Abstract
Background
Genetic studies have implicated Disrupted-in-Schizophrenia-1 (DISC1) as a risk factor for a wide range of mental conditions, including schizophrenia. Since NMDA receptor (NMDAR) dysfunction has been strongly linked to the pathophysiology of these conditions, we examined whether the NMDAR is a potential target of DISC1.
Methods
DISC1 was knocked down with a small inference RNA. NMDAR-mediated currents were recorded and NMDAR expression was measured.
Results
We found that cellular knockdown of DISC1 significantly increased NMDAR currents in cortical cultures, which were accompanied by an increase in the expression of NMDAR subunit, GluN2A. NMDAR-mediated synaptic response in prefrontal cortical pyramidal neurons was also increased by DISC1 knockdown in vivo. The effect of DISC1 knockdown on NMDAR currents in cortical cultures was blocked by protein kinase A (PKA) inhibitor, occluded by PKA activator, and prevented by phosphodiesterase 4 (PDE4) inhibitor. Knockdown of DISC1 caused a significant increase of cAMP response element-binding protein (CREB) activity. Inhibiting CREB prevented the DISC1 deficiency-induced increase of NMDAR currents and GluN2A clusters.
Conclusions
Our results suggest that DISC1 exerts an important impact on NMDAR expression and function through a PDE4/PKA/CREB-dependent mechanism, which provides a potential molecular basis for the role of DISC1 in influencing NMDAR-dependent cognitive and emotional processes.
Keywords: DISC1, NMDA receptors, GluN2A, PKA, CREB, schizophrenia
Since the Disrupted in Schizophrenia 1 (DISC1) gene was identified at the breakpoint of a balanced t(1;11) translocation that segregates with major mental illnesses in a large Scottish pedigree (1), this molecule has been extensively studied as a promising molecular lead for schizophrenia and mood disorders (2). Animal models that perturb DISC1 have shown endophenotypes relevant to schizophrenia and depression (3–10). Furthermore, human brain imaging studies have found that common polymorphisms in the DISC1 gene are associated with the dysfunction of various critical brain circuits relevant to major mental illnesses (2).
To understand how DISC1 regulates neuronal functions, we need to determine the potential targets of DISC1 that are important in modulating human cognition and emotion. The complexity of DISC1 biology makes this task quite challenging (2). There are many DISC1 transcripts now known and their function is poorly understood at present (11). In addition, DISC1 is located in multiple subcellular domains, such as mitochondria, nucleus, and synapse, and the role of different variants in these regions is poorly understood (12,13). Previous studies have started to reveal the function of DISC1 in the developing brain, including neuronal migration, neurite outgrowth, and neurogenesis (14–17). From the network of protein-protein interactions around DISC1, DISC1 has also been implicated in processes of gene transcription, intracellular transport, and synaptic activity (18,19). Although DISC1 may be intimately linked to synapse function, the role of DISC1 in regulating synaptic proteins is just being understood and there are many gaps in our knowledge (20,21).
Convergent findings suggest that dysfunction of glutamatergic transmission, particularly aberrant NMDAR signaling, is a core pathology of mental disorders (22–24). In this study, we investigated whether DISC1 plays a role in regulating NMDARs. Understanding the synaptic functions of DISC1 may reveal important mechanistic insights into schizophrenia and related mental disorders (2).
Methods and Materials
Primary Neuronal Culture
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo. Rat cortical cultures were prepared as previously described (25,26). Briefly, frontal cortex was dissected from SD rat embryos (E18), and cells were dissociated using trypsin and titurated through a Pasteur pipette. The neurons were plated on coverslips coated with poly-L-lysine in DMEM with 10% fetal calf serum at a density of 1×105 cells/cm2. When neurons attached to the coverslip within 24 hr, the medium was changed to Neurobasal media with B27 supplement. Neurons were maintained for 2–3 weeks before being used for recordings.
Small Interfering RNA
To knockdown the expression of DISC1 in cultured neurons, we used a short-hairpin RNA (shRNA) against DISC1 tagged with eGFP (Dharmacon RNA technologies, Lafayette, CO), which has been shown to be a strong suppressor of DISC1 (15,20). Cultured cortical neurons (DIV 12–14) were transfected with a control (scrambled) shRNA or with DISC1 shRNA using the Lipofectamine 2000 method. To suppress CREB expression or function, we transfected cultures with CREB-1 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) or dominant negative CREB (DN-CREB) (a gift from Dr. David Ginty at Johns Hopkins University). Neurons were used for experiments 2 days after transfection.
Antibody Production
Rat DISC1 (C-terminal amino acids 598–854) was cloned into pET15b expressed in E. coli BL21 and purified similarly to what was described before for human DISC1 (598–854) (27). See Supplementary Methods for details.
Lentiviral Production and Lentivirus Infection in vitro and in vivo
See Supplementary Methods for details of DISC1 shRNA lenti virus production and infection.
Western blotting
See Supplementary Methods for details.
Electrophysiological Recordings
Recordings of whole-cell ion channel currents in cultured neurons (DIV 14–17) used standard voltage-clamp techniques (25,28,31). See Supplementary Methods for details.
Biochemical Measurement of Surface Proteins
Surface NMDA receptors were detected as described previously (28,32,33). See Supplementary Methods for details.
Immunocytochemical Staining
See Supplementary Methods for details.
Statistics
All data are expressed as the mean ± SEM. Experiments with two groups were analyzed statistically using unpaired Student's t tests. Experiments with more than two groups were subjected to one-way ANOVA, followed by post hoc Tukey tests.
Results
Knockdown of DISC1 induces an increase of NMDAR-mediated currents in vitro
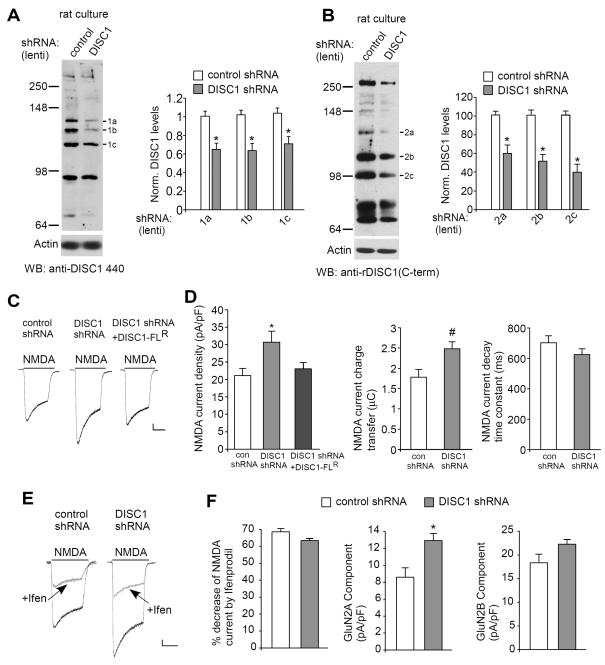
Previous studies indicate that the chromosomal translocation causes a reduction of DISC1 expression (34), so we took a strategy to reduce DISC1 levels using RNA interference (similar to a haploinsufficent DISC1 model) and examined its influences on NMDA receptors. As shown in Fig. 1A, the expression of endogenous DISC1, which was recognized by anti-DISC1 440 as three major bands, were markedly diminished in neuronal cultures infected with DISC1 shRNA lentivirus (band-1a: 0.65 ± 0.07 of control; band-1b: 0.64 ± 0.08 of control; band-1c: 0.71 ± 0.08 of control, n = 8, p < 0.01, t-test), similar to previous reports (20,21,29,30).
Fig. 1.
Knockdown of DISC1 increases NMDAR current density and synaptic response in vitro. A, B, Immunoblots and quantification analysis of DISC1 (A: anti-DISC1 440; B: anti-rDISC1 C-term) in rat cortical cultures infected with control shRNA or DISC1 shRNA lentivirus. *: p < 0.01. C, Representative NMDA (100 μM)-elicited current traces in cultured cortical neurons transfected with control shRNA, DISC1 shRNA or DISC1 shRNA plus DISC1-FLR, a full-length DISC1 rescue construct that is insensitive to the DISC1 shRNA. Scale bar: 100 pA, 1 s. D, Cumulative data (mean ± SEM) of NMDAR current density, charge transfer and decay time constant in neuronal cultures with different transfections. *: p < 0.01. #: p < 0.05. E, Representative NMDAR current traces in the absence or presence of ifenprodil (10 μM, a GluN2B blocker) in cultured cortical neurons transfected with control shRNA or DISC1 shRNA. Scale bar: 100 pA, 1 s. F, Cumulative data (mean ± SEM) of GluN2A and GluN2B components in neuronal cultures with different transfections. *: p < 0.01.
To further confirm the effectiveness of DISC1 knockdown, we used a newly developed DISC1 antibody, anti-rDISC1 C-term. The specificity of this DISC1 antibody was first examined with brain lysates from DISC1 knockout rats, which possess a 20 bp deletion within exon 5 of DISC1, resulting in an early stop codon in exon 6 (Product #: TGRA3640, SAGE® Labs, Sigma). As shown in Fig. S1 in Supplement 1, the 3 bands detected with anti-rDISC1 C-term in wild-type rats were largely lost in DISC1 knockout rats. When anti-rDISC1 C-term was used to measure DISC1 expression in rat primary cultures infected with DISC1 shRNA lentivirus, the 3 bands were also significantly diminished (Fig. 1B, band-2a: 0.60 ± 0.09 of control; band-2b: 0.52 ± 0.07 of control; band-2c: 0.40 ± 0.08 of control, n = 5, p < 0.01, t-test).
In DISC1 shRNA-transfected cortical cultures, the whole-cell NMDAR current density (pA/pF) was significantly increased (Fig. 1C and 1D, control shRNA: 21.1 ± 2.1, n = 13; DISC1 shRNA: 30.3 ± 3.1, n = 15, p < 0.05, ANOVA). To rule out the possibility of “off-target” effects of the DISC1 shRNA, we performed control experiments where we transfected neurons with a DISC1 rescue construct (DISC1R) that is insensitive to the shRNA (20). As shown in Fig. 1C and 1D, the enhancing effect of DISC1 shRNA on NMDAR current density was prevented by DISC1R (23.0 ± 1.8, n = 15, Fig. 1C), suggesting that the finding is due to the selective knockdown of DISC1.
The changes in NMDAR channel properties were also investigated. As shown in Fig. 1D, NMDAR current charge transfer (μC) was significantly increased in DISC1 shRNA-transfected neurons (control shRNA: 1.8 ± 0.2, n = 14; DISC1 shRNA: 2.5 ± 0.2, n = 14, p < 0.05, t-test), and NMDAR current decay time constant (ms) was slightly decreased (control shRNA: 703.5 ± 45.3, n = 14; DISC1 shRNA: 624.4 ± 39.3, n = 14, p > 0.05, t-test, Fig. 1D).
To determine which subpopulations of NMDARs were targeted by DISC1 shRNA, we applied the selective GluN2B inhibitor ifenprodil (10 μM). As shown in Fig. 1E and 1F, ifenprodil caused a similar reduction of the whole-cell NMDAR current amplitudes in both groups (control shRNA: 68.6 ± 1.5%, n = 10; DISC1 shRNA: 63.4 ± 1.2%, n = 12, p > 0.05, t-test). The current density (pA/pF) mediated by GluN2A component (ifenprodil-insensitive) was markedly increased in neurons infected with DISC1 shRNA lentivirus (control shRNA: 8.6 ± 1.1, n = 10; DISC1 shRNA: 12.9 ± 0.8, n = 12, p < 0.01, t-test), while the current density (pA/pF) mediated by GluN2B component (ifenprodil-sensitive) was largely unchanged (control shRNA: 18.3 ± 1.8, n = 10; DISC1 shRNA: 22.3 ± 1.0, n = 12, p > 0.05, t-test). These results suggest that the enhanced NMDAR response induced by DISC1 knockdown is mainly mediated by GluN2A subunits.
Knockdown of DISC1 induces an increase of NMDAR-EPSC in vivo
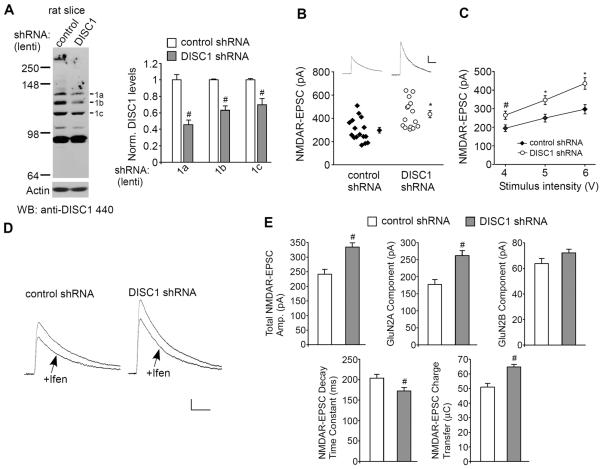
There are certain experimental limitations with these observations, namely the changes seen with in vitro DISC1 knockdown may be a phenomenon specific to neuronal cultures and the NMDA-elicited whole-cell current is mediated by both synaptic and extrasynaptic NMDARs. It is necessary to know whether similar changes also happen at the level of synaptic NMDAR responses with in vivo DISC1 knockdown. To address this, we performed stereotaxic injections of DISC1 shRNA lentivirus into the rat medial PFC (28). The in vivo knockdown effectiveness was confirmed with anti-DISC1 440 (Fig. 2A, band-1a: 0.46 ± 0.06 of control; band-1b: 0.62 ± 0.06 of control; band-1c: 0.70 ± 0.07 of control, n = 4, p < 0.05, t-test).
Fig. 2.
Knockdown of DISC1 increases NMDAR-EPSC in PFC pyramidal neurons in vivo. A, Immunoblots and quantification analysis of DISC1 (detected with anti-DISC1 440) in rat PFC slices taken from animals with stereotaxical injections of control shRNA or DISC1 shRNA lentivirus. #: p < 0.05. B, Dot plots showing the amplitude of NMDAR-EPSC in control shRNA or DISC1 shRNA lentivirus-infected PFC pyramidal neurons. The average data are also shown. *: p < 0.01. Inset: representative NMDAR-EPSC traces. Scale bar: 100 pA, 200 ms. C, Summarized input-output curves of NMDAR-EPSC in pyramidal neurons from rats with the PFC injection of control shRNA or DISC1 shRNA lentivirus. *: p < 0.01, #: p < 0.05. D, Representative NMDAR-EPSC traces in the absence or presence of ifenprodil (10 μM) in PFC pyramidal neurons infected with control shRNA or DISC1 shRNA lentivirus. Scale bar: 50 pA, 100 ms. E, Cumulative data (mean ± SEM) of total NMDAR-EPSC amplitude, GluN2A or GluN2B components, decay time constant and charge transfer in neurons with different viral infections. #: p < 0.05.
We then examined the impact of DISC1 knockdown in vivo on NMDAR-mediated excitatory postsynaptic currents (EPSC) in PFC slices. As shown in Fig. 2B, the amplitude of NMDAR-EPSC evoked by the same stimulus was significantly bigger in PFC pyramidal neurons with DISC1 knockdown (control shRNA: 297.2 ± 24.7 pA, n = 16; DISC1 shRNA: 435.5 ± 31.2 pA, n = 15; p < 0.01, t-test). DISC1 knockdown also caused a substantial increase of the input/output curves of NMDAR-EPSC induced by a series of stimulus intensities (Fig. 2C, 35–45% increase, p < 0.01, ANOVA, n = 15–16 per group). The amplitudes of GluN2A-mediated NMDAR-EPSC (ifenprodil-insensitive) were significantly increased in PFC pyramidal neurons infected with DISC1 shRNA lentivirus (Fig. 2D and 2E, control shRNA: 177.5 ± 13.8, n = 8; DISC1 shRNA: 262.2 ± 14.7, n = 9, p < 0.05, t-test), while GluN2B-mediated NMDAR-EPSC (ifenprodil-sensitive) was largely unchanged (control shRNA: 63.8 ± 3.8, n = 8; DISC1 shRNA: 72.2 ± 2.7, n = 9; p > 0.05, t-test). In addition, a significant reduction of the decay time constant (control shRNA: 203.1 ± 8.9 ms, n = 8; DISC1 shRNA: 172.2 ± 8.7 ms, n = 9; p < 0.05, t-test) and a significant increase of the transfer charge (control shRNA: 50.9 ± 2.6 μC, n = 8; DISC1 shRNA: 64.7 ± 1.8 μC, n = 9; p < 0.05, t-test) of NMDAR-EPSC were observed in neurons with DISC1 knockdown. It suggests that the enhanced synaptic NMDAR response induced by DISC1 knockdown is mediated by GluN2A subunits.
Knockdown of DISC1 increases the level of total and surface GluN2A subunits
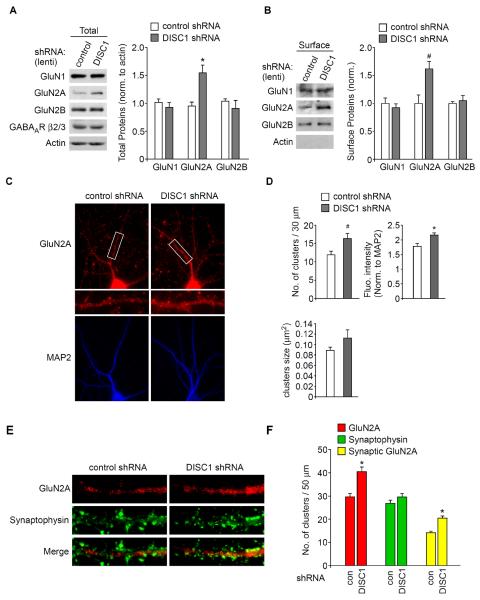
The enhancement of NMDAR responses by DISC1 knockdown could result from increased NMDAR expression or surface delivery/stability. Thus, we examined NMDAR subunits in neurons with DISC1 knockdown. As shown in Fig. 3A and 3B, the total and surface levels of GluN2A were significantly elevated in PFC cultures infected with DISC1 shRNA lentivirus (total GluN2A: 1.7 ± 0.2 fold of control; n = 5, p < 0.01, t-test; surface GluN2A: 1.6 ± 0.1 fold of control; n = 6, p < 0.05, t-test). The levels of GluN1, GluN2B, or GABAAR β2/3 subunits were largely unchanged (Fig. 3A and 3B).
Fig. 3.
Knockdown of DISC1 increases NMDAR subunit GluN2A expression. A, Immunoblots and quantification analysis of GluN1, GluN2A, GluN2B, GABAAR β2/3 and actin in cultured cortical neurons infected with control shRNA or DISC1 shRNA lentivirus. *: p < 0.01. B, Immunoblots and quantification analysis of surface GluN1, GluN2A, and GluN2B in cultured cortical neurons infected with control shRNA or DISC1 shRNA lentivirus. Actin was used as a control. *: p < 0.05. C, Immunocytochemical images of NMDAR GluN2A subunits and MAP2 in cortical cultures transfected with a control shRNA or DISC1 shRNA. D, Quantitative analysis of GluN2A clusters (density, intensity, size) along the dendrites in control shRNA or DISC1 shRNA-transfected neurons. *: p < 0.01, #: p < 0.05. E, F, Immunocytochemical images (E) and quantitative analysis (F) of synaptic GluN2A clusters (synaptophysin co-localized, yellow puncta) in cortical cultures transfected with control shRNA or DISC1 shRNA. *: p < 0.01.
Immunocytochemical studies (Fig. 3C and 3D) also indicated that neurons transfected with DISC1 shRNA showed a significant increase in the GluN2A cluster density (number of clusters/30 μm) (control shRNA: 11.9 ± 1.0, n = 26; DISC1 shRNA: 16.4 ± 1.4, n = 22, p < 0.05, t-test) and GluN2A cluster intensity (control shRNA: 1.8 ± 0.1, n = 26; DISC1 shRNA: 2.2 ± 0.1, n = 22, p < 0.01, t-test). The size (μm2) of GluN2A clusters was not significantly affected by DISC1 shRNA (control shRNA: 0.09 ± 0.01, n = 26; DISC1 shRNA: 0.1 ± 0.02, n = 22, p > 0.05, t-test).
To provide more direct evidence on DISC1 regulation of NMDARs at synapses, we measured synaptic NMDAR clusters, as indicated by GluN2A co-localized with the synaptic marker synaptophysin. As shown in Fig. 3E and 3F, in DISC1 shRNA-transfected neurons, a significant increase of synaptic GluN2A (yellow puncta) cluster density (number of clusters/50 μm) was observed (control shRNA: 14.1 ± 1.6, n = 36; DISC1 shRNA: 20.4 ± 1.7, n = 26, p < 0.01, t test), whereas synaptophysin clusters were not altered (control shRNA: 26.9 ± 1.9, n = 36; DISC1 shRNA: 29.5 ± 2.2, n = 26, p > 0.05, t test). The increased GluN2A synaptic expression could underlie the increased NMDAR response by DISC1 knockdown.
Finally, to test whether DISC1 regulation of NMDARs is via a direct physical interaction, we performed co-immunoprecipitation experiments. We did not find any DISC1 co-precipitating with NMDA NR1 subunits from rat cortical slices (Fig. S2 in Supplement 1).
DISC1 regulation of NMDARs is dependent upon a PDE4/PKA/CREB dependent pathway
Next we examined the potential mechanism underlying DISC1 regulation of NMDARs. It has been shown that DISC1 interacts with phosphodiesterase 4 (PDE4) isoforms (34), an enzyme that inactivates cAMP and orchestrates downstream signaling via cAMP effectors such as protein kinase A (PKA) (35). We hypothesize that DISC1 knockdown may change cAMP-PKA signaling via PDE4 modification.
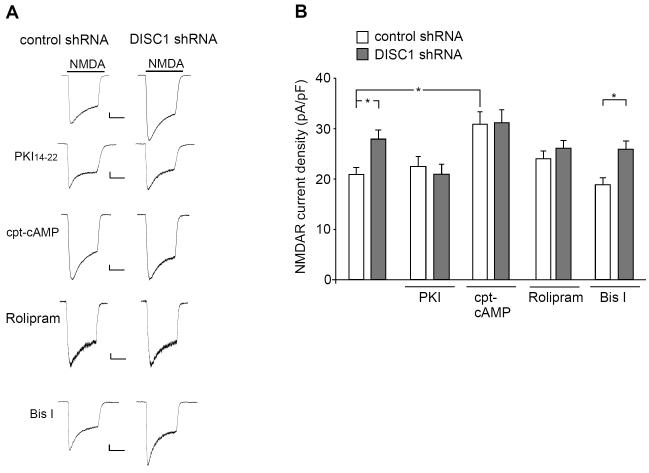
To test this, we first examined the involvement of PKA in DISC1 regulation of NMDARs. Cultured neurons were treated with a specific PKA inhibitor or PKA activator during DISC1 shRNA transfection (48-hr). As shown in Fig. 4A and 4B, DISC1 shRNA failed to increase NMDAR current density (pA/pF) in the presence of the PKA inhibitor PKI (0.2 μM) (control shRNA: 22.5 ± 2.0, n = 10; DISC1 shRNA: 20.9 ± 1.9, n = 10, p > 0.05, ANOVA), which was significantly different from untreated neurons (control shRNA: 20.7 ± 1.3, n = 20; DISC1 shRNA: 27.7 ± 1.8, n = 23, p < 0.05, ANOVA). Treatment with the PKA activator 8-cpt-cAMP (50 μM) induced a significant increase of NMDAR current density (control shRNA: 23.7 ± 1.6, n = 30; control shRNA+cAMP: 30.9 ± 2.5, n = 22, p < 0.01, ANOVA), and occluded the enhancing effect of DISC1 shRNA (31.2 ± 2.6, n = 17). Moreover, in the presence of rolipram (0.1 μM, 48-hr), a selective inhibitor of PDE4, particularly the PDE4B subtype (36,37), DISC1 shRNA failed to enhance NMDAR current density (control shRNA: 22.7 ± 1.6, n = 12; DISC1 shRNA: 22.5 ± 2.6, n = 9, p > 0.05, ANOVA).
Fig. 4.
PKA activation is required for DISC1 regulation of NMDARs. A, Representative whole-cell NMDAR current traces in cultured cortical neurons transfected with a control shRNA and DISC1 shRNA in the absence or presence of PKI (0.2 μM, a PKA inhibitor), 8-cpt-cAMP (50 μM, a PKA activator), rolipram (0.1 μM, a PDE4 inhibitor), and Bisindolylmaleimide I (Bis I, 0.5 μM, a PKC inhibitor). Scale bar: 100 pA, 1 s. B, Cumulative data (mean ± SEM) of NMDAR current density in transfected neurons with different treatments. *: p < 0.05.
Since PKC has been shown to enhance NMDAR-mediated current (38), we also tested its role in DISC1 regulation of NMDARs. As shown in Fig. 4A and 4B, in the presence of the specific PKC inhibitor Bisindolylmaleimide I (0.5 μM, 48-hr), the enhancing effect of DISC1 knockdown on NMDAR current density (pA/pF) was intact (control shRNA: 18.8 ± 1.4, n = 22; DISC1 shRNA: 25.5 ± 1.6, n = 19, p < 0.05, ANOVA). Taken together, these results suggest that DISC1 shRNA increases NMDAR currents via a mechanism at least partially dependent on elevated PKA activity.
To find out whether the DISC1 knockdown-induced increase of NMDAR currents is due to PKA phosphorylation of NMDAR subunits, we examined phospho-S897GluN1 levels (39). As shown in Fig. S3 (Supplement 1), pGluN1 levels were largely unchanged in neuronal cultures infected with DISC1 shRNA lentivirus (1.0 ± 0.2 fold of control, n = 8, p > 0.05, t-test).
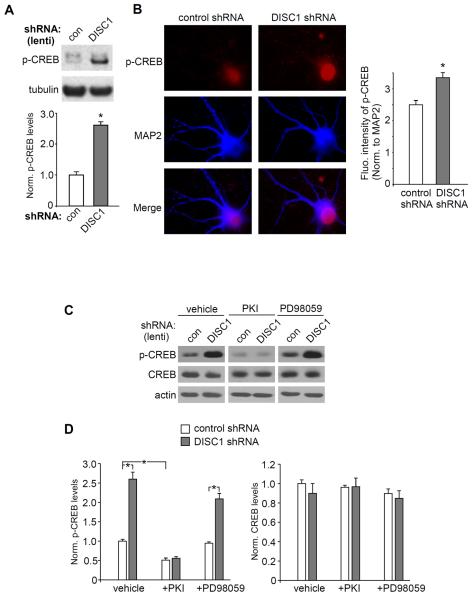
One of the key downstream targets of PKA is the cAMP response element-binding protein (CREB). CREB is phosphorylated at residue Serine-133 (S133) by multiple protein kinases, including PKA and Ca2+/calmodulin-dependent protein kinases (40). Once CREB is phosphorylated, it is translocated from the cytosol to the nucleus, binding to the cAMP-response element (CRE) on the promoter region of many target genes to modulate their transcription. Our Western blot assays indicate that the level of Ser133phospho-CREB (active form of CREB) was significantly increased in PFC cultures infected with DISC1 shRNA lentivirus (Fig. 5A, 2.6 ± 0.2 fold of control, n = 3, p < 0.01, t-test). To further test the impact of DISC1 knockdown on CREB activation, immunocytochemical experiments were performed in cultured PFC neurons transfected with DISC1 shRNA. As shown in Fig. 5B, the nuclear p-CREB staining intensity was significantly increased in DISC1 shRNA-transfected neurons (control shRNA: 2.5 ± 0.1, n = 27; DISC1 shRNA: 3.3 ± 0.2, n = 45, p < 0.01, t-test), suggesting that DISC1 knockdown indeed induced CREB activation and nuclear translocation.
Fig. 5.
DISC1 knockdown induces an increase of CREB activity. A, Immunoblots and quantification showing the level of p-CREB in cortical cultures infected with control shRNA or DISC1 shRNA lentivirus. Tubulin was used as a control. *: p < 0.01. B, Immunocytochemical images and quantitative analysis of p-CREB in PFC cultures transfected with a control shRNA or DISC1 shRNA. MAP2 was co-stained. *: p < 0.01. C, D, Immunoblots and quantification showing the level of p-CREB and CREB in cortical cultures infected with control shRNA or DISC1 shRNA lentivirus in the presence of vehicle, PKI (0.2 μM) or PD98059 (20 μM, an ERK inhibitor). *: p < 0.001.
We further investigated the signaling molecules involved in DISC1 regulation of CREB activity. As shown in Fig. 5C and 5D, PKI treatment (0.2 μM, 48-hr) decreased pSer133-CREB level and blocked the enhancing effect of DISC1 shRNA lentivirus (PKI+control shRNA: 0.5 ± 0.06 fold of control, n = 11, PKI+DISC1 shRNA: 0.6 ± 0.05 fold of control, n = 9), while treatment with the ERK inhibitor, PD98059 (20 μM, 48-hr), was ineffective (PD98059+control shRNA: 0.9 ± 0.04 fold of control, n = 15; PD98059+DISC1 shRNA: 2.1 ± 0.14 fold of control, n = 6, p < 0.001, ANOVA). Treatment with 8-cpt-cAMP (50 μM, 48-hr) also increased the pCREB level (Fig. S4 in Supplement 1, 1.6 ± 0.1 fold of control; n = 5, p < 0.01, t-test). CREB levels were largely unchanged by these treatments. These results suggest that DISC1 knockdown induces the up-regulation of CREB activity via a PKA-dependent mechanism.
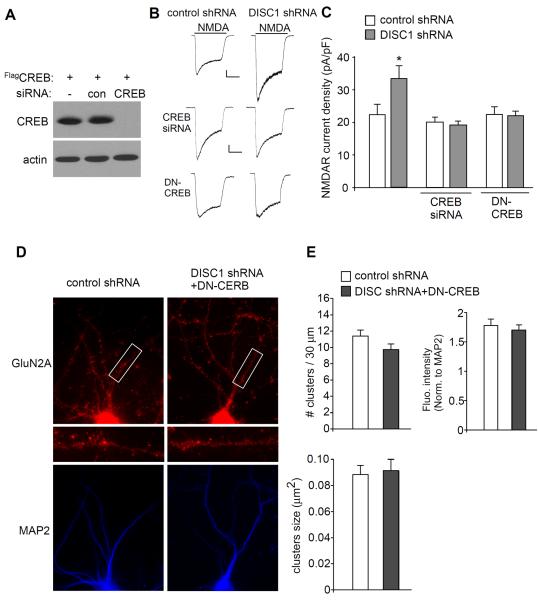
To directly test the role of CREB in DISC1 regulation of NMDARs, we inhibited CREB function by either knocking down its expression (Fig. 6A) or by transfecting a dominant-negative CREB (DN-CREB) construct (S133A mutation). As shown in Fig. 6B and 6C, the enhancing effect of DISC1 shRNA on NMDAR current density (pA/pF) was lost in the presence of CREB siRNA (control shRNA+CREB siRNA: 20.1 ± 1.5, n = 17; DISC1 shRNA+CREB siRNA: 19.3 ± 1.1, n = 15, p > 0.05, ANOVA), or DN-CREB (control shRNA+DN-CREB: 22.5 ± 2.3, n = 11; DISC1 shRNA+DN-CREB: 22.1 ± 1.4, n = 15, p > 0.05, ANOVA), which was significantly different from neurons without CREB inhibition (control shRNA: 22.4 ± 3.2, n = 7; DISC1 shRNA: 33.5 ± 4.1, n = 7, p < 0.05, ANOVA). Immunocytochemical studies (Fig. 6D and 6E) also indicated that DN-CREB blocked the effect of DISC1 shRNA on GluN2A cluster density (control shRNA: 11.4 ± 0.7, n = 21; DISC1 shRNA+DN-CREB: 9.7 ± 0.7, n = 23, p > 0.05, t-test) and GluN2A cluster intensity (control shRNA: 1.8 ± 0.1, n = 21; DISC1 shRNA+DN-CREB: 1.7 ± 0.1, n = 23, p > 0.05, t-test). The size (μm2) of GluN2A clusters was not significantly affected by DISC1 knockdown (control shRNA: 0.089 ± 0.01, n = 21; DISC1 shRNA+DN-CREB: 0.091 ± 0.01, n = 23, p > 0.05, t-test). Taken together, these results suggest that DISC1 depletion drives an enhanced NMDAR response, at least in part via a mechanism depending on PKA/CREB activation.
Fig. 6.
Inhibiting CREB blocks DISC1 regulation of NMDA receptors. A, Western blots in HEK293 cells transfected with FLAG-tagged CREB in the absence or presence of a control siRNA or CREB siRNA. B, Representative whole-cell NMDAR current traces in cultured cortical neurons transfected with a control shRNA or DISC1 shRNA in the absence or presence of CREB siRNA or DN-CREB. Scale bar: 100 pA, 1 s. C, Cumulative data (mean ± SEM) showing NMDAR current density in neurons with different transfections. *: p < 0.05. D, Immunocytochemical images of GluN2A subunits and MAP2 in cortical cultures transfected with a control shRNA or co-transfected DN-CREB with DISC1 shRNA. E, Quantitative analysis of GluN2A clusters (density, intensity, size) along the dendrites in transfected neurons.
Finally, we examined the potential downstream target of CREB involved in DISC1 regulation of NMDARs. BDNF is a possible candidate, because CREB activation induces the increased expression of BDNF, which is essential for neuronal maturation (41). However, no significant increase was observed on BDNF expression by DISC1 knockdown (Fig. S5 in Supplement 1, 0.9 ± 0.1 fold of control, n = 8, p > 0.05, t-test).
Discussion
Since the DISC1 gene was identified, extensive molecular, cell biological, animal model and human genotype-phenotype studies have been conducted to address multiple roles and mechanisms of DISC1 in the brain (9,42–44). Because DISC1 is thought to drive a range of endophenotypes that underlie major mental conditions (45), elucidating the precise biological functions of DISC1 has become an intensely studied topic (2). In recent years the possible role of DISC1 in regulating synapse formation and function has gained critical experimental support. Our own work has shown that DISC1 plays a critical role in regulating excitatory synaptic function through the molecules kalirin and TNIK (20,21). Building on these studies, we have now gone on to show that DISC1 regulates NMDAR function. Our results suggest that loss of DISC1 in vitro leads to increased NMDAR current densities in cortical cultures. In vivo knockdown of DISC1 also results in potentiated NMDAR synaptic responses in layer V pyramidal neurons from prefrontal cortical slices. Whether this effect of DISC1 knockdown on NMDARs is universal in all cortical regions/layers/neuronal types awaits further investigation. Given the critical role of NMDAR in various mental disorders (22–24,46,47), our results could provide a potential molecular mechanism underlying the behavioral phenotypes seen in animals and humans with DISC1 genetic variations.
The functional NMDAR complex is composed of two GluN1 and two GluN2A/B subunits. The GluN1 subunit is normally present in excess, so the determining factor for channel abundance is the GluN2 subunit (48). The DISC1 knockdown-induced enhancement of NMDAR-mediated current is accompanied by a selective increase of GluN2A protein expression and GluN2A synaptic clusters, indicating that DISC1 deficiency leads to an increased number of GluN1/GluN2A channels, which consequently elevates NMDAR responses. This is the first report of such an effect, but there have been previous reports implicating a role in NMDAR regulation by DISC1. For example, DISC1 exon 2 and 3 knock-out mice were shown to display a modified LTP response, suggestive of altered NMDAR function (49).
Our data show that the increased NMDAR current densities in DISC1 deficient cells are caused by the CREB-dependent elevation of GluN2A expression. Putative cAMP response elements (CRE) have been found in the promoter of GluN2A (50), and the activity-dependent developmental increases in GluN2A is mediated by a PKA/CREB pathway (51). It is known that GluN2A and GluN2B, which have distinct synaptic localizations and channel kinetics, play different roles in synaptic plasticity (52,53). A recent report showed that forebrain-specific over-expression of GluN2A led to deficits in certain forms of LTD and long-term memory (54). Thus, the DISC1 knockdown-induced selective increase in GluN2A could lead to aberrant NMDAR-dependent synaptic plasticity and cognitive processes.
DISC1 has been shown to interact with many proteins, and its interactome has provided a powerful framework to understand DISC1 function from initially an in silico perspective to confirmation in cellular and in vivo contexts (2,18). One of the critical binding partners is the cyclic-AMP phosphodiesterase PDE4 that hydrolyses cAMP specifically and down-regulates cAMP-dependent pathways including PKA (34,55,56) and the transcription factor ATF4/CREB2 that controls gene expression (19). DISC1 was thought to sequester PDE4B in resting cells and release it in an activated state in response to elevated cAMP (34). PDE4 isoforms other than PDE4B can also be sequestered by DISC1, and these are not dynamically released, probably because they bind in different fashions to DISC1 (56), which is consistent with the hypothesis that targeted PDE4 family proteins are involved in the control of spatially defined signaling complexes (35). Previous studies have shown that DISC1 mutation (truncation) leads to the reduction of PDE4B expression and elevated cAMP-PKA signaling (57). Mice with mutant DISC1 proteins, which exhibit reduced binding of DISC1 to PDE4B, have lower PDE4B activity (4). Consistently, our data suggest that DISC1 knockdown results in the upregulated PKA-CREB signaling, which is likely via the inhibition of PDE4 function.
There is likely to be additional complexity to the DISC1-NMDA interaction though. Recently DISC1 has been shown to bind and regulate serine-racemase (SR) in astrocytes (58). The primary function of SR is to catalyze the conversion of L-serine to D-serine. D-serine is thought to the obligatory co-agonist of the NMDAR in most brain regions. This could potentially have some role in our recording experiments. In addition, it has been reported that there is a loss of DISC1 from the synapse in GluN1 knockdown mice (29). It is clear there is a complex but critical partnership between the NMDAR and DISC1. The role we have carved out for the GluN2A subunit could be critical in this.
Conclusion
In summary, we have revealed how DISC1 regulates the NMDA receptor, a key synaptic target involved in cognitive and emotional processes under normal and pathological conditions. Our results could help to understand the synaptic functions of DISC1 in neurons and may clarify the role of DISC1 in schizophrenia and related psychiatric disorders (2,59–61).
Supplementary Material
Acknowledgments
We would like to thank Xiaoqing Chen for her excellent technical support. This work was supported by NIH grants to Z.Y. (MH-084233 and MH-085774) and A.S. (MH-094268 and MH-069853), and NEURON-ERANET DISCover / BMBF 01EW1003 to C.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–23. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 2.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A. 2006;103:3693–7. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andradé M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lönnqvist J, Huttunen MO, Kaprio J, Trachtenberg JT, Silva AJ, Cannon TD. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104:18280–5. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–86. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 8.Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, Chatzi C, He S, Mackie I, Brandon NJ, Marquis KL, Day M, Hurko O, McCaig CD, Riedel G, St Clair D. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, Hiyama H, Huang B, Kohda K, Noda Y, O'Donnell P, Nakajima K, Sawa A, Nabeshima T. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–9. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly MP, Brandon NJ. Taking a bird's eye view on a mouse model review: a comparison of findings from mouse models targeting DISC1 or DISC1-interacting proteins. Future Neurology. 2011;6(5):661–77. [Google Scholar]
- 11.Newburn EN, Hyde TM, Ye T, Morita Y, Weinberger DR, Kleinman JE, Lipska BK. Interactions of human truncated DISC1 proteins: implications for schizophrenia. Transl Psychiatry. 2011;1:e30. doi: 10.1038/tp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James R, Adams RR, Christie S, Buchanan SR, Porteous DJ, Millar JK. Disrupted in Schizophrenia 1 (DISC1) is a multicompartmentalized protein that predominantly localizes to mitochondria. Mol Cell Neurosci. 2004;26:112–122. doi: 10.1016/j.mcn.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructuraly level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 14.Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, Okawa M, Yamada N, Hatten ME, Snyder SH, Ross CA, Sawa A. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A. 2003;100:289–94. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, Ross CA, Hatten ME, Nakajima K, Sawa A. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 16.Ishizuka K, Kamiya A, Oh EC, Kanki H, Seshadri S, Robinson JF, Murdoch H, Dunlop AJ, Kubo K, Furukori K, Huang B, Zeledon M, Hayashi-Takagi A, Okano H, Nakajima K, Houslay MD, Katsanis N, Sawa A. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011;473:92–6. doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, Feighery E, Lu B, Rujescu D, St Clair D, Christian K, Callicott JH, Weinberger DR, Song H, Ming GL. Interplay between DISC1 and GABA Signaling Regulates Neurogenesis in Mice and Risk for Schizophrenia. Cell. 2012;148:1051–64. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in schizophrenia 1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 19.Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K, Shimoda M, Toda H, Sawamura-Yamamoto T, Makuch LA, Hayashi A, Ishizuka K, Cascella NG, Kamiya A, Ishida N, Tomoda T, Hai T, Furukubo-Tokunaga K, Sawa A. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry. 2008;13:1138–1148. doi: 10.1038/mp.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Ishizuka K, Makino Y, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Regulation of spine dynamics of glutamate synapse by Disrupted in Schizophrenia-1 (DISC1) Nature Neuroscience. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Charych EI, Pulito VL, Lee JB, Graziane NM, Crozier RA, Revilla-Sanchez R, Kelly MP, Dunlop AJ, Murdoch H, Taylor N, Xie Y, Pausch M, Hayashi-Takagi A, Ishizuka K, Seshadri S, Bates B, Kariya K, Sawa A, Weinberg RJ, Moss SJ, Houslay MD, Yan Z, Brandon NJ. The psychiatric disease risk factors DISC1 and TNIK interact to regulate synapse composition and function. Mol Psychiatry. 2011;16:1006–23. doi: 10.1038/mp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharm. Toxicol. 2002;42:165–79. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 23.Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–16. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 24.Kantrowitz J, Javitt DC. Glutamatergic transmission in schizophrenia: from basic research to clinical practice. Curr Opin Psychiatry. 2012;25:96–102. doi: 10.1097/YCO.0b013e32835035b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J, Liu W, Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. J. Biol. Chem. 2010;285:26369–76. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottis P, Bader V, Trossbach S, Kretzschmar H, Michel M, Leliveld SR, Korth C. Convergence of two independent mental disease genes on the protein level: recruitment of dysbindin to cellinvasive DISC1 aggresomes. Biol Psychiatry. 2011;70:604–10. doi: 10.1016/j.biopsych.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–77. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey AJ, Milenkovic M, Oliveira AF, Escobedo-Lozoya Y, Seshadri S, Salahpour A, Sawa A, Yasuda R, Caron MG. Impaired NMDA receptor transmission alters striatal synapses and DISC1 protein in an age-dependent manner. Proc Natl Acad Sci U S A. 2011;108:5795–800. doi: 10.1073/pnas.1012621108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bader V, Tomppo L, Trossbach SV, Bradshaw NJ, Prikulis I, Leliveld SR, Lin CY, Ishizuka K, Sawa A, Ramos A, Rosa I, García Á , Requena JR, Hipolito M, Rai N, Nwulia E, Henning U, Ferrea S, Luckhaus C, Ekelund J, Veijola J, Järvelin MR, Hennah W, Korth C. Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits. Hum Mol Genet. 2012;21:4406–18. doi: 10.1093/hmg/dds273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyszkiewicz JP, Gu Z, Wang X, Cai X, Yan Z. Group II metabotropic glutamate receptors enhance NMDA receptor currents via a protein kinase C-dependent mechanism in pyramidal neurons of prefrontal cortex. J Physiol (Lond) 2004;554:765–777. doi: 10.1113/jphysiol.2003.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. USA. 2009;106:14075–9. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu Z, Liu W, Wei J, Yan Z. Regulation of NMDA receptors by metabotropic glutamate receptor 7. J. Biol. Chem. 2012;287:10265–75. doi: 10.1074/jbc.M111.325175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 35.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–59. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS drug reviews. 2001;7:387–98. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y, Jover-Menqual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci U S A. 2006;103:19902–7. doi: 10.1073/pnas.0609924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–66. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 40.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–61. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 41.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–26. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 42.Singh KK, De Rienzo G, Drane L, Mao Y, Flood Z, Madison J, Ferreira M, Bergen S, King C, Sklar P, Sive H, Tsai LH. Common DISC1 polymorphisms disrupt Wnt/GSK3β signaling and brain development. Neuron. 2011;72:545–58. doi: 10.1016/j.neuron.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. J Neurosci. 2012;32:738–45. doi: 10.1523/JNEUROSCI.5036-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou M, Li W, Huang S, Song J, Kim JY, Tian X, Kang E, Sano Y, Liu C, Balaji J, Wu S, Zhou Y, Zhou Y, Parivash SN, Ehninger D, He L, Song H, Ming GL, Silva AJ. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron. 2013;77:647–54. doi: 10.1016/j.neuron.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porteous DJ, Millar JK, Brandon NJ, Sawa A. DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol Med. 2011;17:699–706. doi: 10.1016/j.molmed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park SG, Lee JS, Lee K, Kim D, Bae YC, Kaang BK, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–5. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 47.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck St, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–60. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 48.Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–58. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- 49.Kuroda K, Yamada S, Tanaka M, Iizuka M, Yano H, Mori D, Tsuboi D, Nishioka T, Namba T, Iizuka Y, Kubota S, Nagai T, Ibi D, Wang R, Enomoto A, Isotani-Sakakibara M, Asai N, Kimura K, Kiyonari H, Abe T, Mizoguchi A, Sokabe M, Takahashi M, Yamada K, Kaibuchi K. Behavioral alterations associated with targeted disruption of exons 2 and 3 of the Disc1 gene in the mouse. Hum Mol Genet. 2011;20:4666–83. doi: 10.1093/hmg/ddr400. [DOI] [PubMed] [Google Scholar]
- 50.Desai A, Turetsky D, Vasudevan K, Buonanno A. Analysis of transcriptional regulatory sequences of the N-methyl-D-aspartate receptor 2A subunit gene in cultured cortical neurons and transgenic mice. J Biol Chem. 2002;277:46374–84. doi: 10.1074/jbc.M203032200. [DOI] [PubMed] [Google Scholar]
- 51.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–94. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–58. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–8. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui Z, Feng R, Jacobs S, Duan Y, Wang H, Cao X, Tsien JZ. Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci Rep. 2013;3:1036. doi: 10.1038/srep01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millar JK, Mackie S, Clapcote SJ, Murdoch H, Pickard BS, Christie S, Muir WJ, Blackwood DH, Roder JC, Houslay MD, Porteous DJ. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol. 2007;584(Pt 2):401–405. doi: 10.1113/jphysiol.2007.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–24. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kvajo M, McKellar H, Drew LJ, Lepagnol-Bestel AM, Xiao L, Levy RJ, Blazeski R, Arguello PA, Lacefield CO, Mason CA, Simonneau M, O'Donnell JM, MacDermott AB, Karayiorgou M, Gogos JA. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. Proc Natl Acad Sci U S A. 2011;108:E1349–58. doi: 10.1073/pnas.1114113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma TM, Abazyan S, Abazyan B, Nomura J, Yang C, Seshadri S, Sawa A, Snyder SH, Pletnikov MV. Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion. Mol Psychiatry. 2012;18:557–67. doi: 10.1038/mp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 60.Maycox PR, Kelly F, Taylor A, Bates S, Reid J, Logendra R, Barnes MR, Larminie C, Jones N, Lennon M, Davies C, Hagan JJ, Scorer CA, Angelinetta C, Akbar T, Hirsch S, Mortimer AM, Barnes TR, de Belleroche J. Analysis of gene expression in two large schizophrenia cohorts identifies multiple changes associated with nerve terminal function. Mol Psychiatry. 2009;14:1083–94. doi: 10.1038/mp.2009.18. [DOI] [PubMed] [Google Scholar]
- 61.Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, Markx S, Lieberman JA, Javitch JA. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–90. doi: 10.1016/j.tips.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.