molecules which are not easily suppressed such as methemogolbin and fat may lead to erroneous regions of vascular obscuration or conversely false areas of perceived flow 25. Again because TOF MRA relies on dynamic flow, it does not deliver a true anatomic representation of vascular occlusion as can be achieved by CTA. Potential overestimation of degree of stenosis or occlusion is demonstrated in figure 4 as both slow flow as well as no flow render vascular signal diminution. Phase contrast MRA, which computes the alteration in transverse magnetization between moving and stationary tissue, may be advantageous compared to TOF MRA. Not only can this technique display the direction of flow, it can also improve on TOF MRA by showing blood which is flowing slowly given that saturation effects can be better avoided using this technique. One limitation that is posed by use of phase contrast MRA is that through its processing their results signal loss around areas of turbulent flow and areas of stenoses 25. MRA can also be performed in conjunction with gadolinium contrast administration. Figure 5 shows an example of contrast enhanced MRA (CE MRA) in a patient presenting with symptoms of acute stroke. Following a bolus of intravenous gadolinium, this gradient recalled echo sequence display opacified vessels as T1 hyperintense. The use of contrast allows for shorter acquisition times and hence less likelihood of motion artifact, yet it does have a limitation in that acquisition must be completed in the time window of arterial enhancement and may not be repeated until contrast has cleared 25.
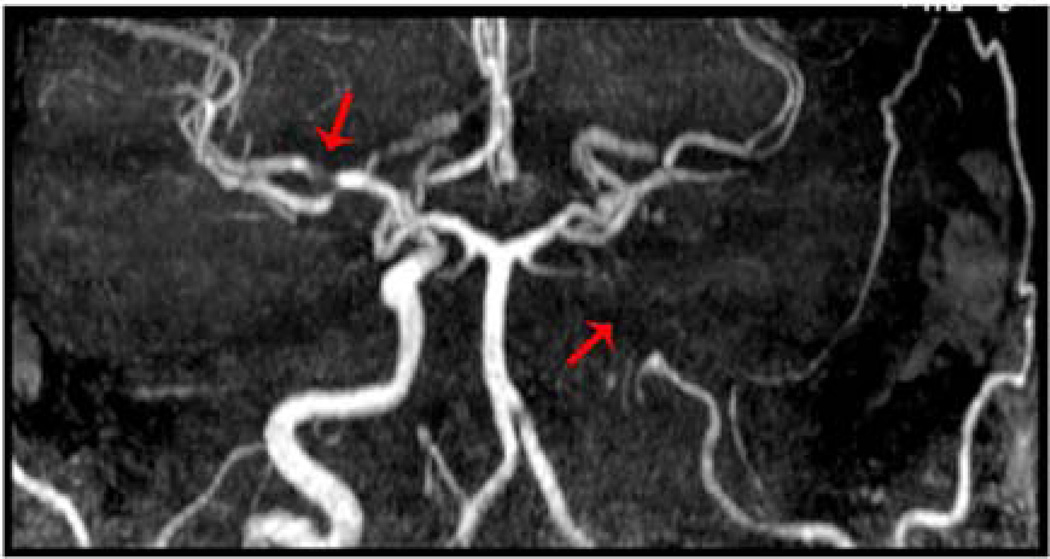
Figure 4. TOF MRA May Overestimate Degree of Vascular Stenosis.
Relying on flow, rather than anatomical representation of vascular occlusion, TOF MRA may overestimate the degree of vascular stenosis in patients. Shown is TOF MRA of a patient presenting with acute infarction involving multiple areas in the left M2 territory with MRA demonstrating Occlusion of the posterior division left M2 division of MCA artery with paucity of vessels demonstrated (arrow). Imaging also suggests a flow-dependent degree of stenosis in right MCA, which was not clinically symptomatic during his presentation (arrow).
Figure 5. Contrast-Enhanced MRA.
The clarity of the neurovascular tree imaged using CE MRA is demonstrated in this figure. Opacified vessels are T1 hyperintense.
In patients with a clinical history of trauma or neck manipulation for whom a vascular dissection is suspected, an additional angiography sequence is employed, namely fat-saturated axial T1 sequence imaging of the neck, obtained pre-gadolinium bolus. Also as compared to non-contrast MRA, more anatomic information can be gleaned from contrast enhanced sequences given that images represent contrast in the lumen of vessels imaged 25. In patients with a clinical history of trauma or neck manipulation for whom a vascular dissection is suspected, an additional angiography sequence is employed, namely fat-saturated axial T1 sequence imaging of the neck, obtained pre-gadolinium 25.
In the case of severe vascular stenosis or total occlusion, imaging clues can be helpful in offering likely prognosis and may even predict recanalization. This finding is described as ‘tram tracking’ which indicates a minimal degree of flow in occluded lesions 26. Not only does revascularization and recanalization play a role in the reperfusion of ischemic tissue but collateral circulation is also thought to interplay with the vascular milieu and contribute to the support of tissue perfusion 27,28.
Perfusion Imaging
Perfusion imaging has served an invaluable role in directing therapeutic intervention in stroke patients. Along with DWI, the identification of ischemic core and direct comparison with cerebral perfusion, helps identify vascular territory that is further compromised or at risk (diffusion-perfusion mismatch model). These data may not only shed light on diagnosis in terms of better understanding the etiology of stroke and providing a dynamic view of tissue integrity to influencing treatment decision, for example based on this data, knowledge of a large territory at risk may support the decision for further revascularization therapy. Perfusion imaging alone is limited by not detailing changes which may occur with respect to changes in vascular collateral or compensatory changes in circulation 29, however with the advent of new technical post processing strategies, perfusion maps may be generated from angiographics maps and in this case information is yielded from both vascular changes as well as tissue bed perfusion30.
Arterial spin labeling, as a non-invasive fMRI perfusion technique, does not rely on contrast agent administration. This imaging modality captures patterns of CBF and hence perfusion by making use of arterial water as a tracer. It is thought to offer a repeatedly reliable measure of CBF both spatially as well as temporally, yet its use has been limited as compared to contrast-based perfusion techniques 31. More recently, groups have been sharing their positive experience with reproducible, and widely applicable results using this perfusion methodology 32,33.
Routinely, aside from the technique of arterial spin labeling MRI perfusion, both multimodal imaging modalities, CT and MRI, require contrast agent for visualization of perfusion portion of the study. In CT technology, labeling is achieved by use of iodinated contrast agents while MRI relies on a gadolinium-based contrast agent. CBF and blood volume may then be extrapolated based on the anatomical mapping and distribution of contrast flow through vasculature and cerebral tissue beds. With the use of contrast comes the risk of allergic reaction, contrast extravasation, nephrotoxicity and their limited use specifically in patients with compromised renal function, but not in patients without concomitant renal disease. Albeit a small risk, there appears to be lower risk and less adverse allergic reaction with gadolinium when compared to iodinated contrast agents 34. With regards to risk for contrast extravasation, owing to the high volume of material administrated and the rapid rate at which it is infused, iodinated contrast is associated with a higher overall probability of occurrence 34. The controversy of risk for developing contrast material–induced nephropathy (CIN) associated with iodinated contrast agents and nephrogenic systemic fibrosis (NSF) as in patients given gadolinium contrast is essentially only an issue for patients with renal disease. Renal disease exists within a spectrum and patients within this spectrum can be managed based on the following guidelines based on the severity of their disease: In patients with glomerular filtration rate (GFR) values between 30 and 60 ml/min, the recommendation is typically for the administration of gadolinium as their risk for NSF is low and the risk for CIN at this stage of their renal disease exists. In patients not dependent on hemodialysis and with GFR <30, the risk for both CIN as well as NSF exist. If the need for contrast material is essential and would change their medical management whether it be in diagnosis, allowing for prognostication or in altering the course of therapeutic management, CT with iodinated contrast is preferred as patient may be prophylactically treated with sodium bicarbonate and N-acetylcysteine and well hydrated prior to the procedure to decrease the risk of CIN. Additionally, even if CIN occurs in such chronic renal disease patient, dialysis may be used to treat. In the case of NSF, while occurring at a frequency of 5% in these patients, the outcome can be lethal 34. Lastly, patients whom are in renal failure and are hemodialysis dependent, there exists a large risk of developing NSF with exposure to gadolinium contrast, as such, in these patients, iodinated contrast is preferred. Given that there does not appear to be an adverse consequence of iodinated contrast administration to worsening renal function, the only risk is in the form of volume overload or overhydration. Therefore, in this cohort of patients, there is no restriction on iodinated contrast administration if it is clinically necessary and it need not be coordinated with dialysis schedule 34.
Patients who are pregnant also fall under a high-risk category for contrast administration. In the case of pregnancy, although it is unlikely for gadolinium to adversely harm the fetus or mother, given the theoretical possibility for gadolinium to become retained, uneliminated then unconjugated and therefore toxic in the amniotic fluid, most academic institutions in the United States recommend against its use. The risk of iodinated contrast material however is known to be a state of hypothyroidism for the fetus which is amenable to therapeutic correction at delivery, and as such guidelines recommend CT with iodinated contrast in pregnant patients if their medical condition necessitates it 34.
Nuclear Neuroimaging
Although no longer logistically a first line option particularly in hyperacute presentations of stroke, nuclear imaging provides valuable information with regard to tissue fate. In the early setting example, investigating regional CBF as well as nuclear imaging including positron emission tomography (PET) and single-photon emission computed tomography (SPECT) has served well as surrogate prognostic markers for patient outcomes 35–38. Nuclear neuroimaging can be used as an important tool in imaging of the hypoperfused, yet possibly salvageable ischemic penumbra in acute stroke. This can be performed by imaging by evaluation perfusion and metabolism, the neuronal integrity or through the use of hypoxic markers 39. 15O-PET as a methodology has been very effective in simultaneous assessment of perfusion as well as oxygen metabolism taking into account CBF and cerebral metabolic rate of oxygen 39. Studies have also demonstrated the use of SPECT in assessing the ischemic penumbra using of tracers such as 99mTc-hexamethylprpyleneamine oxime and 99mTc-ethyl cysteinate dimer 40–42. Imaging neuronal integrity to yield information about the ischemic penumbra has been demonstrated by the use of a central-type benzodiazepine receptor as a marker given that changes in the expression of this receptor in the cerebral cortex can serve as a marker of irreversible neuronal damage 43. It is important to note that in animals models, this receptor was not thought to be expressed in the cerebellum or in the subcortical parenchyma 44,45. Hypoxic markers have also been used to follow the active changes in the brain parenchyma following ischemia in acute stroke, specifically 18F-labeled misonidazole in conjunction with PET imaging 46–48. SPECT, being more widely available, has been suggested as a valuable technique for identifying patients at high risk of reperfusion injury following ischemia. This is extrapolated from that fact studies such as the one performed by Ueda and colleagues demonstrated the use of SPECT technology to evaluate the risk of hemorrhagic transformation following reperfusion therapy 49,50.
Imaging the Sequelae of Ischemia: Evolution and Repair
Hemorrhagic transformation as one of the later consequences of acute ischemic stroke has been a challenge from an imaging standpoint. This entity is different from primary hemorrhagic stroke, which will not specifically be addressed in this chapter. The transformation of ischemic parenchyma to areas of intracerebral hemorrhage has been described in the past to occur both with and without patient exposure to medical therapy in the form of intravenous tPA or in the form of endovascular intervention 51. In terms of approaches to imaging, non-contrast CT, for example, has been demonstrated to be unclear in the differentiation between primary hemorrhage and hemorrhagic transformation of an ischemic lesion 52. Much like in SPECT studies in the case of nuclear neuroimaging, using imaging modalities as a guide to patients who may be at risk for hemorrhagic transformation following reperfusion owing to reperfusion injury, will be clearly beneficial in guiding treatment decision making in the acute setting. Factors that may play a role in the incidence of hemorrhage may include integrity of parenchymal tissue, the severity and extent of ischemia, the level of reperfusion achieved following vascular occlusion as well as alterations in serum blood sugar, namely hyperglycemia. In the setting of MRI assessment, predictors of hemorrhagic transformation include extensive DWI hyperintensity with associated ADC hypointensity and concomitant low CBF 53–55 as well as perhaps the finding of focal FLAIR hyperintensity in the ischemic lesion 56. An important predictor of ischemia reperfusion injury is a disturbance in the permeability of the blood brain barrier (BBB), and such an imaging modality that may identify this finding would be very informative in guiding decisions for revascularization given the concomitant risk of hemorrhagic transformation following reperfusion 57–61 . These derangements can be demonstrated using either CT or MRI techniques.
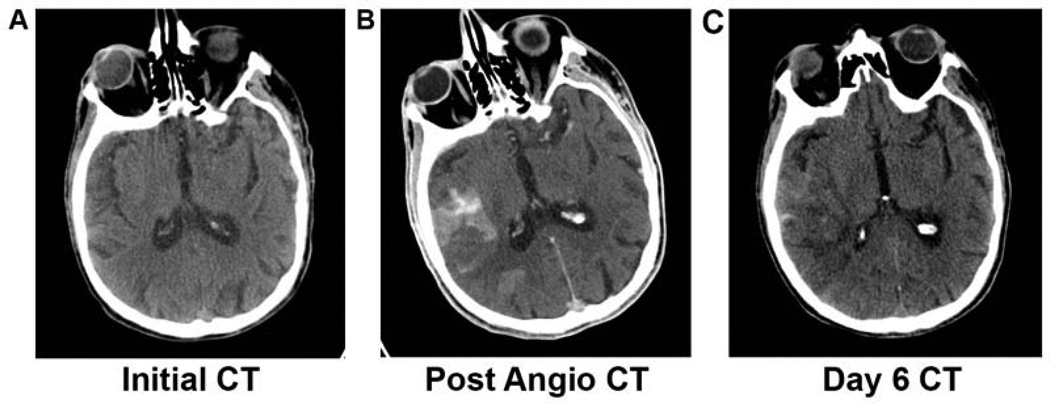
Imaging of suspected cerebral ischemia extends far beyond a go-no go decision during the hyperacute treatment. Special guidance must be employed in the interpretation of early serial imaging following contrast-enhanced studies accounting for the phenomenon of contrast retention, an example of which is shown in figure 6. In CT imaging this may translate into hyperdensity while on T1-weight MRI, this may be visualized as a hyperintensity. This phenomenon on CT imaging may be confounded with possible hemorrhagic transformation. For this reason, clinicians have been evaluating alternative imaging techniques in order to avoid misinterpretation during serial imaging, one of which is dual-energy CT which was shown to be both sensitive and specific in differentiating between iodinated contrast material staining and hemorrhage following initial ischemia 62.
Figure 6. Contrast Retention and Hemorrhage.
(A) Non-contrast CT of a patient presenting with right MCA stroke. No obvious signs of hypodensity present on initial left hemiplegic presentation at onset of acute ischemia. (B) Non-contrast CT following interventional angiography and mechanical revascularization therapy showing multiple foci of hyperdensity in which it is difficult to differentiate between hemorrhagic transformation versus post-procedural contrast retention. (C) Non-contrast CT 6 days following ischemic event showing less burden of hyperdensity than previously demonstrated at initial presentation following angiography.
A second outcome of ischemia, followed by reperfusion, may be reperfusion injury in the form of tissue death in spite of successful recanalization resulting from proposed radical free oxide damage shown in multiple models of stroke 12,63–65 and clinically demonstrated using voxel-based analysis of tissue fate in recent studies 66. Another MRI technique was has been investigated in animal models 65 for its effectiveness in assessing reperfusion injury is diffusion tensor imaging (DTI) which features the changes in brain parenchyma based on the assessment of changes in the features of diffusion of water molecules taking into account both magnitude as well as directionality of change and such exquisitely sensitive particularly in white matter 65,67,68.
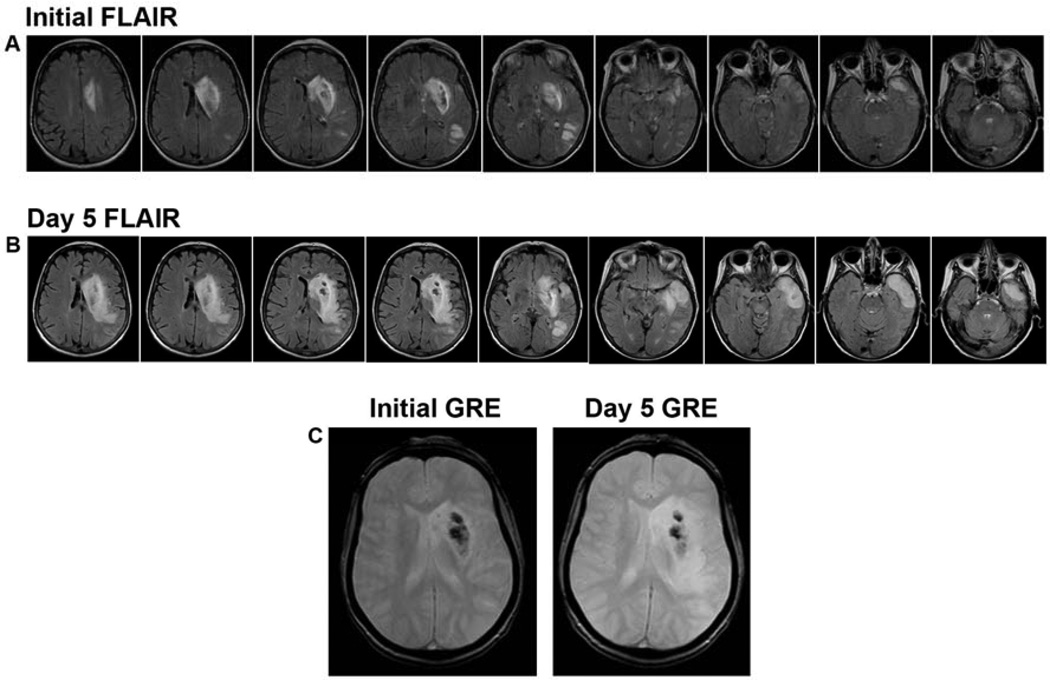
Serial imaging in the evolution of stroke can yield very valuable information to the outpatient clinician, which in combination with the patient’s clinical exam, may serve to predict and oversee functional recovery while guiding therapeutic management and monitoring for cerebrovascular disease reoccurrence. Functional recovery from deficits related to ischemic event is thought to stem from both a reorganization of brain cells as well as the strengthening of brain plasticity 69–72. Repeat imaging taken into context with initial neuroimaging evaluation yields insight into the fate of tissue in the initial peri-infarct zone and ischemic penumbra. MRI images in figure 7 demonstrate the significant evolution of parenchymal findings from initial presentation to 5 days following ischemic stroke in a patient with MCA occlusion.
Figure 7. Serial MRI Imaging of Stroke Evolution.
(A) FLAIR sequence throughout the cerebral parenchyma at initial presentation of acute ischemic stroke as a result of left MCA occlusion. (B) At 5 days following ischemic insult, FLAIR sequences demonstrate the evolution in intensity of lesion burden and the completion of infarction throughout the affected territories (C) GRE hypointensity showing stability of initial hemorrhagic transformation over time.
Capitalizing on the strengths and avoiding the limitations of the available imaging techniques provides incredible insight on the pathophysiology of ischemia and ultimately guides tailored therapeutic approaches.
Ischemic Disorders, the Continuum from Acute to Chronic
Clinically, cerebral ischemia exists on a continuum ranging from the most acute or hyperacute presentation as in the case of ischemic stroke with focal hypoperfusion or cardiac arrest leading to a sudden onset decrease in global cerebral perfusion. Neuroimaging plays a pivotal role in the assessment of ischemic patterns, determining the age of ischemia, understanding disease mechanism and thereby guiding therapeutic management, response and longitudinal follow-up. In ischemic stroke, the categories of disease with variable underlying mechanisms include small vessel, large vessel and thromboembolic disease as well as the category termed cryptogenic although unconfirmed, likely a permutation of the major mechanisms.
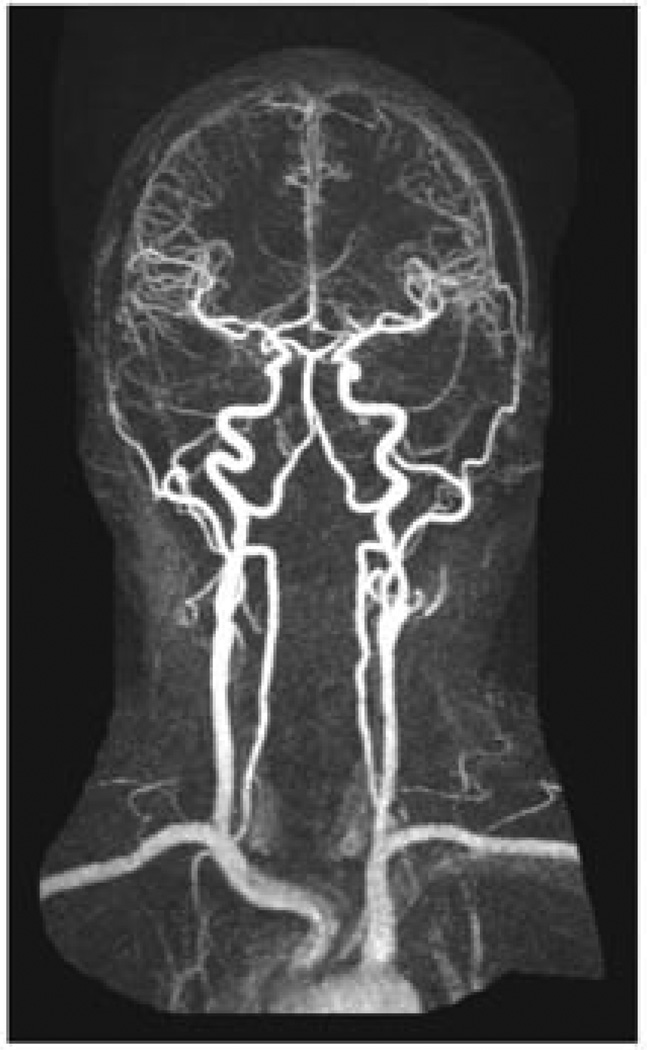
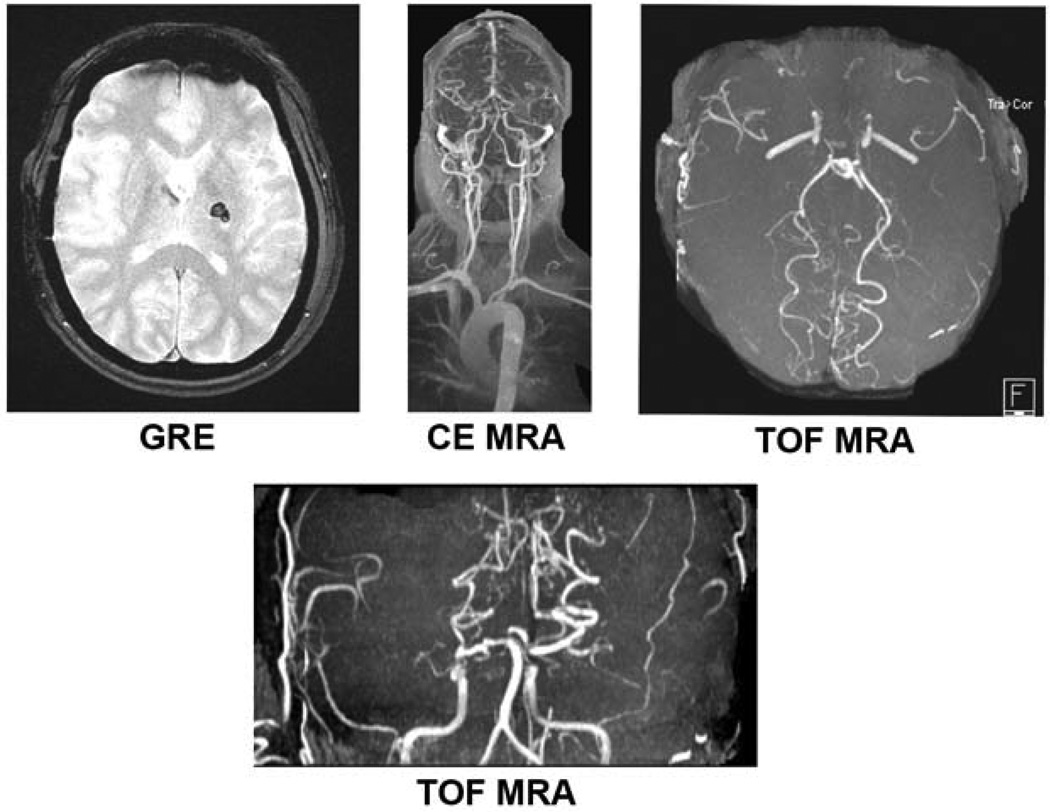
Aside from parenchymal imaging, as discussed previously as part of multimodal imaging, angiography can provide crucial information in identifying disease pathology and specific mechanisms. This is of particular importance in the diagnosis of stroke resulting from as moyamoya, vasculitis and fibromuscular dysplasia as well as stroke resulting from venous pathology such as cerebral venous thrombosis. Moyamoya is a pattern of arterial occlusive disease with an increased incidence in Asian patients, and those with sickle cell and neurofibromatosis. This occlusive vasculopathy is progressive in nature typically that begins by affecting the internal carotid artery then evolves to involve the anterior and middle cerebral arteries proximally with compensatory dilation in collaterals 25. The manifestations of this vascular disease in children versus adults appears to be more ischemic in nature in the former, and presenting with parenchymal hemorrhage in the latter 73. Figure 8 demonstrates multimodal MRI imaging in an adult diagnosed with moyamoya, presenting with acute hemorrhage. The imaging modality which is instrumental in making the diagnosis is MRA, which can both demonstrate the vaso-occlusive phenomenon as well as demonstrating the dilation of collaterals 74,75. Yamada et al., in their evaluation of 26 patients found this imaging technique to yield 73% sensitivity and 100% specificity in diagnosing the disease 76. The pattern of collaterals also has been reported to correlate with the onset of subsequent vascular events and as such MRA has also been instrumental in guiding the treatment of these patients as part of evaluation for revascularization therapy 25. In the case of vasculitis, for which etiology is broad, parenchymal imaging may reveal both ischemic and hemorrhagic lesions and MRA may be clinically useful although less sensitive than digital subtraction angiography imaging (DSA) 77. MRA may also serve an important role in delineating the mimicking pathology conditions such as vascular hypoplasia or dissection, although important to note that as a limitation of TOF MRA slice artifacts may be mistaken for alternating stenoses 25.
Figure 8. Multimodal MRI of Moyamoya Disease.
Imaging of a 44 year-old woman with history of moyamoya presenting with numbness, tingling and weakness in the right upper extremity. GRE hypointensity shows left basal ganglia hemorrhage. CE MRA and TOF MRA images demonstrate MRAs of the neck and head demonstrate occlusion of bilateral supraclinoid internal carotid arteries with distal reconstitution secondary to vascular collaterals as well as narrowing of bilateral internal carotid arteries.
Imaging of Cerebral Ischemia Synopsis.
Cerebral ischemia manifests itself widely in terms of patient symptoms. Along with the clinical exam, imaging serves as a powerful tool throughout the course of ischemia from acute onset to evolution. A thorough understanding of imaging modalities, their strengths and limitations is imperative for capitalizing on the benefit of this complimentary source of information in understanding mechanism of disease, making therapeutic decisions and monitoring patient response over the duration of time.
Key Points in Imaging of Cerebral Ischemia.
Cerebral ischemia spans a temporal continuum from hyperacute presentation and extends into acute, subacute and chronic phases.
Serial imaging of patients throughout the dynamic course of ischemia is highly informative.
Selection of an appropriate imaging modality to answer the clinical question and to compliment the clinical exam is crucial.
A number of neuroimaging modalities exist which yield information regarding the integrity of brain parenchyma, changes in tissue demands and metabolism, severity of vascular pathology and neuronal repair over the duration of time.
Taken into the appropriate context with well-informed selection of imaging tests clinicians can effectively gain insight into disease mechanism, tailor therapeutic decision-making and monitor patients for progression of disease over time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: DSL is a consultant to Stryker, Inc., and Covidien, Inc.
References
- 1.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovascular and brain metabolism reviews. 1990;2(2):161–192. [PubMed] [Google Scholar]
- 2.Shapiro HM, Stromberg DD, Lee DR, Wiederhielm CA. Dynamic pressures in the pial arterial microcirculation. The American journal of physiology. 1971;221(1):279–283. doi: 10.1152/ajplegacy.1971.221.1.279. [DOI] [PubMed] [Google Scholar]
- 3.Finnerty FA, Witkin L, Fazekas JF. Cerebral hemodynamics during cerebral ischemia induced by acute hypotension. The Journal of clinical investigation. 1954;33(9):1227–1232. doi: 10.1172/JCI102997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennett WB, Harper AM, Gillespie FC. Measurement of regional cerebral blood-flow during carotid ligation. Lancet. 1966;2(7474):1162–1163. [PubMed] [Google Scholar]
- 5.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke; a journal of cerebral circulation. 12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 6.Marchal G, Beaudouin V, Rioux P, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke; a journal of cerebral circulation. 1996;27(4):599–606. doi: 10.1161/01.str.27.4.599. [DOI] [PubMed] [Google Scholar]
- 7.Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Annals of neurology. 1996;40(2):216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- 8.Marchal G, Benali K, Iglesias S, Viader F, Derlon JM, Baron JC. Voxel-based mapping of irreversible ischaemic damage with PET in acute stroke. Brain: a journal of neurology. 1999;122(Pt 1):2387–2400. doi: 10.1093/brain/122.12.2387. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard L, Jespersen SN, Mouridsen K, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013 doi: 10.1038/jcbfm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nature reviews. Molecular cell biology. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annual review of physiology. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Stevenson GD, Barber C, et al. Imaging of rat cerebral ischemia-reperfusion injury using(99m)Tc-labeled duramycin. Nuclear medicine and biology. 2012 doi: 10.1016/j.nucmedbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broughton BRS, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke; a journal of cerebral circulation. 2009;40(5):e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 14.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Annals of neurology. 2000;47(4):462–469. [PubMed] [Google Scholar]
- 15.Fiehler J, Foth M, Kucinski T, et al. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke; a journal of cerebral circulation. 2002;33(1):79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- 16.Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovascular diseases (Basel, Switzerland) 2006;21(3):145–153. doi: 10.1159/000090791. [DOI] [PubMed] [Google Scholar]
- 17.Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet neurology. 2011;10(11):978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 18.Selco SL, Liebeskind DS. Hyperacute imaging of ischemic stroke: role in therapeutic management. Current cardiology reports. 2005;7(1):10–15. doi: 10.1007/s11886-005-0004-8. [DOI] [PubMed] [Google Scholar]
- 19.Becker H, Desch H, Hacker H, Pencz A. CT fogging effect with ischemic cerebral infarcts. Neuroradiology. 1979;18(4):185–192. doi: 10.1007/BF00345723. [DOI] [PubMed] [Google Scholar]
- 20.Skriver EB, Olsen TS. Transient disappearance of cerebral infarcts on CT scan, the so-called fogging effect. Neuroradiology. 1981;22(2):61–65. doi: 10.1007/BF00344775. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien P, Sellar RJ, Wardlaw JM. Fogging on T2-weighted MR after acute ischaemic stroke: how often might this occur and what are the implications? Neuroradiology. 2004;46(8):635–641. doi: 10.1007/s00234-004-1230-2. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir O, Leung A, Bussiére M, Hachinski V, Pelz D. Hyperdense internal carotid artery sign: a CT sign of acute ischemia. Stroke; a journal of cerebral circulation. 2008;39(7):2011–2016. doi: 10.1161/STROKEAHA.107.505230. [DOI] [PubMed] [Google Scholar]
- 23.Krings T, Noelchen D, Mull M, et al. The hyperdense posterior cerebral artery sign: a computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke; a journal of cerebral circulation. 2006;37(2):399–403. doi: 10.1161/01.STR.0000199062.09010.77. [DOI] [PubMed] [Google Scholar]
- 24.Josser E, Delgado Almandoz, Shervin Kamalian R, Gilberto González, Michael H, Lev, Javier M, Romero . In: Acute Ischemic Stroke. González RG, Hirsch JA, Lev MH, Schaefer PW, Schwamm LH, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 57–82. [Google Scholar]
- 25.Anne Catherine Kim, David Vu, Gilberto González R, Pamela W, Schaefer LH. In: Acute Ischemic Stroke. González RG, Hirsch JA, Lev MH, Schaefer LH, Schwamm LH, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 123–144. [Google Scholar]
- 26.Otsuka Y, Waki R, Yamauchi H, et al. Angiographic demarcation of an occlusive lesion may predict recanalization after intra-arterial thrombolysis in patients with acute middle cerebral artery occlusion. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2008;18(4):422–427. doi: 10.1111/j.1552-6569.2007.00209.x. [DOI] [PubMed] [Google Scholar]
- 27.Liebeskind DS. Collateral perfusion: time for novel paradigms in cerebral ischemia. International journal of stroke: official journal of the International Stroke Society. 2012;7(4):309–310. doi: 10.1111/j.1747-4949.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebeskind DS. Reperfusion for acute ischemic stroke: arterial revascularization and collateral therapeutics. Current opinion in neurology. 2010;23(1):36–45. doi: 10.1097/WCO.0b013e328334da32. [DOI] [PubMed] [Google Scholar]
- 29.Liebeskind DS. Imaging the future of stroke: I. Ischemia. Annals of neurology. 2009;66(5):574–590. doi: 10.1002/ana.21787. [DOI] [PubMed] [Google Scholar]
- 30.Liebeskind DS, Black SEBB. [Accessed March 19, 2013];Abstract - Perfusion angiography: A novel technique for characterization of perfusion in cerebral ischemia. Available at: http://research.baycrest.org/publication/view/id/7917.
- 31.Borogovac A, Asllani I. Arterial Spin Labeling (ASL) fMRI: advantages, theoretical constrains, and experimental challenges in neurosciences. International journal of biomedical imaging. 2012;2012:818456. doi: 10.1155/2012/818456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendrikse J, Petersen ET, Golay X. Vascular disorders: insights from arterial spin labeling. Neuroimaging clinics of North America. 2012;22(2):259–269. doi: 10.1016/j.nic.2012.02.003. x-xi. [DOI] [PubMed] [Google Scholar]
- 33.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. AJNR. American journal of neuroradiology. 2008;29(8):1428–1435. doi: 10.3174/ajnr.A1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halvorsen RA. Which study when? Iodinated contrast-enhanced CT versus gadolinium-enhanced MR imaging. Radiology. 2008;249(1):9–15. doi: 10.1148/radiol.2491080593. [DOI] [PubMed] [Google Scholar]
- 35.Friedman PJ, Davis G, Allen B. Semi-quantitative SPECT scanning in acute ischaemic stroke. Scandinavian journal of rehabilitation medicine. 1993;25(3):99–105. [PubMed] [Google Scholar]
- 36.Marchal G, Bouvard G, Iglesias S, Sebastien B, Benali K, Defer G, Viader FBJ. Predictive value of (99m)Tc-HMPAO-SPECT for neurological Outcome/Recovery at the acute stage of stroke. Cerebrovasc Dis. 2000;10(1):8–17. doi: 10.1159/000016019. [DOI] [PubMed] [Google Scholar]
- 37.Alexandrov AV, Bladin CF, Ehrlich LE, Norris JW. Noninvasive assessment of intracranial perfusion in acute cerebral ischemia. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 1995;5(2):76–82. doi: 10.1111/jon19955276. [DOI] [PubMed] [Google Scholar]
- 38.Giubilei F, Lenzi GL, Di Piero V, et al. Predictive value of brain perfusion single-photon emission computed tomography in acute ischemic stroke. Stroke; a journal of cerebral circulation. 1990;21(6):895–900. doi: 10.1161/01.str.21.6.895. [DOI] [PubMed] [Google Scholar]
- 39.Oku N, Kashiwagi T, Hatazawa J. Nuclear neuroimaging in acute and subacute ischemic stroke. Annals of nuclear medicine. 2010;24(9):629–638. doi: 10.1007/s12149-010-0421-7. [DOI] [PubMed] [Google Scholar]
- 40.Mahagne M-H, David O, Darcourt J, et al. Voxel-based mapping of cortical ischemic damage using Tc 99m L,L-ethyl cysteinate dimer SPECT in acute stroke. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2004;14(1):23–32. [PubMed] [Google Scholar]
- 41.Hatazawa J, Shimosegawa E, Toyoshima H, et al. Cerebral blood volume in acute brain infarction: A combined study with dynamic susceptibility contrast MRI and 99mTc-HMPAO-SPECT. Stroke; a journal of cerebral circulation. 1999;30(4):800–806. doi: 10.1161/01.str.30.4.800. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe Y, Takagi H, Aoki S, Sassa H. Prediction of cerebral infarct sizes by cerebral blood flow SPECT performed in the early acute stage. Annals of nuclear medicine. 1999;13(4):205–210. doi: 10.1007/BF03164893. [DOI] [PubMed] [Google Scholar]
- 43.Sette G, Baron JC, Young AR, et al. In vivo mapping of brain benzodiazepine receptor changes by positron emission tomography after focal ischemia in the anesthetized baboon. Stroke; a journal of cerebral circulation. 1993;24(12):2046–2057. doi: 10.1161/01.str.24.12.2046. discussion 2057–8. [DOI] [PubMed] [Google Scholar]
- 44.d’Argy R, Persson A, Sedvall G. A quantitative cerebral and whole body autoradiographic study of a intravenously administered benzodiazepine antagonist 3H-Ro 15-1788 in mice. Psychopharmacology. 1987;92(1):8–13. doi: 10.1007/BF00215472. [DOI] [PubMed] [Google Scholar]
- 45.Hantraye P, Kaijima M, Prenant C, et al. Central type benzodiazepine binding sites: a positron emission tomography study in the baboon’s brain. Neuroscience letters. 1984;48(2):115–120. doi: 10.1016/0304-3940(84)90005-3. [DOI] [PubMed] [Google Scholar]
- 46.Markus R, Reutens DC, Kazui S, et al. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke; a journal of cerebral circulation. 2003;34(11):2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- 47.Read SJ, Hirano T, Abbott DF, et al. The fate of hypoxic tissue on 18F–fluoromisonidazole positron emission tomography after ischemic stroke. Annals of neurology. 2000;48(2):228–235. [PubMed] [Google Scholar]
- 48.Read SJ, Hirano T, Abbott DF, et al. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology. 1998;51(6):1617–1621. doi: 10.1212/wnl.51.6.1617. [DOI] [PubMed] [Google Scholar]
- 49.Ueda T, Sakaki S, Yuh WT, Nochide I, Ohta S. Outcome in acute stroke with successful intraarterial thrombolysis and predictive value of initial single-photon emission-computed tomography. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19(1):99–108. doi: 10.1097/00004647-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Ueda T, Hatakeyama T, Kumon Y, Sakaki S, Uraoka T. Evaluation of risk of hemorrhagic transformation in local intra-arterial thrombolysis in acute ischemic stroke by initial SPECT. Stroke; a journal of cerebral circulation. 1994;25(2):298–303. doi: 10.1161/01.str.25.2.298. [DOI] [PubMed] [Google Scholar]
- 51.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke; a journal of cerebral circulation. 2007;38(2):431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 52.Lovelock CE, Anslow P, Molyneux AJ, et al. Substantial observer variability in the differentiation between primary intracerebral hemorrhage and hemorrhagic transformation of infarction on CT brain imaging. Stroke; a journal of cerebral circulation. 2009;40(12):3763–3767. doi: 10.1161/STROKEAHA.109.553933. [DOI] [PubMed] [Google Scholar]
- 53.Campbell BCV, Christensen S, Butcher KS, et al. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2010;41(1):82–88. doi: 10.1161/STROKEAHA.109.562116. [DOI] [PubMed] [Google Scholar]
- 54.Lee S-H, Kim BJ, Ryu W-S, et al. White matter lesions and poor outcome after intracerebral hemorrhage: a nationwide cohort study. Neurology. 2010;74(19):1502–1510. doi: 10.1212/WNL.0b013e3181dd425a. [DOI] [PubMed] [Google Scholar]
- 55.Singer OC, Humpich MC, Fiehler J, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Annals of neurology. 2008;63(1):52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 56.Cho A-H, Kim JS, Kim S-J, et al. Focal fluid-attenuated inversion recovery hyperintensity within acute diffusion-weighted imaging lesions is associated with symptomatic intracerebral hemorrhage after thrombolysis. Stroke; a journal of cerebral circulation. 2008;39(12):3424–3426. doi: 10.1161/STROKEAHA.108.516740. [DOI] [PubMed] [Google Scholar]
- 57.Aviv RI, d’Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250(3):867–877. doi: 10.1148/radiol.2503080257. [DOI] [PubMed] [Google Scholar]
- 58.Bang OY, Saver JL, Alger JR, et al. Patterns and predictors of blood-brain barrier permeability derangements in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2009;40(2):454–461. doi: 10.1161/STROKEAHA.108.522847. [DOI] [PubMed] [Google Scholar]
- 59.Fiehler J, Remmele C, Kucinski T, et al. Reperfusion after severe local perfusion deficit precedes hemorrhagic transformation: an MRI study in acute stroke patients. Cerebrovascular diseases (Basel, Switzerland) 2005;19(2):117–124. doi: 10.1159/000083180. [DOI] [PubMed] [Google Scholar]
- 60.Hom J, Dankbaar JW, Soares BP, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR. American journal of neuroradiology. 2011;32(1):41–48. doi: 10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassner A, Thornhill R. Measuring the integrity of the human blood-brain barrier using magnetic resonance imaging. Methods in molecular biology (Clifton, N.J.) 2011;686:229–245. doi: 10.1007/978-1-60761-938-3_10. [DOI] [PubMed] [Google Scholar]
- 62.Gupta R, Phan CM, Leidecker C, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology. 2010;257(1):205–211. doi: 10.1148/radiol.10091806. [DOI] [PubMed] [Google Scholar]
- 63.Bolaños JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochimica et biophysica acta. 1999;1411(2–3):415–436. doi: 10.1016/s0005-2728(99)00030-4. [DOI] [PubMed] [Google Scholar]
- 64.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke; a journal of cerebral circulation. 1994;25(8):1658–1664. doi: 10.1161/01.str.25.8.1658. discussion 1664–5. [DOI] [PubMed] [Google Scholar]
- 65.Guo J, Zheng H-B, Duan J-C, et al. Diffusion tensor MRI for the assessment of cerebral ischemia/reperfusion injury in the penumbra of non-human primate stroke model. Neurological research. 2011;33(1):108–112. doi: 10.1179/016164110X12761752770177. [DOI] [PubMed] [Google Scholar]
- 66.Nour M, UCLA, Scalzo F, Liebeskind DS. Insight Into Human Ischemia Reperfusion Injury in Acute Stroke: Voxel-Based MRI Analysis of Tissue Fate. International journal of stroke: official journal of the International Stroke Society. 2013 [Google Scholar]
- 67.Granziera C, Ay H, Koniak SP, Krueger G, Sorensen AG. Diffusion tensor imaging shows structural remodeling of stroke mirror region: results from a pilot study. European neurology. 2012;67(6):370–376. doi: 10.1159/000336062. [DOI] [PubMed] [Google Scholar]
- 68.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 69.Lee RG, Van Donkelaar P. Mechanisms underlying functional recovery following stroke. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1995;22(4):257–263. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- 70.Steinberg BA, Augustine JR. Behavioral, anatomical, physiological aspects of recovery of motor function following stroke. Brain research. Brain research reviews. 1997;25(1):125–132. doi: 10.1016/s0165-0173(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 71.Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke; a journal of cerebral circulation. 2000;31(1):223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- 72.Seil FJ. Recovery and repair issues after stroke from the scientific perspective. Current opinion in neurology. 1997;10(1):49–51. [PubMed] [Google Scholar]
- 73.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. The New England journal of medicine. 2009;360(12):1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 74.Yamada I, Matsushima Y, Suzuki S. Moyamoya disease: diagnosis with three-dimensional time-of-flight MR angiography. Radiology. 1992;184(3):773–778. doi: 10.1148/radiology.184.3.1509066. [DOI] [PubMed] [Google Scholar]
- 75.Yamada I, Nakagawa T, Matsushima Y, Shibuya H. High-Resolution Turbo Magnetic Resonance Angiography for Diagnosis of Moyamoya Disease. Stroke. 2001;32(8):1825–1831. doi: 10.1161/01.str.32.8.1825. [DOI] [PubMed] [Google Scholar]
- 76.Yamada I, Suzuki S, Matsushima Y. Moyamoya disease: comparison of assessment with MR angiography and MR imaging versus conventional angiography. Radiology. 1995;196(1):211–218. doi: 10.1148/radiology.196.1.7784569. [DOI] [PubMed] [Google Scholar]
- 77.Demaerel P, De Ruyter N, Maes F, Velghe B, Wilms G. Magnetic resonance angiography in suspected cerebral vasculitis. European radiology. 2004;14(6):1005–1012. doi: 10.1007/s00330-004-2239-y. [DOI] [PubMed] [Google Scholar]