Abstract
Multifocal and recurrent epithelial tumors, originating from either dormant or de novo cancer cells, are major causes of morbidity and mortality. The age-dependent increase of cancer incidence has long been assumed to result from the sequential accumulation of cancer driving or facilitating mutations with induction of cellular senescence as a protective mechanism. However, recent evidence suggests that the initiation and development of epithelial cancer results from a close interplay with its altered tissue microenvironment, with chronic inflammation, stromal senescence, autophagy, and activation of cancer associated fibroblasts (CAFs) playing possible primary roles. We will discuss recent progress in these areas, and highlight how this understanding may be used for devising novel preventive and therapeutic approaches to the epithelial cancer problem.
Keywords: stromal microenvironment, senescence, SASP, skin cancer, autophagy
Drivers of epithelial carcinogenesis
The accumulation of genetic and epigenetic changes in target cells is commonly viewed as the primary cause of epithelial cancer development. However, the analysis of surgically excised non-tumor areas as well as the increased use of non-invasive imaging techniques has revealed a surprisingly high incidence of precancerous epithelial lesions, which might never progress into malignancy [1, 2]. Many genetic mutations implicated as "drivers" of the carcinogenic process can be found in these lesions, bringing into question whether such changes are of primary causative significance for cancer outgrowths [3]. Also, for reasons that are not understood, epithelial cancers of various types are often multifocal, with secondary primary tumors frequently developing at a significant distance from the primary tumors [4].
Substantial evidence suggests that development of epithelial neoplastic lesions involves alterations of a normal stromal environment. The stromal environment consists of several cell types (BOX1), which can often become dysregulated due to aging, environmental changes, and inflammation. As discussed further below, fibroblasts can become senescent through aging and environmental stress and these senescent fibroblasts can contribute to a tumor permissive environment by secreting specific cytokines, growth factors and metalloproteases, which constitute the Senescence Associated Secretory Phenotype (SASP) [5, 6]. Furthermore, as cancer has often been classified as a “wound that never heals” [7], similarities exist between the stromal microenvironment during wound healing and cancer progression [8] (Box 2). Similar to wound healing, resident fibroblasts in a tumor microenvironment convert into contractile myofibroblasts termed cancer associated fibroblasts (CAFs) [9], which are capable of enhancing tumorigenic behavior of neighboring cells [9, 10]. In addition, recent studies show that there is a close association between the lysosomal degradative process known as autophagy and the tumor microenvironment. Both CAFs and senescent cells display altered autophagy, which can fuel and mold the stromal environment [6, 11] leading to tumor progression.
BOX1: RESIDENT STROMAL CELLS.
Substantial similarities exist in the stromal compartment of various organs, including the bone marrow [114]. Besides providing basic physical support, stromal cells play an essential function in development and organogenesis, contributing to tissue repair and disease development [115]. The stroma is composed of a complex and loosely organized network of multiple cell types embedded in an extracellular matrix that provides structural support and participates in the control of cellular signaling. The stroma is composed of the following major cell types: fibroblasts, pericytes, smooth muscle and endothelial cells, pre-adipocytes, and mesenchymal stem cells.
Fibroblasts share a commonality of function, i.e. extracellular matrix production for tissue maintenance and repair, but can have heterogeneous properties even within the same organs, that can be maintained with cultivation [116, 117]. These cells are key in orchestrating stromal composition and contribute to tissue repair through several mechanisms including conversion into contractile myofibroblasts [42].
Pericytes, smooth muscle and endothelial cells are stromal components which play a role in angiogenesis. While pro-angiogenic cytokines are produced by epithelial and stromal cells at early cancer stages or as a consequence of inflammation [10], increased angiogenesis is usually considered a late event in carcinogenesis [10].
While generally assumed to be confined to adipose tissue, pre-adipocytes can also be found in the bone marrow [118] and stromal compartments of other organs such as skin [119]. A possible involvement of these cells in carcinogenesis has not been assessed, but it seems likely in view of their involvement during wound healing [119]. There are also interesting similarities between the gene expression program of pre-adipocytes and macrophages with the first cell type having the potential of converting into the other [120, 121]. A functional interconnection also exists between mesenchymal stroma and underlying adipose tissue, which can be a source of mesenchymal stem cells as well as cytokines triggering stromal cell senescence and/or inflammation [122].
BOX2: THE CONNECTION BETWEEN CANCER ASSOCIATED FIBROBLASTS AND WOUND HEALING.
Many similarities exist between cancer associated tissue alterations and acute wound healing, including a common gene expression signature of surrounding fibroblasts [8]. Cancer has been defined as a "wound that never heals" [7] and wound healing can promote cancer formation [123]. In the skin, the wound healing reaction is associated with the conversion of stromal fibroblasts into contractile myofibroblasts, which, upon successful healing, normally disappear as a consequence of cytokine -induced apoptosis (e.g.IL1, TGF-ß) or senescence [42]. Cancer Associated Fibroblasts (CAFs) can be viewed as myofibroblasts that persist under pathological conditions [9], and α-smooth muscle actin (α-SMA) is a common marker used for the identification of both myofibroblasts and CAFs. Importantly, however, α-SMA is expressed only in CAFs of certain cancer types [124, 125], consistent with organ specific differences of resident fibroblast populations. As a general definition, CAFs consists of fibroblasts capable of enhancing tumorigenic behavior of neighboring epithelial cells [9, 10]. As discussed, these cells may not only promote but initiate epithelial cancer.
The contribution of stromal alterations to cancer progression and metastasis has been covered by excellent reviews (see for instance [9, 10, 12]). Here we intend to provide selective highlights on how stromal changes caused by chronic inflammation and activation of cancer associated fibroblasts, in association with stromal cell senescence and/or increased autophagy, may contribute to initiation of epithelial carcinogenesis. We will further discuss how this knowledge may be used for development of novel preventive and therapeutic approaches to epithelial tumors, including multifocal and recurrent forms.
Stromal tissue inflammation and epithelial cancer
Chronic inflammation plays an important role in cancer initiation. For instance, patients with ulcerative colitis have an increased incidence of colon cancer. Similarly, mice administered dextran sodium sulfate (DSS), a pro-inflammatory tissue-damaging agent devoid of mutagenic activity, form colon cancer [13]. In addition, inflammation due to Helicobacter pilori infection may also be a likely mediator of stomach cancer development [14].
In both normal and cancer settings, inflammation involves a complex orchestration of cells of the innate and acquired immune systems. Neutrophils are the first cells recruited to sites of acute inflammation [15]. Production of interleukin 6 (IL6) and macrophage colony stimulating factor 1 (CSF-1) by these cells or by the incipient cancer cells can push myeloid precursors towards the macrophage lineage [16]. Macrophages play a key role as both suppressors and enhancers of carcinogenesis [17]. These cells are very dynamic and can assume a “killing” M1 phenotype, for elimination of pathogens and cancer cells, or a permissive M2 phenotype, for resolution or containment of acute inflammation [18]. Notably, within chronic inflammatory stroma both M1 and M2 macrophage populations coexist and most macrophages can possess a mixed phenotype [19]
Macrophages can also regulate the inflammatory microenvironment by generating reactive oxygen species (ROS), reactive nitrogen species (RNS) and prostaglandins, of which their synthesis is the target of many pharmacological approaches (TABLE 1) [20]. Indeed, genetic and pharmacologic suppression of the enzyme Cyclooxygenase 2 (COX2), which is responsible for prostaglandin production, can significantly delay cancer development in a number of experimental and clinical settings. Inhibition of the COX2-dependent pathway suppresses both tumor-associated angiogenesis and tumor growth in mice [21, 22], and mice lacking COX2 are resistant to colorectal cancer development [6, 23, 24]. In humans, epidemiological studies have shown that COX2 inhibitors can reduce the risk of several types of cancer [22]. It is believed that inflammation can increase chromosomal instability through the production of ROS and RNS, thus linking inflammation to cancer initiation (Fig. 1) [25].
Table 1.
Molecular targets and pharmacologic modulators for cellular microenvironment
| Signaling Pathway/ Molecular Target |
Agent(s) | Effect on target | Cancer cell type involved (in vivo or cell line) |
|---|---|---|---|
| INFLAMMATION | |||
| NFKB | N-acetyl cysteine, Resveratrol, Sutinib | Inhibitory [1–3] | RCC, GIST [4, 5] |
| COX1/2 | Celecoxib, Aspirin, other NSAIDs | Inhibitory [6, 7] | Colon polyp recurrence, Breast, Oesophageal, Gastric, Biliary [8, 9] |
| NOS | L-NAME | Inhibitory [10] | Breast [10] |
| AP1 signaling | PYC71N, JIP peptide, Resveratrol | Inhibitory [2, 11, 12] | Breast [13] |
| NRF2 | Sulphoraphane, Dimethyl fumarate | Activation [14] | Prostate [15] |
| NRF2 | Brusatol | Inhibitory [16] | Lung [16] |
| MMPs | Marimastat, MMP3 inhibitor peptide | Inhibitory [17, 18] | Lung, Colon [17, 19] |
| TGF β signaling | AP12009, LY 364947, SB 525334 | Inhibitory [20–22] | Prostate, Glioma [23, 24] |
| SENESCENCE | |||
| Notch/CSL | SAHM1, MK0752, RO4949079 | Inhibitory [25, 26] | Breast, Prostate [24, 27] |
| p21 | Senexin A | Inhibitory [28] | Adenocarcinoma [28] |
| MEK | Trametenib, PD035901, UO126, Pimasertib | Inhibitory [29–31] | Melanoma, Lung, Colon [32–34] |
| AUTOPHAGY | |||
| mTORC complex | Rapamycin, Torin, CCI-779, RAD001, AP23573 | Activation [30, 35] | Glioma, Prostate, RCC [24, 36, 37] |
| AMPK | Metformin, AICAR, A-769662 | Activation [30, 38] | Breast, Colon [39, 40] |
| BH3 | ABT 737, GX15-070 | Activation [41, 42] | Pancreas, Leukemia [43, 44] |
| HDAC | Sperimidine, Apicidin, SAHA, Sulphoraphane | Activation [14, 45, 46] | Breast, CML [47–49] |
| Autophagosome/ly sosome | Bafilomycin A1, Chloroquine | Inhibitory [50, 51] | Breast, Prostate, Glioma [47, 52, 53] |
| Vps34, PI3Kγ | 3-methyl-adenine | Inhibitory [50, 51] | Colon, Breast [54, 55] |
• This Table indicates potential molecules that might be targeted in the stroma for cancer preventive-therapeutic purposes as well as some type of cancers or cell lines where certain indicated drugs have been found to be effective, and is not meant to be a comprehensive list of inhibitors/activators.
• Note that some agents directly target the indicated molecules while others act modulating the pathway leading to the molecular target.
List of abbreviation used:
EGCG (epigallocatechin-3-gallate); Celecoxib (sulfonamide nonsteroidal anti-inflammatory drug, NSAID); NSAIDs (non-steroidal anti-inflammatory drugs); AICAR (5-amino-1-β-ribofuranosylimidazole-4-carboxamide); Vps34 (phosphatylinositol 3-kinase class III); PI3Kγ (phosphoinositide 3-kinase gamma ); Senexin A (inhibitors of p21-dependent transcription); Resveratrol (3,5,4'-trihydroxy-trans-stilbene); Sutinib (targets multiple receptor tyrosine kinases. Including all receptors for platelet-derived growth factor (PDGF-Rs) and vascular endothelial growth factor receptors); L-NAME (N-nitro-L-arginine methyl ester); Sulphoraphane (1-Isothiocyanato-4-methylsulfinylbutane, polyvalent molecule derived from vegetables, activates NRF2); SAHM1 (hydrocarbon-stapled peptides that disrupt a critical protein-protein interaction in the Notch/CSL transcription factor complex); Marimastat (BB2516, broad spectrum MMP inhibitor); Torin (mTOR kinase activity inhibitor); CCI-779, RAD001 and AP23573 (mTOR inhibitors analog to rapamycin); Brusatol (selectively inhibits NRF2 ); JIP (Jun interacting protein1); MMP3 inhibitor I (Ac-Arg-Cys-Gly-Val-Pro-Asp); Spermidine (polyamine compound, HDAC inhibitor)
COX2 (cyclooxygenase 2); TGFβ (transforming growth factor-β); mTOR (mammalian target of rapamycin); AMPK (AMP activated kinase); MEK (mitogen-activated protein kinase); MMPs (metalloproteinase); AP1 (activating complex protein 1); NFκB (nuclear factor κB); HDAC (histone deacetylase); KEAP1 (kelch-like ECH-associated protein 1); p21 (cyclin dependent inhibitor waf1); HDAC (Histone deacetylases ); BH3 (bcl2 homology domain 3); CML (chronic myeloid leukemia); RCC (renal cell carcinoma); GIST (gastrointestinal stromal tumors)
References
Pfeffer, U., et al. (2005) Molecular mechanisms of action of angiopreventive anti-oxidants on endothelial cells: microarray gene expression analyses. Mutat Res 591, 198–211
Manna, S.K., et al. (2000) Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 164, 6509–6519
Miller, S.C., et al. (2010) Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action. Biochem Pharmacol 79, 1272–1280
Demetri, G.D., et al. (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368, 1329–1338
Motzer, R.J., et al. (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356, 115–124
Karin, M. and Greten, F.R. (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5, 749–759
Mann, J.R., et al. (2005) Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol 2, 202–210
Chow, L.W., et al. (2005) Current directions for COX-2 inhibition in breast cancer. Biomed Pharmacother 59 Suppl 2, S281–284
Algra, A.M. and Rothwell, P.M. (2012) Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 13, 518–527
Jadeski, L.C. and Lala, P.K. (1999) Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol 155, 1381–1390
Shi, L., et al. (2012) Activation of JNK signaling mediates connective tissue growth factor expression and scar formation in corneal wound healing. PLoS One 7, e32128
Ngoei, K.R., et al. (2011) Characterization of a novel JNK (c-Jun N-terminal kinase) inhibitory peptide. Biochem J 434, 399–413
Kaoud, T.S., et al. (2011) Development of JNK2-selective peptide inhibitors that inhibit breast cancer cell migration. ACS Chem Biol 6, 658–666
Osburn, W.O. and Kensler, T.W. (2008) Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res 659, 31–39
Gibbs, A., et al. (2009) Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci U S A 106, 16663––16668
Ren, D., et al. (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A 108, 1433–1438
Goffin, J.R., et al. (2005) Phase I Trial of the Matrix Metalloproteinase Inhibitor Marimastat Combined with Carboplatin and Paclitaxel in Patients with Advanced Non,ÄìSmall Cell Lung Cancer. Clinical Cancer Research 11, 3417–3424
Fisher, J.F. and Mobashery, S. (2006) Recent advances in MMP inhibitor design. Cancer and Metastasis Reviews 25, 115–136
Primrose, J.N., et al. (1999) Marimastat in recurrent colorectal cancer: exploratory evaluation of biological activity by measurement of carcinoembryonic antigen. Br J Cancer 79, 509–514
Akhurst, R.J. and Hata, A. (2012) Targeting the TGFβ signalling pathway in disease. 1–22
Sawyer, J.S., et al. (2003) Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem 46, 3953–3956
Grygielko, E.T., et al. (2005) Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther 313, 943–951
Hau, P., et al. (2007) Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 17, 201–212
Efstathiou, E. and Logothetis, C.J. (2010) A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res 16, 1100–1107
Moellering, R.E., et al. (2009) Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188
Tolcher, A.W., et al. (2012) Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 30, 2348–2353
Kondratyev, M., et al. (2012) Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene 31, 93–103
Porter, D.C., et al. (2012) Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities. Proc Natl Acad Sci U S A 109, 13799–13804
Wang, J.Y., et al. (2007) Recent advances of MEK inhibitors and their clinical progress. Curr Top Med Chem 7, 1364–1378
Bain, J., et al. (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408, 297–315
Davies, S.P., et al. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351, 95–105
Flaherty, K.T., et al. (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367, 107–114
Martinelli, E., et al. (2013) Antitumor activity of pimasertib, a selective MEK 1/2 inhibitor, in combination with PI3K/mTOR inhibitors or with multi-targeted kinase inhibitors in pimasertib-resistant human lung and colorectal cancer cells. Int J Cancer, 0
Engelman, J.A., et al. (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14, 1351–1356
Faivre, S., et al. (2006) Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 5, 671–688
Takeuchi, H., et al. (2005) Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 65, 3336–3346
Fishman, M.N., et al. (2013) Phase Ib study of tivozanib (AV-951) in combination with temsirolimus in patients with renal cell carcinoma. Eur J Cancer 49, 2841–2850
Steinberg, G.R. and Kemp, B.E. (2009) AMPK in Health and Disease. Physiol Rev 89, 1025–1078
Zakikhani, M., et al. (2006) Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 66, 10269–10273
Buzzai, M., et al. (2007) Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 67, 6745–6752
van Delft, M.F., et al. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10, 389–399
Nguyen, M., et al. (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 104, 19512- 19517
Martin, A.P., et al. (2009) BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol 76, 327–341
Wei, Y., et al. (2010) The combination of a histone deacetylase inhibitor with the Bcl-2 homology domain-3 mimetic GX15–070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Clin Cancer Res 16, 3923–3932
Kim, H.J. and Bae, S.C. (2011) Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 3, 166––179
Acharya, M.R., et al. (2005) Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol 68, 917–932
Kanematsu, S., et al. (2010) Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res 30, 3381–3390
Shao, Y., et al. (2004) Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A 101, 18030–18035
Carew, J.S., et al. (2007) Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110, 313–322
Klionsky, D.J., et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544
Mizushima, N. and Komatsu, M. (2011) Autophagy: Renovation of Cells and Tissues. Cell 147, 728–741
Fan, Q.W., et al. (2010) Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal 3, ra81
Degtyarev, M., et al. (2008) Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol 183, 101–116
Petiot, A., et al. (2000) Distinct classes of phosphatidylinositol 3'-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275, 992–998
Rahim, R. and Strobl, J.S. (2009) Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs 20, 736–745
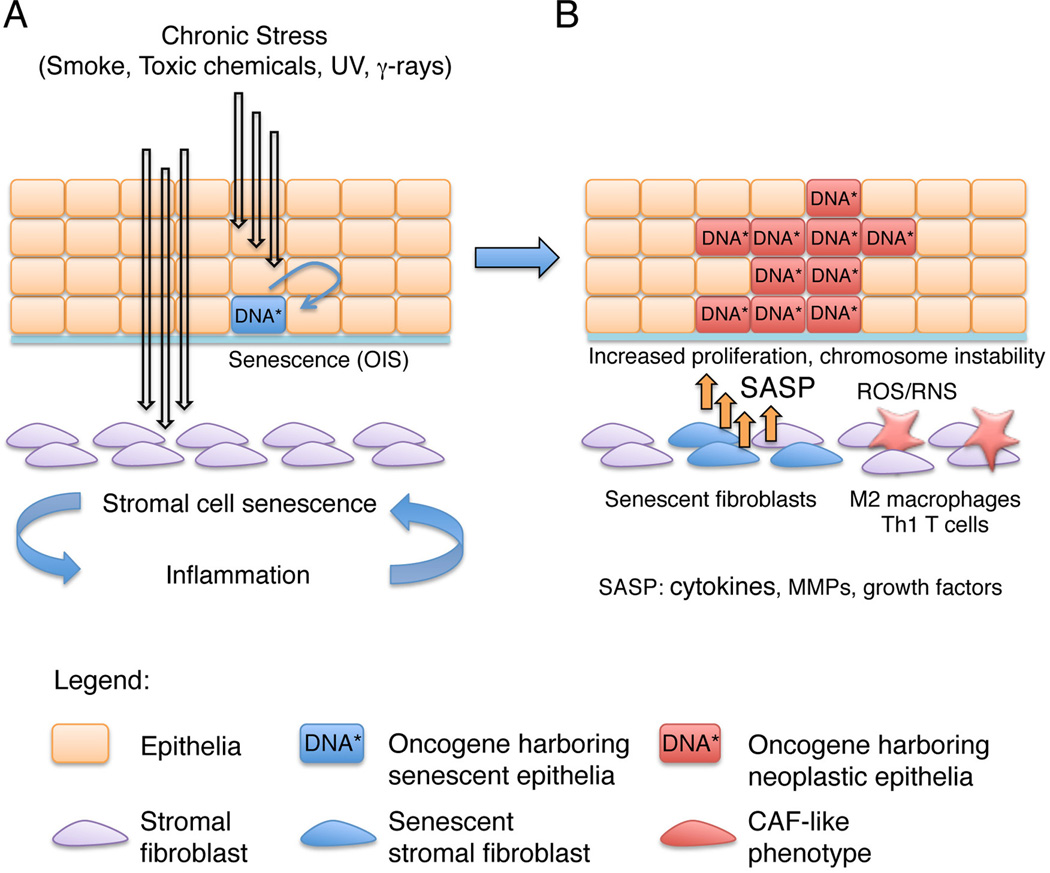
Figure 1. Determining the role of stromal cell senescence and inflammation in initiation of epithelial carcinogenesis.
(A) Surface epithelial tissues like those of the bronchial tree, bladder or skin are exposed to and protected from environmental stressors and carcinogens like smoke, kidney-secreted toxic chemicals, γ-irradiation and UV light. Pro-oncogenic gene mutations induced by these agents are however not sufficient to elicit the carcinogenic process with senescence of oncogene-bearing epithelial cells as a counteracting induced mechanism. The impact of environmental stressors extends however to the underlying stroma, with induction of stromal cell senescence and recruitment of a chronic inflammatory infiltrate as consequences that reinforce each other. (B) Fibroblast senescence produces large amounts of diffusible growth factors, cytokines, and matrix remodeling enzymes (SASP). In addition, chronic inflammation (with prevalent M2 macrophages and Th1 T cells) generates highly reactive small molecular weight molecules (ROS, RNS). Collectively, these factors impinge on the overlying epithelium, with mitogenic and chromosome-destabilizing effects, thus promoting tumorigenic outgrowth. As discussed in this review, an emerging concept is that approaches aimed at suppressing stromal cell senescence and chronic inflammation could be of substantial value for preventing or reversing early stages of epithelial cancer.
The suppression of cancer-promoting chronic inflammation, by itself or coupled with induction of a transient acute inflammatory reaction, provides a promising approach for prevention and treatment of early phases of epithelial cancer [20, 26]. Such intervention modalities are already employed in the skin, in which development of actinic keratosis, a very common precursor of cutaneous squamous cell carcinoma (SCC), is intimately connected with UV light-induced chronic inflammation [27]. These lesions can be effectively prevented by counteracting the UV light effects with agonists of Toll Like Receptors (TLRs) producing a potent acute inflammatory reaction [28]. Thus, an approach aimed to reeducate or redirect the inflammatory response may represent a powerful preventive and therapeutic tool, at least in some cancer settings.
Alterations of mesenchymal stromal cells as primary determinants of epithelial cancer
Substantial evidence supports the notion that cancer progression and metastasis are the result of co-evolving alterations in cancer cells and cells of the surrounding stroma [29–31]. While epigenetic events are an accepted mechanism for changes in in the tumor-surrounding stroma, chromosomal and/or genetic alterations, including loss of p53, have also been reported [32–36], although the general significance of these findings is unclear [31]. Together or preceding inflammation, several lines of evidence discussed below indicate that mesenchymal stromal alterations can also be a trigger for initiation of epithelial cancer.
A number of genetic, human syndromes with intrinsic deregulation of stromal cells result in benign outgrowths and secondary high risk of epithelial cancer, as previously reviewed [37]. For example, patients with Peutz-Jeghers Syndrome (PJS) develop hamartomatous polyps primarily in the intestine, but also at several other sites, and have an increased risk of developing epithelial colon cancer [37]. At the basis of PJS is the germ-line transmission of inactivating mutations of the liver kinase B1 (LKB-1) gene [38]. LKB-1 is best known for controlling 5' AMP-activated protein kinase (AMPK) signaling with a consequent impact on mTOR activity, cellular metabolism and protein synthesis [39]. However, the role of LKB-1 in the colonic mesenchyme has been connected to a different mechanism, involving decreased expression of transforming growth factor β (TGF-β) and consequently impaired myofibroblast differentiation [40]. This in turn may lead to hamartomatous hyperproliferation with secondary increased inflammation and risk of epithelial cancer development.
Other lines of work, based on mouse genetic models, point to the importance of stromal alterations that directly affect adjacent epithelia leading to epithelial dysplasia and neoplasia rather than mesenchymal outgrowths. Recent evidence suggests that activation of TGF-β signaling in the fibroblast can exert a pro-tumorigenic role on adjacent epithelium. TGF-β, a key regulator of stromal fibroblast function, can induce both fibrosis and CAF activation [10, 41, 42]. TGF-β signals downstream via activation of the TGF-β receptor by first binding the TGF-β type II receptor (Tgfr2) and recruiting the TGF-β type I receptor (Tgfr1) [43]. Thus, to assess the role of this pathway in the mesenchymal compartment, mice with fibroblast-specific ablation of the Tgfr2 gene were studied [44]. Epithelial cancerous or precancerous lesions consistently developed in the forestomach and prostate of these mice, with increased proliferation of surrounding fibroblasts [44]. As a possible paracrine mechanism, loss of Tgfr2 specifically in gastric and prostate fibroblasts up-regulated the expression of known epithelial growth promoting factors, such as hepatocyte growth factor and Wnt [45, 46], and induced a cancer-promoting inflammatory reaction [47]. Importantly, these findings are of likely clinical significance as decreased Tgfr2 expression has been observed in the stroma of esophageal squamous cell carcinomas (SCCs) (equivalent to mouse forestomach SCC) [48] as well as in prostate carcinomas [46, 49].
In addition to TFG-β, Notch signaling in the mesenchyme can also modulate the oncogenic potential of adjacent epithelia. Unlike TGF-β, which signals at a distance, Notch signaling is an important form of direct cell to cell communication with a key role in tissue development and homeostasis [50]. Upon activation, the proteolytically cleaved Notch intracellular domain associates with the DNA binding protein CSL, converting it from a transcriptional repressor into an activator [51]. In the epidermal compartment of the skin, the Notch/CSL pathway plays a key role in promoting keratinocyte differentiation and suppressing tumor formation [52]. Loss of the CSL gene in the mesenchymal compartment was also found to cause a skin phenotype with key features of field cancerization, including early and widespread dermal atrophy, followed by expanding areas of inflammation and, as the mice aged, multifocal keratinocyte tumors with features of actinic keratosis or in situ SCCs [53]. All mice developed multiple skin tumors, which were significantly counteracted by pharmacological inhibition of inflammation. In both mouse and human dermal fibroblasts, CSL loss resulted in the activation of a CAF-like phenotype, including growth factor up-regulation, pro-inflammatory cytokine and matrix metalloprotease production. Importantly, this stromal cell phenotype overlapped with the stroma phenotype seen in patients with cutaneous field cancerization [53].
Taken together this evidence indicates that the stromal compartment can be a primary determinant and important target for prevention and treatment of early stages of epithelial cancer. Besides stromal inflammation, there is the attractive possibility of intervening on other important stromal processes discussed below.
Stromal cell senescence and the senescence associated secretory phenotype
Cellular senescence is a terminal cell fate characterized by an irreversible withdrawal from the cell cycle. In vivo, senescent cells accumulate with age and senescence-related events are thought to be a key driver of tissue aging [54]. During transformation, cells need to overcome oncogene-induced senescence (OIS) as an intrinsic fail safe mechanism to prevent tumor development [55]. However, senescent cells have also been implicated in regulating cancer initiation and progression through modulation of neighboring cells [55].
Senescence can result from intrinsic, time-dependent, shortening of telomeres, from inappropriate mitogenic stimulation (as in OIS), accumulation of genomic or mitochondrial DNA mutations, or other degenerative events, such as protein glycosylation and lipid oxidation [55–57]. Senescence can also be triggered by a variety of exogenous insults and chronic stress, including UV exposure, gamma-irradiation, smoke-derived compounds and other toxic chemicals, which may lead to cancer (Figure 1). For instance, UVA exposure results in ROS production that can affect both epidermal and dermal compartments leading to skin cancer [58]. Similarly, smoking can lead to an increased risk of cancer in the lungs, and other organs such as the oral mucosa, bladder, liver, kidney, prostate and breast [59– 61]. Besides the epithelium, a recent report has suggested that smoke-derived chemicals can target resident stromal fibroblasts, causing metabolic changes, DNA damage and accelerated senescence [62].
Pro-senescence signals converge on either the p53 or p105Rb tumor suppressor pathways, with p16 (ink4a) and p21Waf1/Cip1 [63], or miRNA 34 [64] and lncRNA p21 [65] as effectors. Senescent cells also undergo extensive chromatin remodeling resulting in drastic changes in gene expression [66]. An important consequence of senescence is the acquisition of a SASP [5]. SASP factors are potent tools by which senescent cells modify the tissue microenvironment [5, 67]. They can be divided into three categories: 1) soluble signaling factors (interleukins (IL), chemokines, and growth factors) 2) secreted proteases, and 3) extracellular matrix components. The most prominent cytokines of the SASP are represented by IL-1, IL-6, IL-8, Granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), interferons, TGF-β, insulin growth factor-binding proteins (IGFBPs) and their regulators (IGFBP-rP1 and IGFBP-rP2, or connective tissue growth factor—CTGF) [67, 68]. Several matrix metalloproteinases (MMPs) are also produced by human and mouse fibroblasts undergoing replicative or stress-induced senescence, including MMP-1 and MMP-3 [5]. Another family of proteases released by senescent cells are serine proteases of the plasminogen activation pathway, urokinase- or tissue-type plasminogen activators (uPA or tPA), which can trigger a mitogenic signal in neighboring cells [69]. Senescent fibroblasts can also modulate the behavior of neighboring cells through a more indirect mechanism, involving production of extracellular matrix proteins like collagen and fibronectin [70, 71].
The SASP has been shown to reinforce senescence of normal epithelial cells and suppress early epithelial transformation [5]. However, emerging evidence suggests senescence of stromal cells can result in their acquisition of a CAF-like phenotype, resulting in the capability of enhancing tumor formation in neighboring epithelial cells [5, 67, 68]. In the breast, premalignant epithelial cells are induced to proliferate by stromal cells that have undergone senescence spontaneously or upon irradiation [72–74] Similarly, in the oral cavity, tobacco-driven senescence of stromal cells stimulates the growth of epithelial cells with loss of epithelial integrity and altered differentiation [75]. In the prostate, senescent fibroblasts can create a local tissue environment that favors epithelial cell hyper-proliferation [76]. In addition, neighboring senescent stromal cells also induces epithelial-to-mesenchymal transition (EMT) in surrounding epithelia [77]. Besides being an important switch that enables cancer cells to migrate and invade [10], EMT was recently linked to the control of cancer stem cell potential [78], suggesting that senescence-induced EMT could be a very early event in the carcinogenic process.
Although the molecular mechanisms linking stromal cell senescence and SASP activation remain to be elucidated, initial studies indicated that persistent DNA damage could be one means of inducing the SASP [79]. However, persistent DNA damage induced by irradiation or oncogenic RAS, was found to be sufficient, but not essential, for the induction of the SASP [80] suggesting that an alternative mechanism independent of DNA damage may also be involved. Consistent with this possibility, fibroblast senescence and SASP was found to be induced by activation of the stress-inducible kinase p38 MAPK independent of DNA damage [81].
Notably, recent evidence indicates that senescence is not necessarily linked to the induction of a SASP. In fact, increased expression of p16 or p21 in human fibroblasts was reported to induce senescence without establishing nor maintaining the SASP [80]. Furthermore, p16 may play an indirect role in repressing the SASP and SASP-dependent effects on epithelial cells [80]. The possibility of dissociating stromal cell senescence from SASP is of great potential therapeutic significance for approaches aiming to selectively interfere or block these processes.
Transcriptional regulators of stromal senescence and cancer-initiating microenvironment
By virtue of their pleiotropic actions, transcription factors have long been considered master regulators of the cellular microenvironment and represent possible targets for intervention. A number of excellent reviews exist on the topic (see, for instance, [10, 20]); therefore, we will only consider a few that have been closely implicated in control of the stromal cell stress response and aging, including the AP1 family, nuclear factor kappa beta (NF-κB), and Nuclear Factor erythroid 2-Related Factor 2 (NRF2).
Transcription factors of the AP1 family (Jun, Fos and ATF) are activated in response to a number of mitogenic and stress derived signals [82, 83]. These factors are master regulators of a large number of SASP genes and, for example, in the skin, are likely to play a key role in chronic inflammation and photo-aging [84]. Indeed, strong evidence indicates that UV-damage is linked to changes in gene expression in the dermal fibroblast compartment by AP-1-regulated MMPs, including MMP-1, MMP-3 and MMP-9 [85]. Seminal studies on mesenchymal-epithelial crosstalk involving AP1 family members were performed by organotypic co-cultures of fibroblasts from Jun −/− and JunB −/− mice with primary human keratinocytes. Loss of mesenchymal Jun resulted in decreased keratinocyte proliferation and differentiation, and was associated with the loss of keratinocyte growth factor (KGF) and GM-CSF expression by fibroblasts. By contrast, Jun B was found to suppress both of these cytokines, and accordingly JunB −/− fibroblasts expressed constitutively high levels of both KGF and GM-CSF, resulting in proliferation and differentiation of overlaying keratinocytes [86, 87]. These findings outline the existence of a mesenchymal-epithelial paracrine signaling loop regulated by the relative abundance of specific AP1 members within the stroma and unveil the presence, within the AP1 family, of an antagonistic mechanism regulating the same target genes, and thus tissue/organ homeostasis. Consistent with this notion, AP-1 up-regulation may partly explain the CAF phenotype and resulting keratinocyte tumor formation caused by loss of Notch/CSL signaling in skin fibroblasts [53].
In addition to AP-1, the transcription factor NF-κB plays a crucial role in inflammation and in establishing a tumor promoting microenvironment [88]. NF-kb primarily controls pro-inflammatory genes and enzymes, of which many are implicated in the SASP, and pro-survival genes such as Bcl-XL [88, 89]. Studies in human mammary fibroblasts and keratinocytes as well as systemic approaches aiming to identify promoters regulating age-related genes across species and tissue have suggested a function for NF-κB [90] [91] [92]. In both human and mice, NF-κB was found to regulate age-associated gene expression programs in vitro, while in vivo its DNA binding activity was found to increase with age in several mouse tissues including skin, liver, heart and spleen [93]. More importantly, a dominant-negative NF-κB mutant was able to revert both age-related phenotypes and the age-dependent gene signature in skin [93]. These findings suggest that pharmacological approaches to inhibit NF-κB signaling, directly or through suppression of inflammatory signals, may serve to reverse age-related changes and associated detrimental effects.
The transcription factor NRF2 is part of a sensor system to detect cellular oxidative stress, and is important for protection of epithelial and stromal tissues from UV irradiation and toxic chemicals, cancer and, possibly, aging [94]. When relieved from inhibition by the kelch-like ECH associated protein 1 (KEAP1), NRF2 upregulates important components of the antioxidant response, including peroxiredoxin 1 and 6 [95]. Given the key role of ROS in cellular DNA and protein damage, compromised NRF2 function could accelerate stromal senescence and aging, and approaches aimed to increase NRF2 activity have been shown to be beneficial [94, 96, 97]. However, NRF2 mutations associated with higher levels of expression have been linked to specific cancer types, including SSC of the esophagus, skin, lung and larynx [94]. In fact, while initially cytoprotective, NRF2 accumulation can promote tumor formation by excessive stress protection [94]. This double role is exemplified by fumarate, a Krebs metabolite known to inhibit chemical carcinogenesis in mouse skin, lung and liver [98, 99]. Recently fumarate has been shown to target KEAP, thus leading to NRF2 activation and providing a mechanism for its anti inflammatory therapeutic use. However, in vivo loss of fumarate processing by fumarate hydratase resulted in fumarate accumulation and chronic NRF2 activation, suggesting NRF2 is a possible cause of renal carcinoma [100]. Recent findings have also implicated NRF2 in promoting colon cancer, as its silencing in cancer cells blocks hypoxia inducible factor (HIF1α) accumulation and suppresses both tumor growth and angiogenesis [101]. Therefore, tissue-specific approaches for drug delivery or action should be considered when targeting Nrf2.
Overall, there are many molecular targets regulating the stromal microenvironment and a growing number of drugs are being used to modulate these targets (Figure 2 and TABLE 1). Rather than focusing on molecules with single modes of action, it is likely that targeting the pleiotropic gene network regulated by transcription factors might represent an efficient approach for senescence modulation and/or cancer prevention.
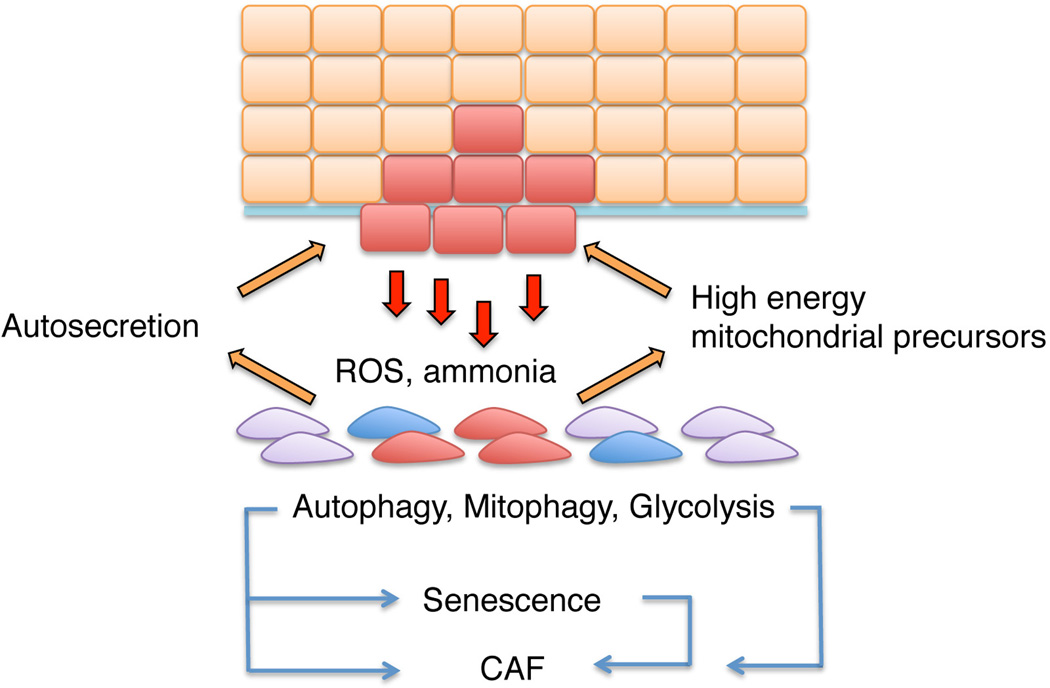
Figure 2. Metabolic crosstalk between epithelial cancer cells and surrounding stroma.
Proposed existence of a two-compartment tumor metabolism whereby epithelial cancer cells, via reactive oxygen species (ROS) and ammonia, released as by-product of preferential glutamine use, stimulate stromal cell autophagy, mitophagy (a form of autophagy targeting selectively the mitochondria) and subsequent glycolysis. Autophagy can lead to a senescent phenotype in stromal fibroblasts, with senescence-dependent or -independent production of various SASP components. In addition, changes in metabolism of stromal cells result in release of high-energy catabolites (e.g. lactate) that fuel-back to the cancer cells (reverse Warburg effect) [6]. As discussed in the text, autosecretion, an autophagy-based unconventional secretion enabling leaderless cytokines to exit the cell without entering the secretory pathway, might contribute to modifying this stromal environment.
Autophagy in control of tissue aging, stromal cell senescence and cancer
Autophagy (self-eating) is a cellular lysosomal degradative process, which maintains energy metabolism and eliminates aged and damaged components, including proteins and organelles [102]. A close association exists between senescence and autophagy; depending on cellular and tissue contexts, autophagy can both trigger senescence and counteract it [103, 104]. In addition, mitigation of tissue aging and associated cellular damage through autophagy is considered a novel mechanism of tumor suppression, while inability to eliminate cellular debris (defective autophagy) can create a damaging environment predisposing cells to chronic inflammation and tumor initiation [11, 105]. The conditional ablation of autophagy regulating genes, like beclin1 or atg7 in the liver and atg16L1 or atg5 in the colon, causes persistence of altered mitochondria and other organelles resulting in cell and tissue dysfunction [11]. This damage is mediated, at least in part, by the deregulated turnover of protein p62 [11], which is an adaptor that regulates various signal transduction pathways. By sequestering KEAP1, p62 controls the levels of stress-responsive NRF2 resulting in an increased production of detoxifying enzymes [106]. Another consequence of deregulated p62 levels results from its direct interaction with TRAF6 and the consequent activation of the NF-κB pro-inflammatory cascade [107].
Thus autophagy can be highly beneficial in preventing and removing age-dependent tissue and cellular damage leading to degeneration and, eventually, cancer. Autophagy however can also play an important pro-senescence function. Oncogene induced senescence in fibroblasts was reported to depend on autophagy [108]. In this system, senescence and expression of two SASP cytokines, IL-6 and IL-8, were delayed by silencing of autophagy-essential genes (atg5 or atg7) [108]. More directly, increased expression of autophagic proteins (BNIP3, CTSS, ATG16L1) in human fibroblasts was sufficient to induce p21 up-regulation and senescence, with a shift towards anaerobic glycolysis, lactate and 3-hydroxybutirate production and a concomitant increase in in vivo tumor enhancing properties [6]. The specific mechanisms linking autophagy to senescence and the SASP are still unclear, but alterations in protein turnover and/or intracellular membrane trafficking are likely involved [108]. A converse link is also possible, whereby senescence of stromal cells induces autophagy. In particular, the expression of cyclin dependent kinase inhibitors (p16, p19 or p21) in immortalized human fibroblasts triggered autophagy, and this "senescence-induced" autophagy enhanced the growth of neighboring tumor cells in vivo [109]. This could be mediated by autophagy-based unconventional secretion, termed "autosecretion", which enables leaderless cytosolic proteins including important cytokines of the SASP (IL-1β, IL-18) to exit the cell without entering the secretory pathway [110].
A novel process called senescence-autophagy transition (SAT) [6] may also occur. During SAT, senescent stromal cells promote epithelial cell growth in the absence of a SASP, by releasing catabolic high energy "mitochondrial fuels” generated as a consequence of a cellular glycolytic switch [6]. In this context, autophagy can be part of a vicious cycle between stromal cells and cancer cells, involving a two-compartment tumor metabolism. Cancer cells, by producing ROS, would stimulate autophagy in the surrounding stroma and this autophagy-dependent senescence of stromal cells in turn would produce high-energy catabolites that fuel back to the adjacent cancer cells (Figure 2) [6, 111]. Consistent with this “reverse Warburg effect”, evidence for a stromal-epithelial lactate metabolic coupling was found in breast cancer - CAFs expressed the transporter implicated in lactate efflux mono-carboxylase (MCT) 4, while adjacent cancer cells expressed the transporter involved in lactate influx MCT-1 [112].
Concluding remarks
As we have discussed, both stromal cell senescence and chronic inflammation have a demonstrated tumor initiating function, with an ensuing reciprocal reinforcement of tumor and stromal alterations (Figure 1). The use of anti-inflammatory agents, specifically COX inhibitors, for prevention of cancer is already strongly supported by a number of experimental and clinical studies [21, 22]. However, given the intrinsic fail safe function that cellular senescence fulfills to restrict cancer cells of origin, the ability to interfere with this process is much more complicated. More specific approaches should be considered such as selectively inhibiting senescence and associated SASP/CAF activation in the stromal mesenchyme while, at the same time, not interfering or enhancing senescence in neighboring cancer precursor cells. Selective targeting of the stroma will be achieved by a better knowledge of the tissue-specific mechanisms at the basis of the senescence program, or use of better, tissue-specific drug delivery approaches, including recently developed multiple needle microinjection techniques [113].
Autophagy is a similar dual edge sword. On one hand, overall enhancement of this process, which can result from caloric restrictions or drugs with similar metabolism-modulatory effects (like metformin or resveratrol), could be beneficial in preventing the overall tissue and organismal aging process (Figure 3A). On the other hand, inhibition of autophagy at both cancer and cancer stromal levels could have very favorable effects in suppressing cellular senescence and associated events like, most prominently, inflammation (Figure 3B). It is hard to predict a priori which of the two functions is predominant. Experiments in cell culture are likely to skew conclusions in favor of the second possibility, while the ultimate, and biologically most relevant tests, will have to be in vivo, especially under conditions in which organ and organismal aging can be taken into consideration. Collectively, alterations of the underlying stroma make it an attractive target for preventive and therapeutic approaches against tumor initiation. In addition, such targets may also be highly beneficial for later stages of epithelial cancer, i.e. invasion and metastasis, in which stromal changes are known to play a critical role [10].
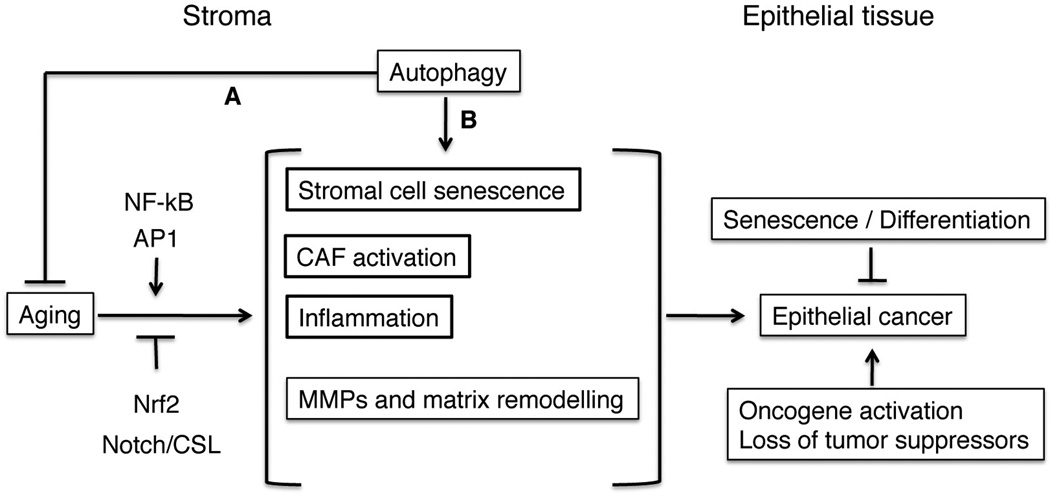
Figure 3. Complex interplay of aging, senescence and autophagy in stromal cell alterations and epithelial cancer initiation.
Aging can lead to senescence of stromal cells in parallel with CAF activation, chronic inflammation and increased MMP production with matrix remodeling. As discussed in the text, the NF-κB, AP1, Nrf2 and Notch/CSL signaling pathways have been implicated in one or more of these processes. Autophagy can delay or limit the negative consequences of tissue / organismal aging (A) while, at the same time, playing a more direct positive role in stromal cell senescence (B). Senescence of stromal cells is linked to CAF activation and chronic inflammation, while, in epithelial cells, it functions in concert with differentiation as intrinsic tumor suppressing mechanism.
Highlights.
-
-
Stromal compartment can be a primary determinant of early stages of epithelial cancer
-
-
Stromal cellular environment can be responsible for recurring cancers
-
-
Senescence and senescence associated secretory phenotype can create a permissive stromal environment
-
-
Tissue chronic inflammation and deregulated autophagy in the stroma can contribute in promoting neoplastic transformation
-
-
The selective targeting of these processes at stromal level might represent an alternative approach in cancer prevention and therapy
Acknowledgements
This work was supported by Grants from the Swiss National Foundation (Grants CRSI33-130576/1; 3100A0-122281/1), Oncosuisse (Grant 02361-02-2009) and NIH (Grant AR39190) to GPD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gatenby R. Perspective: Finding cancer's first principles. Nature. 2012;491:S55. doi: 10.1038/491s55a. [DOI] [PubMed] [Google Scholar]
- 2.Cardiff RD, Borowsky AD. Precancer: sequentially acquired or predetermined? Toxicol Pathol. 2010;38:171–179. doi: 10.1177/0192623309356447. [DOI] [PubMed] [Google Scholar]
- 3.Eifert C, Powers RS. From cancer genomes to oncogenic drivers, tumour dependencies and therapeutic targets. Nat Rev Cancer. 2012;12:572–578. doi: 10.1038/nrc3299. [DOI] [PubMed] [Google Scholar]
- 4.Dakubo GD, et al. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 6.Capparelli C, et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Cell Cycle. 2012;11:2285–2302. doi: 10.4161/cc.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 8.Chang HY, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seril DN, et al. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 14.Wroblewski LE, et al. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 16.Walker F, et al. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood. 2008;111:3978–3985. doi: 10.1182/blood-2007-10-119636. [DOI] [PubMed] [Google Scholar]
- 17.Allavena P, et al. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Ruffell B, et al. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 21.Leahy KM, et al. Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000;7:1163–1170. doi: 10.2174/0929867003374336. [DOI] [PubMed] [Google Scholar]
- 22.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012;47:97–106. doi: 10.1007/s00535-011-0523-6. [DOI] [PubMed] [Google Scholar]
- 24.Oshima M, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 25.Colotta F, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 26.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantisani C, et al. Actinic keratosis: review of the literature and new patents. Recent Pat Inflamm Allergy Drug Discov. 2013;7:168–175. doi: 10.2174/1872213x11307020008. [DOI] [PubMed] [Google Scholar]
- 28.Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs. 2008;17:1051–1065. doi: 10.1517/13543784.17.7.1051. [DOI] [PubMed] [Google Scholar]
- 29.Hu M, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 30.Jiang L, et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg RA. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494–495. doi: 10.1038/ng0508-494. [DOI] [PubMed] [Google Scholar]
- 32.Fukino K, et al. Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res. 2004;64:7231–7236. doi: 10.1158/0008-5472.CAN-04-2866. [DOI] [PubMed] [Google Scholar]
- 33.Kurose K, et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 34.Hill R, et al. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto N, et al. Possible alternative carcinogenesis pathway featuring microsatellite instability in colorectal cancer stroma. Br J Cancer. 2003;89:707–712. doi: 10.1038/sj.bjc.6601141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson RF, et al. Molecular genetic alterations in the laser-capture-microdissected stroma adjacent to bladder carcinoma. Cancer. 2003;98:1830–1836. doi: 10.1002/cncr.11747. [DOI] [PubMed] [Google Scholar]
- 37.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–2538. doi: 10.1101/gad.1593107. [DOI] [PubMed] [Google Scholar]
- 38.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 39.Corradetti MN, et al. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaahtomeri K, et al. Lkb1 is required for TGFbeta-mediated myofibroblast differentiation. J Cell Sci. 2008;121:3531–3540. doi: 10.1242/jcs.032706. [DOI] [PubMed] [Google Scholar]
- 41.Kojima Y, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proceedings of the National Academy of Sciences. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinz B, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 44.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 45.Placencio VR, et al. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res. 2008;68:4709–4718. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, et al. Prostate tumor progression is mediated by a paracrine TGF-beta/Wnt3a signaling axis. Oncogene. 2008;27:7118–7130. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achyut BR, et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-beta signaling. PLoS Genet. 2013;9:e1003251. doi: 10.1371/journal.pgen.1003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukai Y, et al. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer. 2003;104:161–166. doi: 10.1002/ijc.10929. [DOI] [PubMed] [Google Scholar]
- 49.Franco OE, Hayward SW. Targeting the tumor stroma as a novel therapeutic approach for prostate cancer. Adv Pharmacol. 2012;65:267–313. doi: 10.1016/B978-0-12-397927-8.00009-9. [DOI] [PubMed] [Google Scholar]
- 50.Guruharsha KG, et al. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu B, et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149:1207–1220. doi: 10.1016/j.cell.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finkel T, et al. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Otin C, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naylor RM, et al. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin Pharmacol Ther. 2013;93:105–116. doi: 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baraibar MA, et al. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid Med Cell Longev. 2012;2012:919832. doi: 10.1155/2012/919832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Reynolds P, et al. Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol Biomarkers Prev. 2009;18:3389–3398. doi: 10.1158/1055-9965.EPI-09-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brody JS. Transcriptome alterations induced by cigarette smoke. Int J Cancer. 2012;131:2754–2762. doi: 10.1002/ijc.27829. [DOI] [PubMed] [Google Scholar]
- 61.Sasco AJ, et al. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 62.Salem AF, et al. Cigarette smoke metabolically promotes cancer, via autophagy and premature aging in the host stromal microenvironment. Cell Cycle. 2013;12:818–825. doi: 10.4161/cc.23722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Hunten S, et al. The p53/microRNA network in cancer: experimental and bioinformatics approaches. Adv Exp Med Biol. 2013;774:77–101. doi: 10.1007/978-94-007-5590-1_5. [DOI] [PubMed] [Google Scholar]
- 65.Huarte M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sulli G, et al. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rev Cancer. 2012;12:709–720. doi: 10.1038/nrc3344. [DOI] [PubMed] [Google Scholar]
- 67.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 68.Davalos AR, et al. Senescent cells as a source of inflammatory factors for tumor progression. Cancer and Metastasis Reviews. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 70.Cukierman E, et al. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 71.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parrinello S, et al. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krtolica A, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 75.Coppe JP, et al. A role for fibroblasts in mediating the effects of tobacco-induced epithelial cell growth and invasion. Mol Cancer Res. 2008;6:1085–1098. doi: 10.1158/1541-7786.MCR-08-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 77.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coppe JP, et al. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freund A, et al. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 83.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 84.Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 85.Bernerd F, Asselineau D. UVA exposure of human skin reconstructed in vitro induces apoptosis of dermal fibroblasts: subsequent connective tissue repair and implications in photoaging. Cell Death Differ. 1998;5:792–802. doi: 10.1038/sj.cdd.4400413. [DOI] [PubMed] [Google Scholar]
- 86.Szabowski A, et al. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell. 2000;103:745–755. doi: 10.1016/s0092-8674(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 87.Angel P, Szabowski A. Function of AP-1 target genes in mesenchymal-epithelial cross-talk in skin. Biochem Pharmacol. 2002;64:949–956. doi: 10.1016/s0006-2952(02)01158-9. [DOI] [PubMed] [Google Scholar]
- 88.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 89.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 90.Hardy K, et al. Transcriptional networks and cellular senescence in human mammary fibroblasts. Mol Biol Cell. 2005;16:943–953. doi: 10.1091/mbc.E04-05-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bernard D, et al. Involvement of Rel/nuclear factor-kappaB transcription factors in keratinocyte senescence. Cancer Res. 2004;64:472–481. doi: 10.1158/0008-5472.can-03-0005. [DOI] [PubMed] [Google Scholar]
- 92.Adler AS, et al. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adler AS, et al. Motif module map reveals enforcement of aging by continual NF-B activity. Genes & Development. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nature Reviews Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chorley BN, et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braun S, et al. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirota A, et al. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis. J Invest Dermatol. 2005;124:825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 98.Kuroda K, et al. Inhibitory effect of fumaric acid on forestomach and lung carcinogenesis by a 5-nitrofuran naphthyridine derivative in mice. J Natl Cancer Inst. 1982;69:1317–1320. [PubMed] [Google Scholar]
- 99.Kuroda K, et al. Inhibitory effect of fumaric acid on 3-methyl-4'-(dimethylamino)-azobenzene-induced hepatocarcinogenesis in rats. J Natl Cancer Inst. 1983;71:855–857. [PubMed] [Google Scholar]
- 100.Adam J, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim TH, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 2011;71:2260–2275. doi: 10.1158/0008-5472.CAN-10-3007. [DOI] [PubMed] [Google Scholar]
- 102.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rubinsztein DC, et al. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 104.White E, Lowe SW. Eating to exit: autophagy-enabled senescence revealed. Genes Dev. 2009;23:784–787. doi: 10.1101/gad.1795309. [DOI] [PubMed] [Google Scholar]
- 105.Mizushima N, Komatsu M. Autophagy: Renovation of Cells and Tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 106.Jain A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Young ARJ, et al. Autophagy mediates the mitotic senescence transition. Genes & Development. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Capparelli C, et al. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts,"fueling" tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle. 2012;11:3599–3610. doi: 10.4161/cc.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deretic V, et al. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012;22:397–406. doi: 10.1016/j.tcb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinez-Outschoorn UE, et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whitaker-Menezes D, et al. Evidence for a stromal-epithelial"lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim YC, et al. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scadden DT. Rethinking stroma: lessons from the blood. Cell Stem Cell. 2012;10:648–649. doi: 10.1016/j.stem.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 117.Jelaska A, et al. Fibroblast heterogeneity in physiological conditions and fibrotic disease. Springer Semin Immunopathol. 1999;21:385–395. [PubMed] [Google Scholar]
- 118.Crossno JT, Jr, et al. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Charriere GM, et al. Macrophage characteristics of stem cells revealed by transcriptome profiling. Exp Cell Res. 2006;312:3205–3214. doi: 10.1016/j.yexcr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 121.Charriere G, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 122.Tchkonia T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 124.Erez N, et al. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 125.Sugimoto H, et al. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]