Abstract
Mesial temporal lobe epilepsy (MTLE) is the most frequent form of focal epilepsy. At rest, there is evidence that brain abnormalities in MTLE are not limited to the epileptogenic region, but extend throughout the whole brain. It is also well established that MTLE patients suffer from episodic memory deficits. Thus, we investigated the relation between the functional connectivity seen at rest in fMRI and episodic memory impairments in MTLE. We focused on resting state BOLD activity and evaluated whether functional connectivity (FC) differences emerge from MTL seeds in left and right MTLE groups, compared with healthy controls. Results revealed significant FC reductions in both patient groups, localized in angular gyri, thalami, posterior cingulum and medial frontal cortex. We found that the FC between the left non‐pathologic MTL and the medial frontal cortex was positively correlated with the delayed recall score of a non‐verbal memory test in right MTLE patients, suggesting potential adaptive changes to preserve this memory function. In contrast, we observed a negative correlation between a verbal memory test and the FC between the left pathologic MTL and posterior cingulum in left MTLE patients, suggesting potential functional maladaptative changes in the pathologic hemisphere. Overall, the present study provides some indication that left MTLE may be more impairing than right MTLE patients to normative functional connectivity. Our data also indicates that the pattern of extra‐temporal FC may vary as a function of episodic memory material and each hemisphere's capacity for cognitive reorganization. Hum Brain Mapp 34:2202–2216, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: connectivity, resting state, fMRI, episodic memory, epilepsy, functional reorganization
INTRODUCTION
Mesial temporal lobe epilepsy (MTLE) is the most frequent form of refractory epilepsy, and is commonly associated with mesial temporal sclerosis (MTS). However, there is a growing body of evidence that brain abnormalities in MTLE are not limited to the epileptogenic region, but extend into widespread areas of the ipsilateral and contralateral hemispheres [e.g., see Gross, 2011]. These abnormalities have been evident in gray matter, white matter, and metabolic studies. Indeed, structural neuroimaging studies have consistently shown gray matter atrophy in an extensive bilateral extratemporal network in TLE, most reliably in thalami, parietal, and contralateral temporal cortex [see review of Keller and Roberts, 2008]. Other studies have demonstrated extensive bilateral white matter abnormalities in TLE that extend beyond the epileptogenic temporal lobe [Gross, 2011]. Studies using single photon emission computed tomography (SPECT) or positron emission tomography [PET, see review of Shulman, 2000], and spectroscopy [Tasch et al., 1999; Vermathen et al., 2003] have revealed abnormal findings in extratemporal regions, including the contralateral hemisphere, suggesting that seizures in MTLE can exert remote deleterious effects on neural integrity. One possible explanation is that epileptic seizures, through mechanisms such as seizure generalization or secondary epileptogenesis can initiate a neural circuit and lead to aberrant neural communication and maladaptive neural networks involving brain areas outside the epileptogenic region. Ultimately, the exact cause of these wider deficits remains unresolved and unknown.
MTLE patients are well‐known to suffer from several neurocognitive deficits with memory or language impairments most commonly reported [Hermann et al., 2008]. While structural and functional abnormalities in the temporal lobe have been implicated [Arnold et al., 1996; Bonilha et al., 2007a; Guedj et al., 2010; Kilpatrick et al., 1997; Miller et al., 1993], evidence is accruing that these cognitive deficits may be associated with the extratemporal abnormalities noted above. For example, prefrontal hypometabolism in MTLE has been associated with deficits in higher order cognitive abilities such as executive deficits [Jokeit et al., 1997]. Also, atrophy in regions outside the epileptogenic temporal lobe such as the cingulate and orbito‐frontal cortex have also been correlated with the impaired episodic memory in MTLE [Bonilha et al., 2007b].
Task‐driven fMRI imaging has begun to provide evidence that extratemporal abnormalities may be behind the BOLD activation differences that are seen in MTLE outside the epileptogenic temporal lobe, with the implication they are playing a role in the cognitive deficits of these patients. For instance, regarding memory networks, a recent study by Alessio et al. 2011 argued for the presence of functional reorganization of verbal memory processing in left MTLE, exhibiting an extensive network including more bilateral or right lateralized encoding‐related activations than normal controls, potentially due to the failure of left MTL system.
Resting‐state fMRI provides a promising methodology for helping to elucidate the relationship between cognitive dysfunction abnormalities in MTLE and a broader, maladaptive extratemporal brain network. Functional connectivity (FC) measures for resting‐state fMRI highlight the temporal correlations between remote brain regions [Friston, 1994]. In epilepsy, such resting state studies have begun to show functional changes in large‐scale networks, compared with healthy participants. Such investigations have resulted in the description of functional connectivity abnormalities in the whole brain [Liao et al., 2010], and within several functional networks outside the temporal lobe such as the well‐known default‐mode [Zhang et al., 2010], in addition to attentional [Zhang et al., 2009], and language [Waites et al., 2006] networks. To date several studies with MTLE patients have specifically explored the FC emerging from the hippocampus at rest [Bettus et al., 2009; Morgan et al., 2011; Pereira et al., 2010], and tested a relationship to memory functioning [Bettus et al., 2009]. These studies, however, have mainly explored the FC between both temporal lobes, excluding extratemporal regions. For example, Bettus et al. 2010 investigated FC between bilateral temporal regions in a left TLE patient sample. They highlighted reduced FC within the left pathologic temporal lobe, while the right nonepileptic temporal lobe was associated with increased FC, compared to controls. In another study, FC changes between the posterior and anterior parts of the right hippocampus were associated with a working memory quotient in a left MTLE group [Bettus et al., 2009]. Although such a direct relation between neuropsychological and resting state functional parameters has been suggested in previous fMRI studies [such as for language, see Waites et al., 2006], the study of Bettus et al. 2009 is, to our knowledge, the only study describing a direct positive relation between resting state FC values within the mesial temporal lobe and memory functioning, albeit working memory, in MTLE patients with MTS.
In this project, we seek to expand our understanding of spontaneous brain activity in MTLE, with a particular focus on determining if extratemporal functional connectivity changes within the network emerging from the mesial temporal lobe are associated with episodic memory deficits. Moreover, we sought to determine if right and left MTLE differ in their connectivity to extratemporal regions and, in attempt to maximize sensitivity to such lateralized differences, utilize two types of memory material (verbal and nonverbal) to examine if such material‐specific effects alter the associations between functional connectivity and episodic memory performance. We utilize resting state data from groups of right MTLE (RMTLE), left MTLE (LMTLE) patients, and healthy age‐matched controls. First, we investigate changes of functional connectivity based on seeds located in the left or right mesial temporal lobe (MTL) in each patient group compared to controls. Second, we explore the hypothesis that FC abnormalities in a mesial temporal lobe (MTL) network in patients are associated with episodic memory performance, and whether this relation varies as a function of memory material or the side of the ictal generator and MTS pathology. Verbal and nonverbal memory functioning was examined using scores from the immediate and delayed test phases of the Logical Memory and Facial Recognition subtests of the Wechsler Memory Scale III (WMS‐III) [Wechsler, 1997], respectively.
MATERIALS AND METHODS
Participants: MTLE Patients
A total of 21 patients with refractory mesial temporal lobe epilepsy, all with unilateral temporal sclerosis, were recruited from the Thomas Jefferson University Comprehensive Epilepsy Center (9 left and 12 right temporal lobe patients). All participants were recommended for anterior temporal lobe resections (left or right) as treatment for their condition. Details of the Thomas Jefferson Comprehensive Epilepsy Center algorithm for surgical decision making are described in the study of Sperling et al. 1992. A combination of EEG, MRI, PET, and neuropsychological testing was used to lateralize the side of seizure focus. All patient participants met the following inclusion criteria: unilateral temporal lobe seizure onset through surface video/EEG recordings (i.e., a single unilateral mesial temporal lobe focus); MRI evidence of mesial temporal lobe pathology confirming the presence of mesial temporal lobe atrophy in the epileptogenic temporal lobe; concordant PET finding of hypometabolism in the temporal lobe (available for most patients), and no patient had a nonconcordant PET; Full‐Scale IQ of at least 75. MTLE patients were excluded from the study for any of the following: medical illness with central nervous system impact other than epilepsy; head trauma; prior or current alcohol or illicit drug abuse; extratemporal or multifocal epilepsy; contraindications to MRI; psychiatric diagnosis other than a Axis‐I Depressive Disorder; or hospitalization for any Axis I disorder listed in the Diagnostic and Statistical Manual of Mental Disorders, IV. Depressive Disorders were allowed given the high comorbidity of depression and epilepsy [Tracy et al., 2007]. Participants provided written informed consent. The study was approved by the Institutional Review Board for Research with Human Subjects at Thomas Jefferson University. Table 1 outlines the demographic and clinical characteristics of the subjects. The Edinburgh handedness scale was used as a measure of handedness [Oldfield, 1971].
Table 1.
Clinical information and characteristics of MTLE patients and controls
| RMTLE | LMTLE | Controls | |
|---|---|---|---|
| N (female) | 12 (9) | 9 (7) | 12 (9) |
| age (m ± std, years) | 44.8 ± 14.8 | 41.1 ± 7.8 | 43.1 ± 11.9 |
| Edinburg (m ± std, %) | 67 ± 64 | 64 ± 65 | — |
| Right‐Handers (N) | 10 | 8 | |
| Duration of epilepsy (m ± std, y) | 22 ± 14 | 25 ± 17 | — |
| Seizure type | CPS: 25% | CPS: 33% | — |
| CPS/SPS: 25% | CPS/SPS: 11% | — | |
| CPS/SG or GTC: 50% | CPS/SG or GTC: 56% | — | |
| Full Scale IQ (m ± std) | 96.0 ± 16.4 | 95.7 ± 13.6 | — |
| Medicationa | Phenytoin 0% | Phenytoin 11% | |
| Keppra 22% | Keppra 44% | ||
| Tegretal 11% | Tegretal 22% | ||
| Topomax 33% | Topomax 11% | ||
| Lamictal 22% | Lamictal 44% | ||
| Trileptal 22% | Trileptal 11% | ||
| Zonegran 11% | Zonegran 0% | ||
| Neuropsychological performance (m ± std) | |||
| FM I | 36 ± 6b | 42 ± 19c | 37.5 ± 5.0d |
| FM II | 33 ± 6b | 30 ± 10c | 37.5 ± 5.0d |
| LM I | 35 ± 12b | 29 ± 10c | 40 ± 11d |
| LM II | 17 ± 11b | 14 ± 7c | 34 ± 10d |
Medication percentiles do not sum to 100 because some patients were on multiple anticonvulsant medications.
Average on 11 patients.
Average on 8 patients.
Normative results obtained on healthy age‐matched participants (bin = [35–44] years) [Wechsler, 1997]. Abreviations: CPS, complex partial seizures; SPS, simple partial seizures; SG, secondarily generalized seizures; GTC, generalized tonic/clonic seizures; FM, facial memory; LM, logical memory.
EEG Procedures
EEG was obtained using the 10–20 system with anterior temporal electrodes and, at times, sphenoidal electrodes that used a Grass‐Telefactor 32 channel acquisition system. At least 96 h of continuous EEG recording, with selected samples in wakefulness and sleep examined both by registered EEG technologists, board certified electroencephalographers, and through commercial automated spike detection program (SZAC, Grass Telefactor) to determine location and lateralization of interictal spikes and seizures.
Patient MRI Data Acquisition
All patients underwent Magnetic Resonance Imaging on a 3‐T X‐series Philips Achieva clinical MRI scanner (Amsterdam, the Netherlands) using an 8‐channel head coil. A total of 5 min of a resting state condition was collected on all MTLE patients. Anatomical and functional acquisitions were similar for all patients. Single shot echoplanar gradient echo imaging sequence acquiring T2* signal was used with the following parameters: 120 volumes, 34 axial slices acquired parallel to the AC‐PC line, TR = 2.5 s, TE = 35 ms, FOV = 256 mm, 128 × 128 data matrix isotropic voxels, flip angle = 90°, bandwidth = 1.802(±241.1 kHz). The in‐plane resolution was 2 × 2 mm2 and the slice thickness was 2 mm. Prior to collection of the T2* images, T1‐weighted images (180 slices) were collected using an MPRage sequence (256 × 256 isotropic voxels; TR = 640 ms, TE = 3.2 ms, FOV 256 mm, flip angle 8°) in positions identical to the functional scans to provide an anatomical reference. The in‐plane resolution for each T1 slice was 1 mm3 (axial oblique; angle following anterior, posterior commissure line). Survey and field reference inhomogeneity images were collected prior to the start of the study. Each EPI imaging series started with three discarded scans to allow for T1 signal stabilization. Subjects lay in a foam pad to comfortably stabilize the head, were instructed to remain still throughout the scan, not focus on any particular activity or thing, and keep their eyes closed during the entirety of the scan.
Participants: Healthy Controls
Data from healthy normal controls were downloaded from the 1000 Functional Connectomes dataset (http://www.nitrc.org/projects/fcon_1000/). The dataset was selected in order to match as close as possible the functional imaging parameters of the Connectomes dataset with our scanning protocol, though there were some differences (TR for patients = 2.5 s, controls = 2.8 s; number of slices for patients = 34, controls = 31). From this dataset, a sample of 12 healthy subjects was selected according to their age and gender in order to match our MTLE groups. These normal controls were scanned using a 12 channel receive‐only head array on a Siemens Trio 3T scanner (Siemens Medical Solutions, Erlangen). They were instructed to rest with eyes closed and refrain from any voluntary motion. A single shot echoplanar gradient echo imaging sequence acquiring T2* signal was used with the following parameters: 120 volumes (5 min, 36 s), 31 axial slices acquired parallel to the AC‐PC line, TR = 2.8 s, TE = 29 ms, FOV = 256 mm, 128 × 128 data matrix isotropic voxels, bandwidth = 1.954 Hz/pixel. The in‐plane resolution was 2 × 2 mm2 and the slice thickness was 2 mm.
Imaging Processing
Data from the MTLE patients and normal controls were preprocessed identically using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). Slice timing correction was used to adjust for variable acquisition time over slices in a volume, with the middle slice in every volume used as reference. Next, a six‐parameter variance cost function rigid body affine registration was used to realign all images within a session to the first volume. Motion regressors were computed and later used as regressors of no interest. To maximize mutual information, coregistration between functional scans and the MNI305 (Montreal Neurological Institute) template was carried out using six iterations and resampled with a 7th‐Degree B‐Spline interpolation. Functional images were then normalized into standard space (MNI305) to allow for signal averaging across subjects. We utilized the standard normalization method in SPM5, which minimizes the sum‐of‐squared differences between the subject's image and the template (MNI305), while maximizing the prior probability of the transformation. The segmentation of the data in the grey matter, white matter (WM) and cerebro‐spinal fluid (CSF) classes was also realized. All normalized images were smoothed by convolution with a Gaussian kernel, with a full width at half maximum of 8 mm in all directions. Sources of spurious variance were then removed from the data through linear regression: six parameters obtained by rigid body correction of head motion, the CSF and WM signals. For each individual, the time‐courses of both WM and CSF were estimated in the relevant brain tissue classes defined at the segmentation step. Finally, fMRI data were temporally filtered using the REST Toolbox (low cutoff frequency = 0.008 Hz—high cutoff frequency 0.1 Hz) [Song et al., 2011].
Definition of the Seed Regions
We constructed right and left seed regions covering the areas of mesial temporal sclerosis (MTS) in the right and left temporal lobes, i.e., the region of presumptive epileptogenic focus as determined by the Thomas Jefferson University (TJU) presurgical algorithm. Each seed includes the anterior part of hippocampus and of the parahippocampal gyrus (anterior to axis y = −18), and the amygdala (see Fig. 1). The seed region mask was drawn on the MNI305 anatomical template. The same seed regions were used for the healthy controls. Analyses involving the right MTL seed for the RMTLE group may be referred to as the pathologic seed. Conversely, the analyses involving the left MTL seed for the LMTLE group may be referred to as the pathologic seed.
Figure 1.
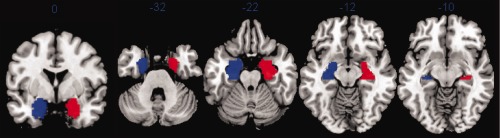
Right (red) and left (blue) MTL seed regions. They are displayed on the MNI template. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Statistical Analyses
First‐level analyses
At the individual level, a correlation map was produced by extracting the BOLD time course from each seed region and then computing the correlation between that time course and the time course from all other brain voxels. Next, this matrix of correlation coefficients was submitted to a Fisher r‐to‐z transformation (Z(r)), yielding an approximate normal distribution for the sampled data. All second or group level statistical analyses were conducted on these transformed data.
Second‐level analyses
To determine the positive functional network correlated with spontaneous activity of each seed region (left MTL, right MTL) within each of our experimental groups (LMTLE, RMTLE, controls), these individual Z(r) values maps were entered into a one‐sample t‐test. Each independent test resulted in a spatial map for each seed within each group. A height threshold at P < 0.0001 (uncorrected, cluster > 10 voxels) was chosen.
Individual Z(r) values maps were entered into a second‐level random‐effects analyses to determine if differences in functional connectivity existed between the experimental groups. We performed two‐sample t‐tests to detect differences in controls versus left MTLE patients, controls versus right MTLE patients and right MTLE patients versus left MTLE patients. The group comparisons were restricted (masked) to the network of significant voxels defined by the one‐sample t‐test, described above, for each group of interest (P < 0.0001 uncorrected). Last, a statistical whole‐brain map was generated, where the height threshold was fixed at P < 0.0001 (uncorrected) and the spatial extent, cluster‐level threshold was set at a corrected alpha level of P < 0.01. A spatial extent threshold consistent with image smoothness and the expected number of voxels per cluster was utilized (k >6). As a subanalysis, we also focused specifically on the FC changes in the contralateral, non‐ictal MTL seed region.
Correlation between Z(r) and neuropsychological memory scores in patients
If two‐sample t test results (controls versus right MTLE patients, or controls versus left MTLE patients) associated with a seed region showed significant clusters, these clusters were extracted as regions of interest (ROIs). The mean time series of these ROIs were computed. The resulted individual Z(r) computed between each ROI and the seed region were then correlated to patients' scores on episodic memory tests using a non parametric Spearman's correlation analysis, run separately for each patient group.
Verbal and nonverbal episodic memory functioning was assessed using scores from the immediate and delayed recall conditions of the Logical Memory and Face Recognition Memory subtests of the Wechsler Memory Scale III (WMS‐III) [Wechsler, 1997], respectively. Standard administration instructions were utilized (e.g., the delayed recall condition was administered ∼30 min after the immediate recall). The Logical Memory subtest is a verbal test that requires the examinee to freely recall a story after it is read aloud by the examiner. The Face Recognition Memory subtest is a visual, nonverbal memory test that requires a yes/no recognition response after two‐second exposure to a series of 24 faces. Recognition testing includes presentation of 24 distractor faces, not previously presented. This task is free from any visuomotor (graphic) reconstruction requirements. These tests were administered as part of the neuropsychological test battery given to all patients undergoing presurgical evaluation at the Thomas Jefferson Epilepsy Center. For the Face Recognition Memory test, three scores were used: the immediate (FM I) and delayed recall (FM II) scores and the rate of retention index (the ratio of FM II to FM I); the same three comparable scores were generated for the Logical Memory test (LM I, LM II, and the ratio of LM II to LM I). The first two variables refer to the number of items remembered correctly while the ratio (retention index) indicates the proportion of items recalled following a delay interval. As the number of items recalled increases the retention index increases, indicating better memory. Because two MTLE patients did not complete neuropsychological testing at our center, analyses involving these cognitive measures were conducted on 11 RMTLE and 8 LMTLE patients. All results reported are at a significance (alpha) level of at least P < 0.005.
RESULTS
No difference of age or gender was found between the experimental groups (RMTLE patients; LMTLE patients; controls) (age: P > 0.8; gender: P > 0.9) (see Table 1). The results of the neuropsychological tests for both patient groups are presented in the Table 1. There was no significant difference between the RMTLE and LMTLE groups (P > 0.05). The performance data listed for the normal controls is based on the normative data provided in the manual [Wechsler, 1997] for an age‐matched [(35–44) years old] healthy sample.
Functional Connectivity Maps Within Each Experimental Group
Seed in the right MTL
As expected, the control group displays the highest level of functional connectivity in the ipsilateral (right) hippocampal and parahippocampal gyri with the right MTL seed (k = 3,147, T = 23.4) (Fig. 2A). The right MTL seed was also positively correlated with the posterior cingulum cortex/precuneus (k = 576, T = 14), the contralateral hippocampus (k = 961, T = 11.9), the medial frontal cortex (k = 61, T = 10.7) and bilateral middle temporal gyri (left: k = 139, T = 7; right: k = 84, T = 6.7).
Figure 2.

Whole brain functional connectivity map with the right MTL seed for the control group (A), the RMTLE group (B), and the LMTLE group (C). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Similarly for the RMTLE patients, the largest cluster correlated with the right (pathologic) MTL seed activity was located in the right parahippocampal gyrus, extending to the hippocampus (k = 4,165, T = 25.1). To a lesser degree, the contralateral region was also significantly correlated with the seed region (Fig. 2B).
For the LMTLE patients, the right (nonpathological) MTL seed was positively correlated with a right cluster, including parahippocampal gyrus, amygdala and the hippocampus (k = 1,970; T = 46.1) (Fig. 2C). The left hippocampus and parahippocampal gyrus were part of this functional network as well (k = 283, T = 20.6).
Seed in the left MTL
Analogous to the positive FC map in controls associated with the right MTL seed, the left MTL seed was associated with the ipsilateral (left) amygdala, the parahippocampal, and hippocampal gyri (k = 1,799; T = 18.4) (Fig. 3A). To a lesser degree, the contralateral (right) hippocampus and parahippocampal gyrus (k = 323, T = 13.8) were also positively correlated with the left seed region, as well as bilateral thalami (k = 682, T = 11.8), right temporal pole (k = 41, T = 6.8), medial frontal cortex (k = 477; T = 10.8), left angular gyrus (k = 83; T = 9.5) and cerebellum (k = 85; T = 9.7).
Figure 3.

Whole brain functional connectivity map with the left MTL seed for the control group (A), the RMTLE group (B), and the LMTLE group (C). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The LMTLE patients showed a functional network correlated with the left pathological seed encompassing much of the limbic system, more extensive in the left (k = 1,070, T = 45.8) than the right hemisphere (k = 306, T = 16.6). In addition, the seed was functionally connected to small bilateral regions in the fusiform cortex (Left: k = 22, T = 10.9; Right: k = 16, T = 7.8) (Fig. 3C).
Finally, the RMTLE patients also showed an extensive functional network associated with their left nonpathologic MTL seed including much of the left (k = 2,844, T = 19.5) and right (k = 768, T = 9.9) limbic systems, including the hippocampal and parahippocampal gyri, amygdala, thalamus, as well as an area in the left superior temporal cortex (k = 119, T = 11.2) (Fig. 3B).
Functional Connectivity Comparisons Between Groups
Seed in the right MTL
Controls versus RMTLE patients
Within the functional network associated with the right MTL seed, a two‐sample t test revealed several significant differences between the controls and the RMTLE patients. The largest difference was detected between each angular gyrus and the (pathologic) seed, revealing significantly reduced FC for the patients (Table 2, Fig. 4A). Moreover, the RMTLE patients also showed weaker FC between the contralateral (left) thalamus and the seed in comparison to the controls. At the whole brain level, this patient group did not show increased FC values with the pathological seed relative to controls. However, when looking specifically in the contralateral MTL region, a part of the contralateral (left) hippocampus (T = 5.37; x = −18, y = −14, z = −18; k = 9) had significantly increased connectivity with the epileptogenic right mesial temporal lobe region compared with controls.
Table 2.
Whole brain functional connectivity differences between our three experimental groups with the right MTL seed
| Contrasts | Location | P corrected | Ke | T | x | y | z | Z(r) Ctls (SD) | Z(r) Pts (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Controls–RMTLE | L angular | <0.001 | 205 | 7.08 | −46 | −62 | 32 | 0.15(0.22) | −0.08(0.16) |
| L angular | 5.39 | −36 | −62 | 30 | — | — | |||
| L Thalamus | 0.004 | 76 | 6.45 | −14 | −30 | 10 | 0.48(0.19) | 0.01(0.19) | |
| L Post cingulum | 4.76 | −8 | −44 | 8 | — | — | |||
| R angular | <0.001 | 135 | 5.72 | 40 | −58 | 26 | 0.26(0.15) | −0.10(0.17) | |
| R angular | 5.13 | 30 | −58 | 40 | — | — | |||
| Controls–LMTLE | L angular | <0.001 | 511 | 9.15 | −28 | −64 | 36 | 0.21(0.22) | −0.22(0.09) |
| L angular | 9.03 | −36 | −62 | 30 | — | — | |||
| L angular | 7.44 | −44 | −60 | 30 | — | — | |||
| R angular | <0.001 | 144 | 6.78 | 32 | −64 | 30 | 0.24(0.15) | −0.15(0.11) | |
| R precuneus | 6.42 | 32 | −58 | 24 | — | — | |||
| R angular | 6.05 | 34 | −60 | 38 | – | – | |||
| Post Cingulum | <0.001 | 142 | 5.82 | −10 | −40 | 10 | 0.47(0.22) | 0.03(0.15) | |
| L hippocampus | 5.6 | −22 | −40 | 12 | — | — | |||
| LMTLE–Controls | Null | ||||||||
| RMTLE–Controls | Null | ||||||||
| RMTLE vs. LMTLE | Null |
Ke: Number of voxels included in the cluster; p corrected at the cluster‐level. All the coordinates are given in the stereotaxic space of the MNI template. The Z(r)s represent the mean FC (SD) computed between the region of interest and the right MTL seed, within the experimental groups. All these regions are displayed on the Figure 4A.
Figure 4.
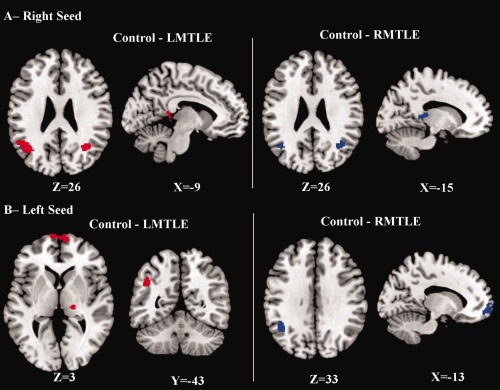
Regions showing reduced functional connectivity with either the right MTL Seed (A) or the left MTL Seed (B) in patients, relative to controls (see Tables 2 and 3, respectively). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Controls versus LMTLE patients
The LMTLE group had also brain areas with a significant reduction of functional connectivity compared with controls with the nonpathologic right MTL seed. Relative to the controls, the LMTLE group showed reduced connectivity to both angular gyri as well as a cluster including left posterior cingulum cortex, extending to the left hippocampus (Table 2, Fig. 4A). This patient group did not show brain areas with a significant increase in functional connectivity in the whole brain analysis involving the seed, nor between the seed and the contralateral MTL region.
Overall, with the right seed, the LMTLE group showed a more extensive network than the RMTLE group compared with the controls' network (from Table 2, 655 versus 415 voxels, respectively, in the contrast with controls). For both patient groups, impaired FC with the seed involved mainly brain regions in the contralateral left hemisphere.
RMTLE versus LMTLE patients
A direct comparison between patient group showed no significant differences in FC with the right MTL seed were observed between patient groups, involving either the whole brain analyses or regional FC with the right MTL seed.
Seed in the left MTL
Controls versus LMTLE patients
The LMTLE group, compared with controls, had several brain areas with significantly reduced FC involving their left pathological MTL (Table 3, Fig. 4B). These regions included the left inferior parietal cortex, right thalamus, medial superior frontal cortex and posterior cingulum cortex (Figs. 4B and 5B). No brain areas had increased FC with the seed in the patients at either the whole brain level or when examining FC to the right contralateral MTL specifically.
Table 3.
Whole brain functional connectivity differences between our three experimental groups with the left MTL seed
| Contrasts | Location | P corrected | Ke | T | x | y | z | Z(r) Ctls (SD) | Z(r) Pts (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Controls−LMTLE | L inf parietal | <0.001 | 122 | 11.4 | −44 | −54 | 36 | 0.16(0.23) | −0.24(0.15) |
| R thalamus | 0.007 | 58 | 8.2 | 16 | −22 | 6 | 0.29(0.22) | −0.10(0.12) | |
| R Med sup frontal | <0.001 | 140 | 6.3 | 10 | 66 | 4 | 0.31(0.14) | −0.16(0.18) | |
| L Med sup frontal | 6.0 | −10 | 72 | 4 | — | — | |||
| R Med frontal | 5.2 | 10 | 60 | −2 | — | — | |||
| R post cingulum | <0.001 | 106 | 5.6 | 2 | −36 | 32 | 0.28(0.13) | −0.19(0.20) | |
| L post cingulum | 5.3 | −2 | −28 | 42 | — | — | |||
| Controls−RMTLE | L Sup Frontal | <0.001 | 234 | 6.1 | −14 | 56 | −10 | 0.37(0.15) | −0.08(0.19) |
| L Sup Frontal | 6.1 | −14 | 64 | −8 | — | — | |||
| Med frontal | 5.6 | 2 | 70 | 0 | — | — | |||
| L angular | <0.001 | 111 | 6.1 | −42 | −56 | 34 | 0.17(0.24) | −0.11(0.19) | |
| L supramarginal | 5.4 | −46 | −44 | 26 | — | — | |||
| L supramarginal | 5.4 | −44 | −44 | 34 | — | — | |||
| RMTLE−Controls | Null | ||||||||
| LMTLE−Controls | Null | ||||||||
| RMTLE vs. LMTLE | Null |
Ke: Number of voxels included in the cluster; p corrected at the cluster‐level. All the coordinates are given in the stereotaxic space of the MNI template. The Z(r)s represent the mean FC (SD) computed between the region of interest and the left MTL seed, within the experimental groups. These regions are displayed on Figures 4B and 5.
Figure 5.

Correlation between FC values with the left seed in RMTLE (A) and in LMTLE (B) with episodic memory scores. A: Reduced FC between left seed (blue) and medial frontal cortex (green, x = −14, y = 56, z = −10; also see Table 3) in RMTLE patients compared with controls (left bottom plot); positive correlation between FC values between these two regions and the FM II (right bottom plot, Spearman correlation, r = 0.78; P = 0.0045). The normative values of the controls are shown through the green data point where the y axis indicates the average FC value of the controls' data and the x axis is the normative value of age‐matched healthy controls of the FM II score [Wechsler, 1997]. Bars indicate standard deviation. B: Reduced FC between left seed (blue) and posterior cingulum (green, x = 2, y = −36, z = 32; also see Table 3) in LMTLE patients compared with controls (left bottom plot); negative correlation between the FC values between these two regions and the ratio LM II/ LM I (right bottom plot, Spearman correlation, r = −0.93; P = 0.001). The normative values of the controls are shown through the green data point where the y axis indicates the average FC value of the controls' data and the x axis is the normative value of age‐matched healthy controls of the LM II/LM I ratio score (Wechsler, 1997). Bars indicate standard deviation. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Controls versus RMTLE patients
With regard to the left nonpathological seed region, compared with the controls, the RMTLE group showed significantly reduced FC between this seed and both the left lateral parietal cortex (including angular and supramarginal gyrus, Fig. 4B) and the left superior medial frontal cortex (Fig. 5A, Table 3). No brain areas had increased FC with the seed in the patients relative to the controls.
Overall, with the left seed, the LMTLE group had a slightly more extensive network than the RMTLE group compared with the controls (see Table 3; 426 versus 345 voxels, respectively; in the contrast with controls). Notable is the differing location of the clusters: the regions are mainly medial for the LMTLE, while almost exclusively in the ipsilateral left nonpathologic hemisphere for the RMTLE.
RMTLE versus LMTLE patients
No significant differences in FC with the right MTL seed were observed between patient groups.
Correlation with Neuropsychological Scores in Patients
Using the regions displaying a significant increase or reduction of FC in the patient groups relative to the controls, we sought to determine whether these observed FC differences were correlated with memory performance in the MTLE patients.
For the RMTLE patients, we found a significant positive correlation involving the FC between the nonpathologic left seed and the medial frontal cortex cluster (Fig. 5A), and the delayed recall score of the facial memory test (FM II; Spearman Correlation: r = 0.78, P = 0.0045). This correlation indicated that patients with higher FM II retention scores had higher FC values between these regions (i.e., values closer to those seen in controls). No significant effect was observed with the right (pathologic) MTL seed.
For the LMTLE patients, we found a significant negative correlation involving the FC values between the (pathologic) MTL seed and the posterior cingulum cluster, which correlated with the rate of retention index on the Logical Memory test (ratio LM II / LM I; Spearman Correlation: r = −0.93, P = 0.001, Fig. 5B). This correlation indicated that patients with lower rates of recall on LM had higher FC values between these regions (i.e., values closer to those seen in controls).
DISCUSSION
In this work, spontaneous fluctuations of the BOLD signal during a conscious resting state were recorded in healthy controls and patients suffering from right or left mesial temporal lobe epilepsy (n.b., mesial temporal sclerosis origin). The aim of this study was to investigate whole brain modulations of resting state functional connectivity emerging from the mesial temporal lobe in MTLE and determine if they are related to objective neuropsychological memory performance.
For each MTL seed, we highlighted a functional brain network for each experimental group at rest. In all three groups, a bilateral network was evident, involving, at least, the seed (namely, hippocampus, amygdale, and parahippocampal gyrus) and contralateral MTL regions to varying degrees.
A qualitative comparison between the patient groups showed that the network of the RMTLE patients was more extensive bilaterally within the limbic system than the one evident within the LMTLE patients. A finding true for both MTL seeds. Despite these differences, a direct statistical comparison (2 sample t‐test) did not reveal any statistically reliable differences between these groups when using whole brain analyses, and when using either the left or the right seed. Our finding stands in contrast to a study of Pereira et al. 2010 which found statistical differences between right and left MTLE patients, regardless the side of the seed. The difference between our study and theirs may be related to methodological differences as they used a less stringent statistical height threshold. Second, these authors used solely the hippocampus as a seed whereas our seed was more extensive, including the amygdala and parahippocampal gyri as well.
Importantly, other quantitative comparisons between the experimental groups highlighted a general pattern of decreased extra‐temporal FC values in the patient groups for each seed relative to normal healthy controls. In examining group differences in the FC emerging from the epileptogenic seed (namely, right seed for the RMTLE and left seed for the LMTLE patients), our data suggests that the epileptogenic MTL impairs heteromodal association regions in both hemispheres for both patient groups. Whereas, in examining group differences in the FC emerging from the non‐epileptic seed region (i.e., left seed for the RMTLE and right seed for the LMTLE patients), the LMTLE group was associated with a more extensive impaired network than the RMTLE group compared to the controls' network, regardless of the seed. This result is consistent with previous work (Pereira et al., 2010). Of note, contrary to the controls, who showed positive FC between these regions and the seeds, almost all these regions had a negative FC with the seed in the patient groups (see Tables 2 and 3). One potential interpretation of these negative correlations, which were exclusive to the patients, is that they represent an inhibitory relationship between the MTL and extra‐temporal regions, an inhibition that was not present in controls. Consistent with this idea, Ge et al. 2011 provided physiological data describing TLE as associated with abnormal reorganization of inhibitory circuits in the hippocampus. Ultimately, given that the meaning of negative FC relationships remains unknown and is still the subject of debate in the recent literature [see Fox et al., 2009; Murphy et al., 2009], we cannot with our data specify the exact nature of these FC relationships. Further investigation is needed to explain such phenomenon both in TLE patients and healthy normals.
Overall, in terms of our interpretation of these findings, decreased functional connectivity is generally considered to result from the disruption of neuronal connections within a functional network, and is commonly considered to reflect cognitive impairments in brain disorders [Greicius, 2008]. Thus, our data indicates that brain spontaneous activity is impaired in the MTLE patients, though at different levels depending on the side of the (epileptogenic) seed. Moreover, the pathologic seed was associated with more extended bilateral impairments, while the healthier MTL seems to have less impaired (though nevertheless impaired) connectivity with the rest of the brain, relative to the controls. This result might be explained by higher negative effects of the epileptogenic region on the rest of the brain, perhaps through seizure generalization and spread, or the start of secondary epileptogenesis. More directly, these functional impairments are likely related to higher structural damage in the epileptogenic compared to the nonepileptogenic hippocampus, decreasing both afferent and efferent communication. Several studies have described the epileptogenic hippocampus as significantly atrophic compared with controls while the contralateral one was either less atrophic [Bonilha et al., 2012] or did not differ from normals [Bonilha et al., 2010; Pereira et al., 2010; Theodore et al., 1999]. Furthermore, regions such as the lateral parietal lobe, thalamus, and cerebellum have been consistently reported as having structural atrophy, gray matter reductions [Bonilha et al., 2007b; Mueller et al., 2010], and a reduction of N‐acetyl aspartate to creatine ratios [Hetherington et al., 2007] in TLE. Therefore, the disrupted FC can be seen as part of the broad downstream and network effects of anatomical injury to an important area of cortex involved in the propagation of seizures.
We also observed a region of increased FC with the epileptogenic MTL seed. Our data indicates that the RMTLE patients had higher FC than the controls between the right seed and the contralateral left hippocampus. This increase is more difficult to understand and explain, but has been previously described by Morgan et al. 2011 as a compensatory phenomenon of the brain, perhaps recruiting the healthy hemisphere to participate in various cognitive and behavioral activities to make up for the impaired hippocampus. It is also possible that this involves a deleterious development in connectivity, bearing some relation to epileptogenesis and seizure spread that current models cannot explain.
For the seed contralateral to the mesial temporal lobe sclerosis (i.e., nonepileptogenic seed), both patient groups showed reductions of functional connectivity in several brain extratemporal regions as well. These findings suggest other factors at work, causing the functional connectivity impairment, that are distinct from structural abnormalities in epileptogenic temporal lobe. These findings are consistent with those of Pereira and colleagues, who found reduced FC associated with the right nonepileptogenic hippocampus for LMTLE patients [Pereira et al., 2010]. However, we extend their result by describing the same phenomenon for the RMTLE patients and the left nonepileptogenic MTL seed. The regions showing decreased FC with the left and right MTL seeds are medial, involving the medial superior frontal (BA 10) and the posterior cingulate cortices (BA 23). Neither of these regions have been commonly described as atrophic in TLE. Therefore, we suspect these results may reflect diaschisis. Diaschisis would cause a damaged region (i.e., the epileptogenic MTL) to disrupt the neuronal communications emerging from remote sites in the connected network such as the contralateral MTL, or the medial regions described above. In other words, if one region within a network is damaged then abnormal responses, or a disconnection in signaling, may ensue for another site within the same network.
Significant associations between neuropsychological scores and functional connectivity have been established in other pathologies with hippocampal atrophy such as Alzheimer's disease [Greicius et al., 2004]. Our data demonstrate such associations exist in MTLE. More specifically, we showed that functional connectivity impairments emerging from the MTL to medial brain regions, outside the temporal lobe, are significantly related to episodic memory performance. The specific engagement of these regions are consistent with their role in episodic memory tasks [Buckner and Carroll, 2007]. Indeed, both the medial frontal cortex and posterior cingulum have been described as part of the default mode network; a network that several workers have argued is directly engaged during episodic memory functions [Buckner et al., 2008; Greicius et al., 2004]. It is important, however, to highlight that these correlations with performance may have different functional causes and implications for performance, based on the absence or presence of epileptogenesis in the seed. For the RMTLE, we showed a positive relation in the nonpathologic left hemisphere, indicating that the patients with lower delayed recall scores on our Facial Memory test had decreased FC values between the left MTL and the posterior cingulum. On the basis of the recent work of Coolican et al. 2008 which argued that the left hemisphere can be recruited for cognitive processes when right hemisphere mechanisms are damaged, our data may, therefore, reflect adaptative change and reorganization within the contralateral nonpathologic MTL network of right temporal lobe epilepsy patients. In such instances, the left hemisphere may come to assume a dominant role for nonverbal episodic memory tasks as a mean of ensuring the preservation of function. This interpretation could also explain why several studies failed to find a direct relation between right hippocampal atrophy and deficits on any kind of episodic memory task [Alessio et al., 2004].
In contrast, for LMTLE, we observed a negative correlation between FC in the pathologic hemisphere and verbal memory performance, indicating that patients with higher rates of recall had lower FC values involving the left MTL and a medial frontal cluster. At first glance, this result in the LMTLE patients appears counter‐intuitive given that higher FC with the left MTL seed (i.e., closer to controls values) was related to lower memory performance. Nevertheless, because this specific relation is present in the pathologic hemisphere, we suggest it represents a maladaptative change in spontaneous activity from seizure, hurting verbal memory performance. Thus, our results suggest that the relationship between FC and cognitive performance may vary by disease state and as a function of the hemisphere possessing the pathology. Consistently with this idea, Zhang et al. 2010 suggested that there are different pathological mechanisms underlying the right and left MTLE.
Both of the associations with memory performance that we obtained involved the left hemisphere MTL seed, independent of the patient groups. It is important to note that the majority of our sample was right‐handed, suggesting left hemisphere dominance for language was prominent in our sample [Corina et al., 1992]. Thus, our data is consistent with evidence indicating that episodic memory, whether verbal or visual spatial, is highly dependent on the language dominant hemisphere. It also strengthens the claim that the left hemisphere plays a crucial role in predicting the association between functional connectivity and both verbal and visual‐spatial memory recall performance in MTLE. This hypothesis has been suggested in several previous studies that investigated the relation between hippocampal sclerosis and memory [Miller et al., 1993; Powell et al., 2007].
Finally, we see our data as suggesting that left mesial temporal lobe epilepsy represents a more severe form of the disease as indicated by the more pervasive FC abnormalities and potential maladaptative functional reorganization. This hypothesis has been suggested in previous work [Pereira et al., 2010]. Thus, our results provide new evidence supporting this idea, contributing to a better understanding of the effects of LMTLE on both functional brain extratemporal activity and episodic memory performance. Moreover, our data are consistent with the possibility that LMTLE patients may be both more prone to maladaptive functional connectivity changes within their left dominant (epileptogenic) hemisphere, and less capable of recruiting other regions to take over functionality and reorganize skills such as verbal episodic memory.
Limitations
As we did not observe the full memory network depicted by other studies such as those involving the Default Mode network [Buckner et al., 2008], our data does not address or imply changes in other regions involved in episodic memory (such as inferior parietal cortex), nor in other networks in which the mesial temporal lobe participates [e.g., for language, see Tracy and Boswell, 2008]. It is not clear, for instance, in the case of the left MTLE patients, why the pathologic MTL would only disrupt FC with a medial frontal region in the context of episodic memory.
Regarding the significant effects we described with neuropsychological tests, it is possible that they are caused by correlation with a third hidden variable that is influencing FC, such as general intellectual abilities or some other measure of general cognitive resources. We should note, however, we observed no such association between IQ and FC (P > 0.05). As described previously, our memory measures have been observed as predictors of structural change. However, our data suggests that functional connectivity and structural measures may have dissociable effects on neurocognitive measures of memory, particularly as one takes into account the type of memory material and the side of ictal activity.
Another limitation is likely technical. Despite the fact that we processed the resting state data of the healthy controls in a fashion identical to the patients (i.e., similar statistical power based on the volumes and scan time, identical degrees of freedom), the data of the healthy controls were acquired with another scanner with slightly different imaging parameters than used those for the patients. However, we assume that this is not an adequate explanation of the effects we reported because our results are generally coherent with the results of previous studies [Morgan et al., 2011; Pereira et al., 2010]. Furthermore, Van Dijk et al. 2010 showed that resting state functional connectivity strength is stabilized after a duration of 5 min, with no substantive effects introduced by varying temporal resolutions (i.e., different TRs).
CONCLUSIONS
Overall, we demonstrated that MTLE patients showed abnormal functional connectivity with both mesial temporal lobes at rest. The present study suggests that a focal epilepsy such at MTLE may cause impaired connectivity in a bilateral extratemporal network, and be linked to both the performance and brain organization of the broader episodic memory network of these patients. We are among the first to highlight a direct relation between episodic memory performance in MTLE and a functional connectivity reduction involving the left MTL and medial extratemporal regions. Our results also suggest that right MTLE may differ as a disease entity in that its functional connectivity disruptions are less pronounced and its capacity for brain reorganization of episodic memory functions may be different than for the left MTLE. Thus, our results suggest that one cannot assume the two temporal lobes are homologous in terms of functional connectivity, implying that the merger of these two populations as a strategy to increase statistical power is flawed. Our findings clarify the utility of resting‐state FC as a technique for identifying aberrant cognitive brain networks, and demonstrate that FC in MTLE is related to neurocognitive performance in ways that are unique and potentially separable from the effects that emerge from structural data on these patients. Our study emphasizes the importance of taking into account functional connectivity differences between the hemispheres, particularly when studying links to neurocognitive functions and broad brain networks. Our data also implies that one must take into account the nature or content of the material (verbal or visuospatial) when evaluating episodic memory as the impact of mesial temporal pathology appears to vary with the type of memory material and has hemisphere‐specific effects impact on functional connectivity, potentially related to differences in each hemisphere's capacity for cognitive reorganization.
REFERENCES
- Alessio A, Damasceno BP, Camargo CH, Kobayashi E, Guerreiro CA, Cendes F (2004): Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav 5:22–27. [DOI] [PubMed] [Google Scholar]
- Alessio A, Pereira FR, Sercheli MS, Rondina JM, Ozelo HB, Bilevicius E, Pedro T, Covolan RJ, Damasceno BP, Cendes F (2011): Brain plasticity for verbal and visual memories in patients with mesial temporal lobe epilepsy and hippocampal sclerosis: An fMRI study. Hum Brain Mapp. doi: 10.1002/hbm.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, Schlaug G, Niemann H, Ebner A, Luders H, Witte OW, Seitz RJ (1996): Topography of interictal glucose hypometabolism in unilateral mesiotemporal epilepsy. Neurology 46:1422–1430. [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F (2009): Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort‐Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M (2010): Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 81:1147–1154. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Alessio A, Rorden C, Baylis G, Damasceno BP, Min LL, Cendes F (2007a): Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp 28:1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Halford JJ, Eckert M, Appenzeller S, Cendes F, Li LM (2007b): Asymmetrical extra‐hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 78:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Edwards JC, Kinsman SL, Morgan PS, Fridriksson J, Rorden C, Rumboldt Z, Roberts DR, Eckert MA, Halford JJ (2010): Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia 51:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Halford JJ, Morgan PS, Edwards JC (2012): Hippocampal atrophy in temporal lobe epilepsy: The ‘generator’ and ‘receiver’. Acta Neurol Scand. 125:105–110. doi: 10.1111/j.1600-0404.2011.01510.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC (2007): Self‐projection and the brain. Trends Cogn Sci 11:49–57. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Coolican J, Eskes GA, McMullen PA, Lecky E (2008): Perceptual biases in processing facial identity and emotion. Brain Cogn 66:176–187. [DOI] [PubMed] [Google Scholar]
- Corina DP, Vaid J, Bellugi U (1992): The linguistic basis of left hemisphere specialization. Science 255:1258–1260. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (1994): Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp 2:56–78. [Google Scholar]
- Ge YX, Liu Y, Tang HY, Liu XG, Wang X (2011): ClC‐2 contributes to tonic inhibition mediated by alpha5 subunit‐containing GABA(A) receptor in experimental temporal lobe epilepsy. Neuroscience 186:120–127. [DOI] [PubMed] [Google Scholar]
- Greicius MD (2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21:424–430. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DW (2011): Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52 (Suppl 4):32–34. [DOI] [PubMed] [Google Scholar]
- Guedj E, Barbeau EJ, Liegeois‐Chauvel C, Confort‐Gouny S, Bartolomei F, Chauvel P, Cozzone PJ, Ranjeva JP, Mundler O, Guye M (2010): Performance in recognition memory is correlated with entorhinal/perirhinal interictal metabolism in temporal lobe epilepsy. Epilepsy Behav 19:612–617. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Jones J (2008): The neurobehavioural comorbidities of epilepsy: Can a natural history be developed? Lancet Neurol 7:151–160. [DOI] [PubMed] [Google Scholar]
- Hetherington HP, Kuzniecky RI, Vives K, Devinsky O, Pacia S, Luciano D, Vasquez B, Haut S, Spencer DD, Pan JW (2007): A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology 69:2256–2265. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Seitz RJ, Markowitsch HJ, Neumann N, Witte OW, Ebner A (1997): Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain 120 ( Part 12):2283–2294. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N (2008): Voxel‐based morphometry of temporal lobe epilepsy: An introduction and review of the literature. Epilepsia 49:741–757. [DOI] [PubMed] [Google Scholar]
- Kilpatrick C, Murrie V, Cook M, Andrewes D, Desmond P, Hopper J (1997): Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure 6:213–218. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Lu G, Chen H (2010): Altered functional connectivity and small‐world in mesial temporal lobe epilepsy. PLoS One 5:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Munoz DG, Finmore M (1993): Hippocampal sclerosis and human memory. Arch Neurol 50:391–394. [DOI] [PubMed] [Google Scholar]
- Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou‐Khalil B (2011): Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia 52:1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, Cardenas‐Nicolson V, Weiner MW (2010): Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia 51:1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9:97–114. [DOI] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F (2010): Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: Evidence from resting state fMRI. BMC Neurosci 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, Koepp MJ (2007): Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 48:1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman MB (2000): The frontal lobes, epilepsy, and behavior. Epilepsy Behav 1:384–395. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, O'Connor MJ, Saykin AJ, Phillips CA, Morrell MJ, Bridgman PA, French JA, Gonatas N (1992): A noninvasive protocol for anterior temporal lobectomy. Neurology 42:416–422. [DOI] [PubMed] [Google Scholar]
- Tasch E, Cendes F, Li M, Dubeau F, Anderman F, Arnold D (1999): Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Annal Neurol 45:568–576. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Bhatia S, Hatta J, Fazilat S, DeCarli C, Bookheimer SY, Gaillard WD (1999): Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology 52:132–136. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Boswell S (2008): Modeling the interaction between language and memory: The case of temporal lobe epilepsy In: Whitaker BSH, editor. Handbook of the Neuroscience of Language. San Diego, CA: Academic Press; pp319–328. [Google Scholar]
- Tracy J, Johnson V, Sperling MR, Cho R, Glosser D (2007): The association of mood with quality of life ratings in epilepsy. Neurology 68:1101–1107. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermathen P, Laxer KD, Schuff N, Matson GB, Weiner MW (2003): Evidence of neuronal injury outside the medial temporal lobe in temporal lobe epilepsy: N‐acetylaspartate concentration reductions detected with multisection proton MR spectroscopic imaging—Initial experience 1. Radiology 226:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD (2006): Functional connectivity networks are disrupted in left temporal lobe epilepsy. Annal Neurol 59:335–343. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997): Wechsler Memory Scale, 3rd ed. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, Chen Z, Shi J, Liu Y (2009): Impaired attention network in temporal lobe epilepsy: A resting FMRI study. Neurosci Lett 458:97–101. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Wang Z, Li K, Chen H, Liu Y (2010): Altered spontaneous neuronal activity of the default‐mode network in mesial temporal lobe epilepsy. Brain Res 1323:152–160. [DOI] [PubMed] [Google Scholar]


