Abstract
Background
Hypertension is associated with cardiovascular stiffening and left ventricular diastolic dysfunction, leading to comorbidities such as heart failure with preserved ejection fraction (HFpEF). It is unknown whether sex and hypertension subtype affect haemodynamics and left ventricular function in older individuals.
Methods
Ninety-five older patients with Stage 1 hypertension (ambulatory awake SBP135–159 mmHg) and 56 normotensive controls were enrolled. Patients were stratified prospectively into isolated systolic hypertension (ISH, DBP <85 mmHg) or systolic-diastolic hypertension (SDH, DBP ≥85 mmHg). Haemodynamics and Doppler variables including early filling (E) and averaged mitral annular (E′mean) velocities were measured during supine rest.
Results
Ambulatory awake blood pressures (BPs) were the highest in SDH, whereas supine SBP was similar in both hypertensive groups. No sex difference was observed in supine or ambulatory awake BPs in all groups. Stroke volume was similar among groups within the same sex, but smaller in women. Women exhibited faster E, slower E′mean and greater E/E′mean, whereas no group difference was observed in E within the same sex. In women, E′mean was significantly slower in SDH (5.9 ± 1.6 vs. 7.4 ± 1.1 cm/s, P < 0.01) and ISH (6.6 ± 1.6 cm/s, P = 0.07) than controls, resulting in the highest E/E′mean in SDH. In men, E′mean and E/E′mean were similar among the three groups.
Conclusion
These results suggest that elderly hypertensive women may have left ventricular early diastolic dysfunction and higher estimated filling pressure, consistent with their susceptibility to HFpEF. Women with SDH seemed to have more left ventricular diastolic dysfunction, which might be explained by the greater cumulative afterload when ambulatory.
Keywords: haemodynamics, left ventricular diastolic function, sex difference, stage 1 hypertension
INTRODUCTION
Hypertension promotes cardiovascular stiffening and impairs left ventricular function [1,2], resulting in increased risks for cardiovascular comorbidities such as heart failure with or without preserved ejection fraction, myocardial infarction and stroke [3]. Isolated systolic hypertension (ISH) is the most common form of hypertension in older individuals [4,5] and is associated with widening pulse pressure due to central artery stiffening [6]. High cardiovascular morbidity [4,7,8] and mortality [4] have been reported in individuals with ISH compared with normotensive individuals. Increased cardiovascular morbidity [9] and mortality [10] have also been observed in individuals with systolic-diastolic hypertension (SDH).
The current JNC-VII HTN guidelines define Stage 1 systolic hypertension as an SBP of 140–159 mmHg in the office, or ambulatory awake SBP ≥135 mmHg [5]. Stage 1 systolic hypertension is subdivided into ISH and SDH, which may be characterized by an increased sympathetic nerve activity, especially in young and middle-aged patients with hypertension [11]. A recent echocardiographic study [12] reported a reduction in left ventricular early diastolic filling in Stage 1 hypertensive patients treated with medications compared with normotensive controls. However, almost all types of antihypertensive drugs can affect the cardiovascular system. It is unknown whether and how hypertension subtype affects haemodynamics and left ventricular function in patients with Stage 1 hypertension.
Sexual dimorphism has been reported in haemodynamics, left ventricular geometry and diastolic function [13,14]. In healthy older individuals, women have more impaired left ventricular diastolic function, higher left atrial pressure and increased left ventricular stiffness than similarly aged men [15,16]. In Stage 1 hypertension, the HyperGEN study previously reported an enhanced left ventricular early filling in middle-aged women compared with men [17]. However, little is known about sex differences in haemodynamics or left ventricular early diastolic function in older individuals with Stage 1 hypertension.
On the basis of the results from the previous and recent studies, we hypothesized that older individuals with Stage 1 hypertension, especially women, would have more impaired left ventricular diastolic function than normotensive controls, and left ventricular diastolic function may be more impaired in Stage 1 patients with ISH than those with SDH.
MATERIALS AND METHODS
Study participants
Individuals were recruited from the Dallas-Fort Worth area. We recruited individuals from the Dallas Heart Study, a population-based, probability sample of 6101 individuals in the Dallas community [18]. In addition, E-mail notices were sent throughout the Texas Health System network informing participants of the study and requesting potential individuals to contact our research team. Approved flyers were also posted in an advertisement format in newspapers and within Dallas Area Rapid Transit locations; buses, light rail trains and stations. Our research nurse performed initial individual contact over the phone and potential individuals were invited to the laboratory for a screening visit. In total, 178 elderly hypertensive patients were screened. Of these, 95 patients (52 women, aged 60–83 years) with unmedicated Stage 1 hypertension (ambulatory awake SBP between 135 and 159 mmHg from 24-h ambulatory BP monitoring) met the inclusion criteria [5,19] and were enrolled in the study. Fifty-six healthy normotensive individuals were also recruited from the Dallas-Forth area as controls. Frequency matching (not 1 : 1 paired matching) for age, BMI, sex and socioeconomic status was employed to achieve balance of these characteristics across the groups. All the individuals were screened with a careful medical history, physical examination, 12-lead electrocardiogram, office (seated) BP measured by auscultation, 24-h ambulatory BP monitoring (Suntech Oscar2; Suntech Medical Instruments, Raleigh, North Carolina, USA) [20,21] and echocardiogram. After screening, patients with previous antihypertensive medications were weaned progressively from these drugs at least for 3 weeks (wash-out period), which was followed by a 3-week run-in period. Twenty-four-hour ambulatory BP measurements were repeated at the end of the run-in period in all the individuals and used for group assignment.
Twenty-four-hour ambulatory blood pressure monitoring
The ambulatory BP monitoring device measured BP every 30 min during the awakening period and every 30–60 min during the sleep period [20,21]. Individuals were instructed to maintain a diary listing the times of retiring to bed at night and awakening in the morning. On the basis of their bed time and awake time out of bed with transition periods surrounding bed and rising times, ambulatory awake BP was determined and used to stratify patients into ISH or SDH.
Individual stratification
Patients with Stage 1 hypertension were stratified into two groups: ISH (ambulatory awake DBP <85 mmHg from 24-h ambulatory BP monitoring) or SDH (ambulatory awake DBP ≥85 mmHg). Exclusion criteria included any evidence of cardiopulmonary disease or secondary hypertension by history or by physical examination, atrial fibrillation/flutter or left bundle branch block, ambulatory awake SBP at least ≥160 mmHg and/or DBP ≥100 mmHg, stenotic mitral or aortic valve, moderate to severe valvular regurgitation, left ventricular wall motion abnormality or impaired systolic function (ejection fraction <50% by echocardiography), BMI ≥35 kg/m2, regular exercise training (>20 min, >3 days per week), chronic kidney disease (serum creatinine >1.5 mg/dl), diabetes mellitus and current smoker. Women taking hormone replacement therapy were excluded. All individuals signed an informed consent form, which was approved by the institutional review boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Study protocol
All the individuals were required to measure BP daily at home using Life Source devices (A & D Engineering, Inc., San Jose, California, USA) [22] and come to our laboratory once a week for a BP check by auscultation during the run-in period. At each visit, patients received counselling and a pamphlet to maintain a healthy lifestyle as recommended by the current JNC VII standard guidelines [5], including maintaining normal body weight; adopting the Dietary Approaches to Stop Hypertension (DASH) eating plan (consume a diet rich in fruits, vegetables and low-fat dairy products); dietary sodium reduction (≤ 6 g sodium chloride per day); aerobic physical activity such as walking; and moderation of alcohol consumption. Patients were excluded from the study and were asked to resume their previous antihypertensive medications immediately if their SBP in the office was at least ≥160 mmHg and/or DBP was ≥100 mmHg. Healthy controls received the same counselling and a pamphlet to maintain a healthy lifestyle during the run-in period.
Individuals were given a constant diet consisting of 100 mEq sodium, which corresponds to approximately 6 g of sodium chloride, 100 mEq potassium and 1000 mg calcium 3 days prior to the haemodynamic study. The diet was provided by the Clinical and Translational Research Center (CTRC) at University of Texas Southwestern Medical Center. On the day of haemodynamic study, individuals had a light breakfast provided by the CTRC, and then came to our laboratory for testing.
Haemodynamic measurements
All experiments were performed in a quiet, environmentally controlled laboratory with an ambient temperature of approximately 25°C. Individuals were relaxed in the supine position. Arm cuff BP was measured by electrosphygmomanometry (model 4240; Suntech Medical Instruments, Raleigh, North Carolina, USA) with a microphone placed over the right brachial artery to detect Korotkoff sounds [23]. Heart rate was detected from lead II of the electrocardiogram (Agilent, Munich, Germany). At least 30 min after the instrumentation, cardiac output was measured by a modification of the acetylene rebreathing method [24,25]. Supine BPs and cardiac output were measured every 5 min at least three times and the average value was recorded. Stroke volume was calculated from cardiac output divided by heart rate. Total peripheral resistance was determined as 80 × mean BP divided by cardiac output. Effective arterial elastance (Ea) was defined as brachial SBP × 0.9 divided by stroke volume [26,27]. Total arterial compliance was calculated as stroke volume divided by pulse pressure. All these variables were normalized by body surface area.
Assessment of left ventricular systolic function and morphology
After the measurements of haemodynamics, a transthoracic echocardiogram was obtained using iE33 echocardiograph (Philips). Left ventricular end-diastolic and end-systolic volumes were determined from the apical four-chamber view by the modified Simpson's method of disks that was used in our previous studies [25]. The peak systolic mitral annular velocity (S′mean) was measured in both septal and lateral sides of the mitral annulus, and the values were averaged. Ejection fraction was assessed as (left ventricular end-diastolic volume − end-systolic volume) divided by left ventricular end-diastolic volume. Left ventricular posterior wall thickness was obtained in the parasternal long-axis view. The within-individual typical error (expressed as coefficient variation) for left ventricular end-diastolic volume measurements was 2.1% (95% confidence interval: 1.8–2.6), and the between-individual typical error was 6.2% (95% confidence interval: 4.5–9.7) in our laboratory.
Assessment of left ventricular diastolic function
Left ventricular early (E) and late (A) filling peak velocities were recorded and the ratio of E/A was used to assess global left ventricular diastolic function [25,28]. The peak early diastolic mitral annular velocity was measured in both septal and lateral sides of the mitral annulus and values were averaged to obtain E′mean [28] and was used to estimate left atrial pressure or left ventricular end-diastolic filling pressure (E/ E′mean) as previously reported [29,30]. Colour M-mode Doppler was obtained and the mitral flow propagation velocity was measured by the slope along the aliasing isovelocity line [25,28]. Isovolumic relaxation time (IVRT) was also determined. To evaluate left ventricular late diastolic function or diastolic stiffness, operant diastolic elastance (Ed) was estimated as E/E′mean divided by absolute left ventricular end-diastolic volume measured by echocardiography [31]. Ed index was estimated as Ed divided by body surface area. The within-individual and between-individual typical errors for E′mean measurements were less than 1%.
Statistical analysis
Statistical analyses were performed using commercially available software. Data were expressed as mean ± SD unless otherwise noted. Haemodynamics and echo data were compared by two-way analysis of variance (ANOVA) with Tukey posthoc analysis and the Kruskal–Wallis test was used for nonnormally distributed data. As age may affect variables such as E′mean, E/E′mean and Ed, data were also compared using two-way analysis of covariance (ANCOVA) with age as a covariate. Simple and multivariate linear regression analyses with forward stepwise procedure (P < 0.10 for entry) were applied to assess the relationships between E′mean and clinical variables. A P value of less than 0.05 was considered significant.
RESULTS
Individual characteristics
There were 95 hypertensive patients (age: 69 ± 5 years) and 56 healthy normotensive controls (age: 69 ± 6 years) in the present study. Antihypertensive medication was previously prescribed in 50 patients. As shown in Table 1, there were no differences in age or BMI among the six groups, while body surface area was larger in men than in women (sex effect P < 0.01). No sex difference was observed in ambulatory awake BPs within the normotensive, SDH or ISH group. However, ambulatory awake SBP was higher in SDH than in ISH and controls in both men and women (P < 0.05).
TABLE 1.
Individual characteristics
| Women |
Men |
Sex effect | Group effect | Interaction effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Normotensive | SDH | ISH | Normotensive | SDH | ISH | |||
| Number | 31 | 13 | 39 | 25 | 22 | 21 | – | – | – |
| Age (years) | 69±7 | 65±6 | 69±5 | 69±5 | 69±4 | 70±5 | 0.12 | 0.11 | 0.39 |
| Body surface area (m2) | 1.78±0.17 | 1.84±0.11 | 1.79±0.15 | 1.98±0.15*** | 2.07±0.18*** | 2.07±0.16*** | <0.01 | 0.08 | 0.37 |
| BMI (kg/m2) | 26.8±4.0 | 29.3±4.7 | 27.8±4.4 | 27.3±3.3 | 27.7±3.2 | 28.3±3.9 | 0.79 | 0.20 | 0.40 |
| Ambulatory SBP (mmHg) | 124±7 | 155±5* | 145±8*,** | 126±6 | 152±7* | 146±9*,** | 0.78 | <0.01 | 0.13 |
| Ambulatory DBP (mmHg) | 72±7 | 92±5* | 75±6** | 74±4 | 89±3* | 79±5*,*** | 0.17 | <0.01 | 0.01 |
| Ambulatory MBP (mmHg) | 89±6 | 113±4* | 98±6*,** | 92±5 | 110±3* | 102±5*,**,*** | 0.27 | <0.01 | 0.02 |
| Ambulatory PP (mmHg) | 52±6 | 63±6* | 70±7*,** | 52±5 | 63±6* | 67±9* | 0.28 | <0.01 | 0.57 |
| Supine SBP (mmHg) | 109±10 | 126±12* | 123±12* | 111±11 | 129±12* | 124±18* | 0.32 | <0.01 | 0.86 |
| Supine DBP (mmHg) | 61±7 | 74±7* | 66±9*,** | 66±7*** | 72±8* | 68±9 | 0.18 | <0.01 | 0.18 |
| Supine MBP (mmHg) | 77±7 | 91±8* | 85±9* | 81±8 | 91±9* | 86±11 | 0.19 | <0.01 | 0.53 |
| Supine PP (mmHg) | 48±9 | 52±10 | 58±10* | 45±6 | 57±8* | 56±13* | 0.86 | <0.01 | 0.18 |
| Supine heart rate (bpm) | 68±7 | 69±8 | 68±10 | 62±9*** | 68±11 | 63±8*** | <0.01 | 0.18 | 0.49 |
| Cardiac index (l/min per m2) | 2.07±0.36 | 2.19±0.35 | 2.09±0.35 | 2.29±0.35*** | 2.37±0.49 | 2.18±0.49 | 0.02 | 0.26 | 0.71 |
| SV index (ml/m2) | 30.6±5.6 | 32.0±7.0 | 30.9±4.4 | 37.7±8.3*** | 35.4±6.7 | 35.3±8.1*** | <0.01 | 0.70 | 0.38 |
| Ea index (mmHg/ml per m2) | 3.3±0.7 | 3.7±0.8 | 3.7±0.6 | 2.8±0.6*** | 3.4±0.8* | 3.3±0.7*,*** | <0.01 | <0.01 | 0.70 |
| TPR index (dyne•s/cm5•m2) | 3046±636 | 3419±667 | 3330±587 | 2894±531 | 3188±644 | 3253±518 | 0.14 | <0.01 | 0.84 |
| TAC index (ml/mmHg•m2) | 0.67±0.20 | 0.65±0.26 | 0.55±0.11* | 0.86±0.27*** | 0.63±0.13* | 0.64±0.18* | <0.01 | <0.01 | 0.04 |
Values are mean±SD. ISH, isolated systolic hypertension; ambulatory SBP, ambulatory awake SBP from 24-h ambulatory blood pressure monitoring; Ea, effective arterial elastance; MBP, mean blood pressure; PP, pulse pressure; SDH, systolic-diastolic hypertension; SV, stroke volume; TAC, total arterial compliance; TPR, total peripheral resistance.
P<0.05 vs. normotensive individuals within the same sex.
P<0.05 vs. patients with SDH within the same sex.
P<0.05 for men vs. women within the same BP group.
Haemodynamic measurements during supine rest
As shown in Table 1, supine SBP was similar between men and women within the three groups. Patients had higher supine SBP than controls. No difference was observed in supine SBP between SDH and ISH. Supine pulse pressure was similar between ISH and SDH in both sexes.
No differences were observed in cardiac or stroke volume indexes among the three groups within the same sex. However, women had smaller cardiac and stroke volume indexes than men (ANOVA P < 0.05), resulting in a tendency towards higher Ea, especially in hypertensive women. Normotensive women had higher Ea and smaller total arterial compliance indexes than normotensive men. In men, Ea was higher and total arterial compliance was smaller in hypertensive patients than in controls. However, Ea was similar among the groups in women.
Cardiac size and left ventricular systolic function
As shown in Table 2, women had smaller left ventricular end-diastolic volume and end-systolic volume indexes than men (sex effect P < 0.05). No significant interaction effects were observed in left ventricular end-diastolic volume or end-systolic volume indexes. S′mean was lower in women (sex effect P < 0.01), whereas ejection fraction was similar (around 70%) in all the six groups (sex effect P = 0.60).
TABLE 2.
Echocardiographic parameters
| Women |
Men |
Sex effect | Group effect | Interaction effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Normotensive | SDH | ISH | Normotensive | SDH | ISH | |||
| LVEDV index (ml/m2) | 43.9±6.5 | 46.4±8.7 | 41.6±7.3 | 48.1±7.5*** | 49.6±6.2 | 47.1±9.6*** | <0.01 | 0.09 | 0.77 |
| LVESV index (ml/m2) | 13.1±4.0 | 14.1±4.1 | 12.6±3.9 | 15.3±3.4*** | 14.9±3.4 | 14.3±4.8 | 0.02 | 0.42 | 0.71 |
| LV ejection fraction (%) | 71±6 | 70±6 | 70±7 | 68±5 | 70±6 | 70±6 | 0.60 | 0.84 | 0.45 |
| LV wall thickness (mm) | 8.6±0.9 | 9.6±1.4* | 9.7±1.1* | 8.7±1.2 | 9.6±1.1* | 9.8±1.1* | 0.61 | <0.01 | 0.95 |
| Peak E wave (cm/s) | 73±16 | 76±12 | 76±18 | 64±12*** | 60±11*** | 65±14*** | <0.01 | 0.61 | 0.55 |
| Peak A wave (cm/s) | 78±22 | 92±23* | 80±15 | 65±16*** | 71±15*** | 71±17*** | <0.01 | 0.04 | 0.35 |
| E/A ratio | 0.97±0.25 | 0.86±0.18 | 0.97±0.25 | 1.03±0.27 | 0.88±0.27 | 0.95±0.23 | 0.65 | 0.06 | 0.78 |
| S′mean (cm/s) | 7.3±0.8 | 6.8±0.7* | 7.2±1.1 | 8.0±1.1*** | 8.3±1.1*** | 8.6±1.3*** | <0.01 | 0.23 | 0.14 |
| E′mean (cm/s) | 7.4±1.1 | 5.9±1.6* | 6.7±1.6 | 7.3±1.2 | 7.2±1.7*** | 7.1±1.6 | 0.04 | 0.05 | 0.07 |
| IVRT (ms) | 113±24 | 129±25* | 111±17** | 123±17 | 134±19 | 122±18*** | 0.01 | <0.01 | 0.82 |
| Vp (cm/s) | 45±13 | 48±15 | 49±17 | 43±12 | 42±10 | 49±13 | 0.26 | 0.07 | 0.58 |
Values are mean±SD. E′mean, average of the peak early velocities of septal and lateral mitral annulus; ESV, end-systolic volume; ISH, isolated systolic hypertension; IVRT, isovolumic relaxation time; LVEDV, left ventricular end-diastolic volume; S′mean, average of the peak systolic velocities of septal and lateral mitral annulus; SDH, systolic-diastolic hypertension; Vp, mitral inflow propagation velocity.
P<0.05 vs. normotensive individuals within the same sex.
P<0.05 vs. patients with SDH within the same sex.
P<0.05 for men vs. women within the same BP group.
Doppler measures of diastolic function
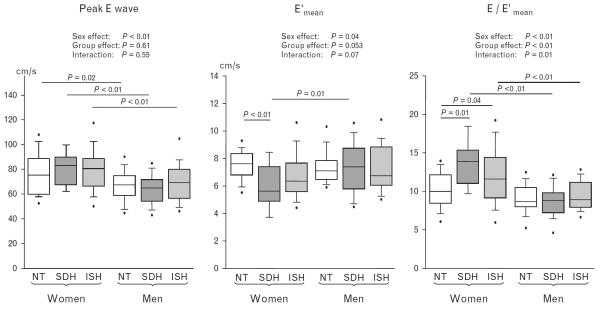
Women exhibited faster peak E wave and shorter IVRT than men (both sex effect P < 0.05, Table 2). When age was adjusted, women also had faster E wave and shorter IVRT than men (both sex effect P < 0.05). However, women had slower E′mean (sex effect P = 0.04), suggesting more impaired left ventricular relaxation in women than in men. In women, patients with SDH had slower E′mean than controls (5.9 ± 1.6 vs. 7.4 ± 1.1 cm/s, P < 0.01), whereas those with ISH showed a tendency towards slower E′mean than controls (6.6 ± 1.6 cm/s, P = 0.07). E/E′mean was the highest in SDH (Fig. 1 and Table 2). In men, E, E′mean and E/E′mean were similar among the three groups.
FIGURE 1.
Peak E wave, E′mean and E/E′mean during the haemodynamic study. ISH, isolated systolic hypertension; NT, normotensive; SDH, systolic-diastolic hypertension.
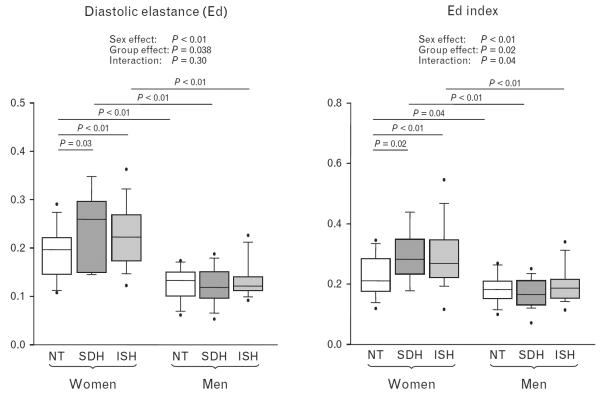
As shown in Fig. 2, Ed and Ed index suggested a stiffer left ventricle in hypertensive patients than normotensive controls in women (P < 0.05), but not in men. After adjusting for age, hypertensive women also had slower E′mean, higher E/E′mean and increased Ed (interaction effect P < 0.05) than other groups.
FIGURE 2.
Operant diastolic elastance (Ed) and Ed index during the haemodynamic study. Ed was significantly greater in women with SDH and ISH than in controls. ISH, isolated systolic hypertension; NT, normotensive; SDH, systolic-diastolic hypertension.
Correlations between left ventricular early diastolic function and arterial stiffness
As shown in Table 3, age and ambulatory awake SBP, but not supine BPs or arterial stiffness, were independently correlated to left ventricular early diastolic dysfunction assessed by E′mean in women. In men, a mild inverse correlation was observed between supine mean BP and E′mean.
TABLE 3.
Correlations between haemodynamics and left ventricular early diastolic function (E′mean) stratified by sex
| Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|
| Simple regression |
Multiple regression |
Simple regression |
Multiple regression |
|||||
| P | r | P | β | P | r | P | β | |
| Age (years) | 0.01 | −0.27 | <0.01 | −0.31 | 0.11 | −0.20 | – | – |
| Supine heart rate (bpm) | 0.99 | −0.001 | – | – | 0.40 | −0.11 | – | – |
| Ambulatory awake SBP (mmHg) | <0.01 | −0.35 | <0.01 | −0.38 | 0.49 | −0.09 | – | – |
| Ambulatory awake MBP (mmHg) | 0.03 | −0.33 | 0.26 | −0.13 | 0.83 | −0.03 | – | – |
| Ambulatory awake PP (mmHg) | 0.04 | −0.22 | 0.22 | 0.14 | 0.25 | −0.14 | – | – |
| Supine SBP (mmHg) | 0.051 | −0.22 | 0.74 | 0.04 | 0.17 | −0.17 | – | – |
| Supine MBP (mmHg) | 0.04 | −0.23 | 0.73 | −0.04 | 0.046 | −0.24 | 0.046 | −0.24 |
| Supine PP (mmHg) | 0.35 | −0.10 | – | – | 0.81 | −0.03 | – | – |
| Ea index (mmHg/ml per m2) | <0.01 | −0.29 | 0.22 | −0.14 | 0.09 | −0.21 | 0.34 | −0.12 |
| TPR index (dyne•s/cm5•m2) | <0.01 | −0.30 | 0.09 | −0.19 | 0.13 | −0.19 | – | – |
| TAC index (ml/mmHg•m2) | 0.06 | 0.21 | 0.72 | 0.04 | 0.67 | 0.05 | – | – |
Values are mean±SD. Correlation coefficients of simple (r) and multiple (β) regression analysis and P values are displayed. E′mean, average of the peak early velocities of septal and lateral mitral annulus; Ea, effective arterial elastance; PP, pulse pressure; TAC, total arterial compliance; TPR, total peripheral resistance.
DISCUSSION
In the present study, we demonstrated in older patients with Stage 1 hypertension that women appeared to have more impaired left ventricular diastolic function and higher estimated filling pressure than men (both P < 0.01). These results suggest that women with SDH seemed to exhibit more impaired left ventricular diastolic function than those with ISH, which may be at least in part explained by the greater cumulative left ventricular afterload when ambulatory.
Supine and 24-h ambulatory blood pressures in systolic-diastolic hypertension and isolated systolic hypertension
ISH is characterized by central artery stiffness and is often observed in older individuals [6]. During supine rest, we observed similar SBP and total peripheral resistance in hypertensive men and women with Stage 1 hypertension, although women had higher Ea index than men, especially in ISH. These findings may suggest stiffer central arterial stiffness in women. In addition to the higher ambulatory DBP by definition, a greater increase in DBP from the supine to ambulatory measurements was observed in SDH compared with ISH in both sexes (18 ± 6 vs. 9 ± 10 mmHg in women, P < 0.05, and 17 ± 7 vs. 12 ± 9 mmHg in men, P = 0.09).
Effects of sex on haemodynamics and left ventricular diastolic function
We observed greater arterial stiffness in normotensive women than in normotensive men, although they had similar blood pressures (BPs) and total peripheral resistance. In men, Stage 1 hypertension was significantly related to the increased arterial stiffness. Conversely, this relationship was not observed in women. These findings suggest that the age-related increase in arterial stiffness is more prominent in older women than in men, and that ageing may have a greater impact on arterial stiffness than on BP in women.
In healthy older individuals, left ventricular diastolic function deteriorates more significantly in women than in men. For example, a greater decline in long-axis velocities during diastole [15], a slower left ventricular relaxation [32] and increased left ventricular stiffness [16] have been reported in older women than in men with no cardiovascular diseases. However, little is known about left ventricular diastolic function in patients with unmedicated Stage 1 hypertension. A few earlier studies in Stage 1 hypertension reported faster E wave in older women (≥65 years) [8] or in middle-aged women than in similarly aged men (77±19 vs. 70±18 cm/s, P<0.001) [17]. As women had shorter isovolumic relaxation time than men (81±17 vs. 85±18 ms, P<0.001), it was speculated that middle-aged women had brisk left ventricular relaxation and more enhanced left ventricular early filling than men [17].
Consistent with previous studies, we observed faster E wave (sex effect P<0.01) and shorter IVRT (sex effect P=0.01) in women than in men. When age was adjusted, the same findings were observed in E wave and IVRT (both sex effect P<0.05). In normotensive individuals, there was no difference in E′mean between men and women, whereas E′mean, which had not been reported in the previous two studies [8,17], was significantly slower in hypertensive women than in hypertensive men (sex effect P=0.04). Therefore, our Doppler results in women with hypertension (faster E, shortened IVRT and slower E′mean) could result from an increase in left atrial driving pressure, but not likely from a decrease in left ventricular minimal pressure due to an improvement in left ventricular relaxation. A higher value for E/E′mean observed in older hypertensive women may support this speculation. A smaller mitral annular size in women might be also related to the faster E wave in women [33].
In previous reports by others, a greater impairment in endothelial function was observed only in women with Stage 1 hypertension [34]. An age-associated decline in E′mean was observed in healthy women after the age of 65, but not in men [32]. In the present study, echo Doppler variables such as S′mean and E′mean were significantly affected by hypertension subtype in women, but not in men. No correlation was observed between E′mean and age in men, although age was inversely correlated to E′mean in women. These findings might partly explain the sex difference in the occurrence of HFpEF in older individuals.
Effects of hypertension subtype on haemodynamics and left ventricular diastolic function
Women with hypertension had significantly impaired left ventricular early and late diastolic function, especially in SDH. Ambulatory BP monitoring can track daily changes in BPs, thus is a better predictor for cardiovascular mortality in hypertensive patients [35]. In the present study, patients with SDH had higher ambulatory awake systolic, diastolic and mean BPs than those with ISH, which appeared to be more affected by the sympathetic nervous system with a larger increase in DBP when ambulatory.
A recent study reported that left ventricular diastolic dysfunction was modestly associated with arterial stiffness measured during supine rest in older men and women [36]. In the present study, we also observed that arterial stiffness was inversely correlated with E′mean by a simple linear regression analysis in older women. However, by a multivariate analysis, ambulatory awake SBP and age were the only independent determinants of impaired left ventricular early diastolic function. In SDH, the aortic valve may open at a higher left ventricular pressure when ambulatory. Thus, it may be possible that this cumulative afterload when ambulatory is responsible for the impaired left ventricular diastolic function obtained during supine rest. Preliminary data from our laboratory showed that older women with Stage 1 SDH had the highest total peripheral resistance after 20 min of 60° upright tilt among patients with different subtypes of hypertension.
The ICARE study enrolled Stage 2 hypertensive patients and observed greater arterial stiffness and left ventricular hypertrophy in ISH than in SDH [37]. In this previous study, supine pulse pressure was significantly higher in ISH than in SDH (87± vs. 65±14mmHg, P<0.001), suggesting the stiffer central artery in Stage 2 ISH than SDH. To our knowledge, there is no report evaluating haemodynamics in ISH and SDH in Stage 1 hypertension. In contrast to previous studies, we observed no differences in SBP, pulse pressure, Ea or total peripheral resistance during the haemodynamic study at supine rest between ISH and SDH. It is possible that some of these variables may be different between hypertension subtypes during ambulatory or during upright posture. SBP continuously increases along with ageing, while DBP increases during middle-age and then begins to decrease during senior years. Therefore, ISH with Stage 1 hypertension may consist of patients with different stages of cardiovascular stiffening. This heterogeneity in ISH with Stage 1 hypertension might be related to our finding of similar or less cardiovascular stiffening in ISH compared with SDH.
Clinical relevance
Our findings that women with Stage 1 SDH had more impaired left ventricular diastolic function and higher estimated left ventricular filling pressure than women with ISH may provide a rationale for the treatment of Stage 1 SDH as well as ISH, especially in older women. In patients with Stage 1 hypertension, thiazide-type diuretics have been used as the basis of antihypertensive treatment [5]. However, thiazide-type diuretics cause exaggerated sympathetic activation [38]. Persistent sympathetic activation may cause left ventricular hypertrophy and end-organ damage. The renin–angiotensin–aldosterone system inhibitors have favourable effects on arterial compliance [39] and left ventricular diastolic function [40], resulting in better clinical outcomes than thiazide monotherapy in patients at lower cardiovascular risks [41]. Moreover, combination therapies of angiotensin receptor blockers and thiazide-type diuretics [39,42], or calcium channel blockers and angiotensin-converting enzyme inhibitors [43] were reported to have more beneficial effects on cardiovascular morbidities [39,42] and outcomes [42,43] in older hypertensive patients. Thus, combined drug treatment might also be recommended for older Stage 1 hypertensive patients, especially women with SDH.
Limitations
There are some limitations in this study. First, our results may reflect a different prevalence of hypertension subtypes between Stage 1 and Stage 2 hypertension in the community. If we follow these ISH patients over time, some patients may experience increases in their BPs, and be categorized as ISH with Stage 2 hypertension [4] or SDH [44]. Second, due to the relatively small sample size and the number of tests carried out, there might be a probability of false positives in our results. Therefore, future studies are required to confirm our findings. Third, the left ventricular filling pressure was estimated by use of E/E′mean. We also used an indirect measure of left ventricular stiffness, although invasive left ventricular pressure–volume loop is the gold standard for assessing left ventricular stiffness. Fourth, we evaluated arterial function by stroke volume and brachial cuff pressures, but not by calibrated central blood pressures using tonometry. Lastly, about a half of our hypertensive patients were previously on antihypertensive medications. All antihypertensive medications significantly affect haemodynamics. However, all patients with previous medications had been free of them at least 3 weeks before the haemodynamic study.
In conclusion, our results suggest that older women with hypertension may have greater left ventricular diastolic dysfunction with higher left ventricular filling pressure and increased left ventricular stiffness than older men. Furthermore, women with Stage 1 SDH appeared to have more impaired left ventricular early diastolic function than women with ISH, which may be due in part to the greater cumulative left ventricular afterload when ambulatory.
ACKNOWLEDGEMENTS
This study was supported by the National Institutes of Health grant R01 HL091078.
Abbreviations
- E′mean
averaged mitral annulus velocity
- Ea
effective arterial elastance
- Ed
operant diastolic elastance
- ISH
isolated systolic hypertension
- S′mean
averaged peak systolic mitral annular velocity
- SDH
systolic-diastolic hypertension
Footnotes
Conflicts of interest
The authors have not declared any conflicts of interest.
REFERENCES
- 1.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43:739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 3.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 4.Sagie A, Larson M, Levy D. The natural history of borderline isolated systolic hypertension. N Engl J Med. 1993;329:1912–1917. doi: 10.1056/NEJM199312233292602. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a `set up' for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 7.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53:458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O'Leary DH, et al. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 9.Papademetriou V, Devereux RB, Narayan P, Wachtell K, Bella JN, Gerdts E, et al. Similar effects of isolated systolic and combined hypertension on left ventricular geometry and function: the LIFE Study. Am J Hypertens. 2001;14:768–774. doi: 10.1016/s0895-7061(01)01292-4. [DOI] [PubMed] [Google Scholar]
- 10.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 11.Grassi G, Colombo M, Seravalle G, Spaziani D, Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension. 1998;31:64–67. doi: 10.1161/01.hyp.31.1.64. [DOI] [PubMed] [Google Scholar]
- 12.Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging. 2009;2:93–99. doi: 10.1161/CIRCIMAGING.108.793190. [DOI] [PubMed] [Google Scholar]
- 13.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127:55–62. doi: 10.1161/CIRCULATIONAHA.112.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Föll D, Jung B, Schilli E, Staehle F, Geibel A, Hennig J, et al. Magnetic resonance tissue phase mapping of myocardial motion: new insight in age and gender. Circ Cardiovasc Imaging. 2010;3:54–64. doi: 10.1161/CIRCIMAGING.108.813857. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Ageand gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 17.Bella JN, Palmieri V, Kitzman DW, Liu JE, Oberman A, Hunt SC, et al. Gender difference in diastolic function in hypertension (the HyperGEN study) Am J Cardiol. 2002;89:1052–1056. doi: 10.1016/s0002-9149(02)02274-9. [DOI] [PubMed] [Google Scholar]
- 18.Victor RG, Haley RW, Willett D, Peshock R, Vaeth P, Leonard D, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 20.Jones SC, Bilous M, Winship S, Finn P, Goodwin J. Validation of the OSCAR 2 oscillometric 24-h ambulatory blood pressure monitor according to the International Protocol for the validation of blood pressure measuring devices. Blood Press Monit. 2004;9:219–223. doi: 10.1097/00126097-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin J, Bilous M, Winship S, Finn P, Jones SC. Validation of the Oscar 2 oscillometric 24-h ambulatory blood pressure monitor according to the British Hypertension Society protocol. Blood Press Monit. 2007;12:113–117. doi: 10.1097/MBP.0b013e3280acab1b. [DOI] [PubMed] [Google Scholar]
- 22.Longo D, Bertolo O, Toffanin G, Frezza P, Palatini P. Validation of the A&D UA-631 (UA-779 Life Source) device for self-measurement of blood pressure and relationship between its performance and large artery compliance. Blood Press Monit. 2002;7:243–248. doi: 10.1097/00126097-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Taylor R, Gallen I. Evaluation of SunTech 4240 during rest and during exercise: a novel automated blood pressure device. J Cardiopulm Rehabil. 1994;14:330–334. [Google Scholar]
- 24.Laszlo G. Respiratory measurements of cardiac output: from elegant idea to useful test. J Appl Physiol. 2004;96:428–437. doi: 10.1152/japplphysiol.01074.2001. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 28.Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, et al. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure and a preserved ejection fraction. Circ Heart Fail. 2010;3:617–626. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 30.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 31.Shah SJ, Blair JE, Filippatos GS, Macarie C, Ruzyllo W, Korewicki J, et al. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am Heart J. 2009;157:1035–1041. doi: 10.1016/j.ahj.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, et al. Ageand gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging. 2009;2:41–46. doi: 10.1161/CIRCIMAGING.108.809087. [DOI] [PubMed] [Google Scholar]
- 33.Gardin JM, Arnold AM, Bild DE, Smith VE, Lima JA, Klopfenstein HS, Kitzman DW. Left ventricular diastolic filling in the elderly: the cardiovascular health study. Am J Cardiol. 1998;82:345–351. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- 34.Routledge FS, Hinderliter AL, Blumenthal JA, Sherwood A. Sex differences in the endothelial function of untreated hypertension. J Clin Hypertens. 2012;14:228–235. doi: 10.1111/j.1751-7176.2012.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Björklund K, Lind L, Zethelius B, Andrén B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107:1297–1302. doi: 10.1161/01.cir.0000054622.45012.12. [DOI] [PubMed] [Google Scholar]
- 36.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, et al. Arterial stiffness and wave reflection: Sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, et al. Cardiovascular remodeling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol. 2002;40:1283–1289. doi: 10.1016/s0735-1097(02)02159-9. [DOI] [PubMed] [Google Scholar]
- 38.Menon DV, Arbique D, Wang Z, Adams-Huet B, Auchus RJ, Vongpatanasin W. Differential effects of chlorthalidone versus spironolactone on muscle sympathetic nerve activity in hypertensive patients. J Clin Endrocrinol Metab. 2009;94:1361–1366. doi: 10.1210/jc.2008-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, Salvetti A. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–1286. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- 40.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 41.Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 42.Ito H, Ishii K, Kihara H, Kasayuki N, Nakamura F, Shimada K, et al. Adding thiazide to a renin-angiotensin blocker improves left ventricular relaxation and improves heart failure in patients with hypertension. Hypertens Res. 2012;35:93–99. doi: 10.1038/hr.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 44.Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, Vasan RS, Levy D. Predictors of new-onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation. 2005;111:1121–1127. doi: 10.1161/01.CIR.0000157159.39889.EC. [DOI] [PubMed] [Google Scholar]