EPIDEMIOLOGY OF LUNG CANCER
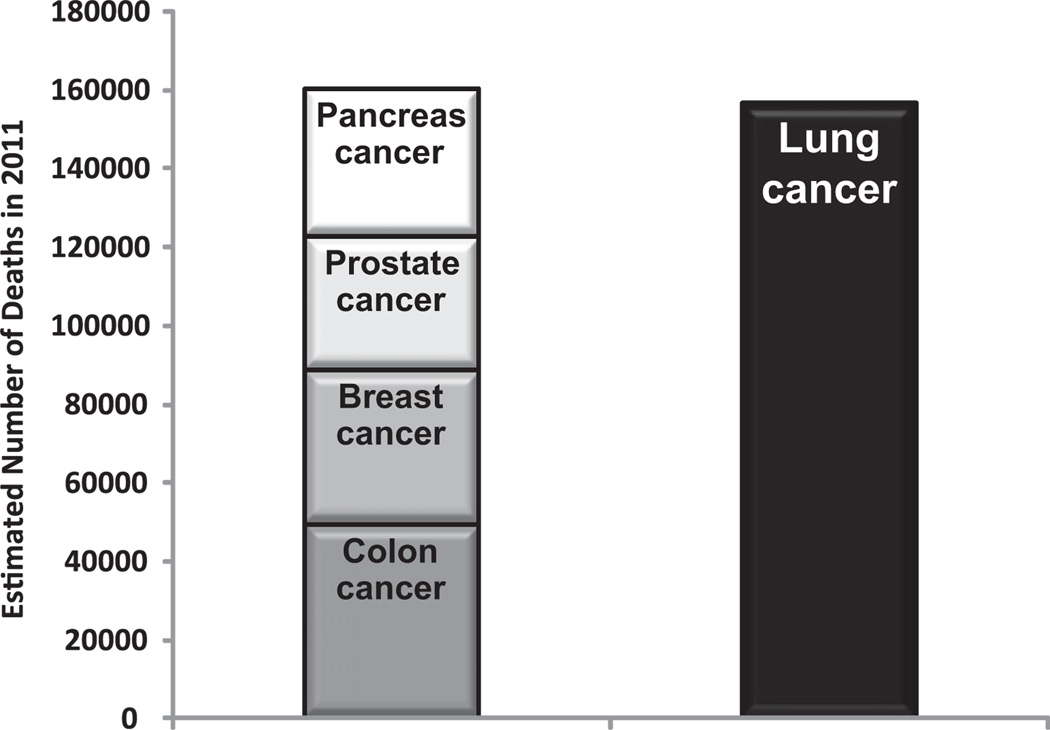
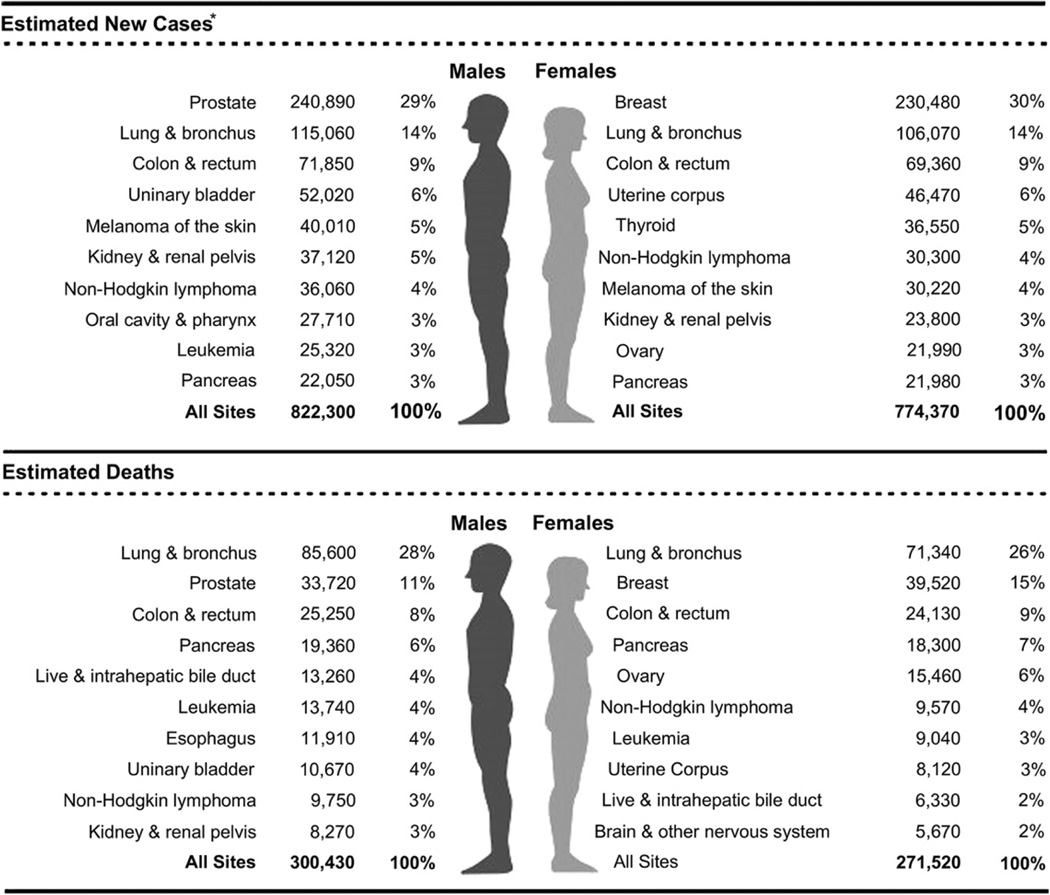
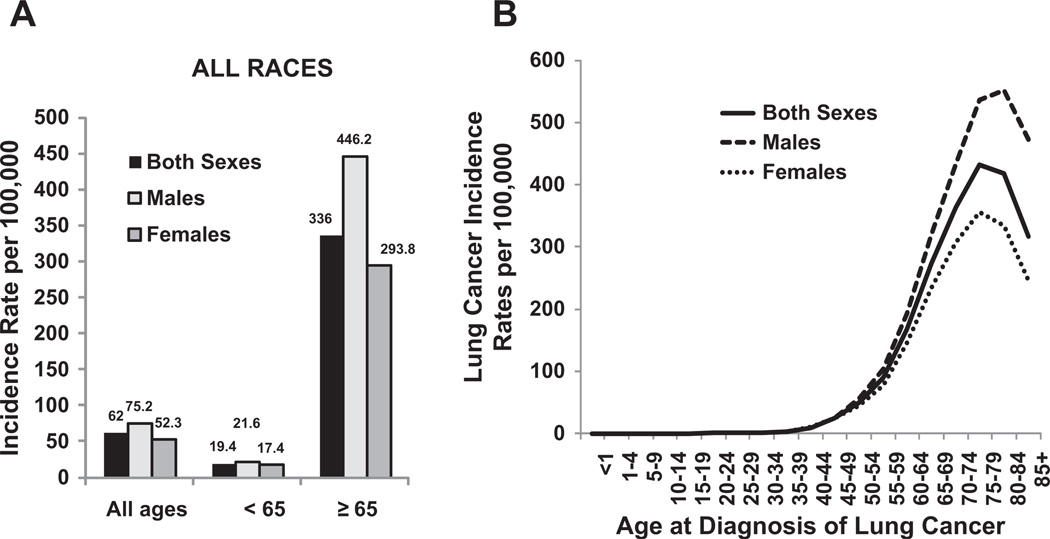
Lung cancer is the leading cause of cancer death in the United States and around the world. Almost as many Americans die of lung cancer every year than die of prostate, breast, and colon cancer combined (Fig. 1).1 Siegel and colleagues1 reviewed recent cancer data and estimated a total of 239,320 new cases of lung cancer and 161,250 deaths from lung cancer in the United States in 2010.2 The statistics reflect data from 2007 and, therefore, likely underestimate the current lung cancer burden. Lung cancer has been the most common cancer worldwide since 1985, both in terms of incidence and mortality. Globally, lung cancer is the largest contributor to new cancer diagnoses (1,350,000 new cases and 12.4% of total new cancer cases) and to death from cancer (1,180,000 deaths and 17.6% of total cancer deaths). The 5-year survival rate in the United States for lung cancer is 15.6%, and although there has been some improvement in survival during the past few decades, the survival advances that have been realized in other common malignancies have yet to be achieved in lung cancer. There has been a large relative increase in the numbers of cases of lung cancer in developing countries. Approximately half (49.9%) of the cases now occur in developing countries whereas in 1980, 69% of cases were in developed countries. The estimated numbers of lung cancer cases worldwide has increased by 51% since 1985 (a 44% increase in men and a 76% increase in women). In the United States, cancer of the lung and bronchus ranks second in both genders, with an estimated 115,060 new cases in men (14% of all new cancers) and 106,070 in women (14% of all new cancers).1,2 The age-adjusted incidence rate of lung cancer is 62 per 100,000 men and women per year in the United States, with the incidence rate higher in men than in women (75.2 vs 52.3 per 100,000).3 Lung cancer in both genders tops the list on the number of estimated deaths yearly (85,600, or 28% of all cancer deaths for men, and 71,340, or 26% of all cancer deaths for women) (Fig. 2).
Fig. 1.
Estimated deaths from lung cancer compared with colon cancer, breast cancer, prostate cancer, and pancreatic cancer combined. (Data from Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61(4):212–36.)
Fig. 2.
Ten leading cancer types for the estimated new cancer cases and deaths categorized by gender. (From Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61(4):212–36; with permission.)
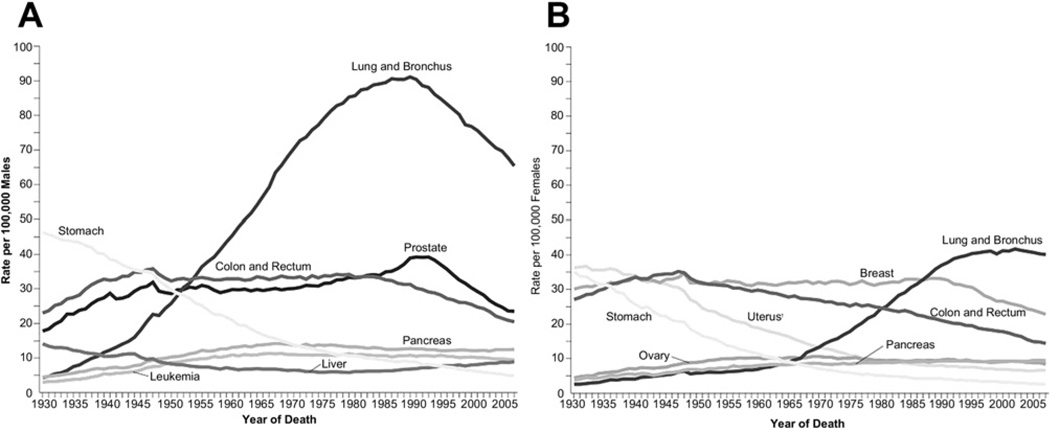
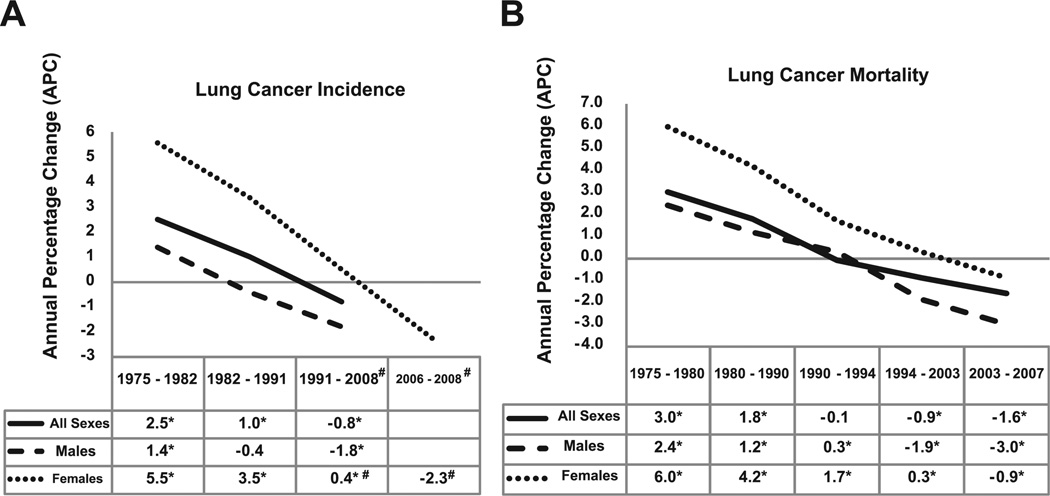
Lung cancer incidence in men in the United States has been decreasing since the early 1980s. The incidence and mortality rates for lung cancer tend to mirror one another because most patients diagnosed with lung cancer eventually die of it. Siegel and colleagues,1 in their review of cancer statistics in 2011, noted decreases in death rates from lung cancer in men by 2.0% per year from 1994 to 2006 (Fig. 3). In women, however, lung cancer death rates continued to increase by 0.3% per year from 1995 to 2005, but more recent data from 2003 to 2006 show a more encouraging trend with a start in decline of 0.9% per year (see Figs. 3 and 4). The lung cancer incidence among women has declined over the past decades, from 5.6% between 1975 and 1982, to 3.4% between 1982 and 1990, to 0.4% between 1991 and 2006, and more recently to −2.3% between 2006 and 2008 (see Fig. 4). Because of the change in lung cancer incidence in women, recent figures show that lung cancer death rates decreased in women for the first time, more than a decade after decreases in men.4 The lag in the decline of lung cancer rates in women compared with men has been attributed to the fact that cigarette smoking in women peaked two decades later than in men. Lung cancer mortality rates thus seem to be reaching a plateau, which is an encouraging change from the steep rise in the 1970s (see Fig. 3).
Fig. 3.
Annual age-adjusted cancer death rates among (A) men and (B) women for selected cancers. Rates are age adjusted to the 2000 US standard population. Due to changes in International Classification of Diseases coding, numerator information has changed over time. Rates for cancers of the uterus, ovary, lung and bronchus, and colon and rectum are affected by these changes. (Source: US Mortality Volumes 1930 to 1959, US Mortality Data, 1960 to 2007. National Center for Health Statistics, Centers for Disease Control and Prevention; 2006.) (From Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61(4):212–36; with permission.)
Fig. 4.
Trends in (A) lung cancer incidence and (B) lung cancer mortality rates in the United States as evaluated by the annual percentage change (APC). A negative APC value means that the trend is a decrease; a positive APC value refers to an increase trend. Asterisk refers to statistically significant APC value; # refers to the APC value of 0.5 for women for the period 1991–2006; and the APC trend value of −2.3 refers to the period 2006–2008. (Data from Howlander N, Noone A, Krapcho M, et al. Cancer of the lung and bronchus [invasive]. In: Institute NC, editor. SEER Cancer Statistics Review 1975–2008; 2011.)
The Surveillance, Epidemiology and End Results (SEER) data from 2004 to 2008 reported the median age at diagnosis for cancer of the lung and bronchus as 71 years (Fig. 5). No cases were diagnosed in patients younger than 20 years (see Fig. 5).3 Approximately 0.2% of lung cancers was diagnosed in patients between age 20 and 34 years; 1.5% between 35 and 44 years; 8.8% between 45 and 54 years; 20.9% between 55 and 64 years; 31.1% between 65 and 74 years; 29% between 75 and 84 years; and 8.3% at 85 years and older.
Fig. 5.
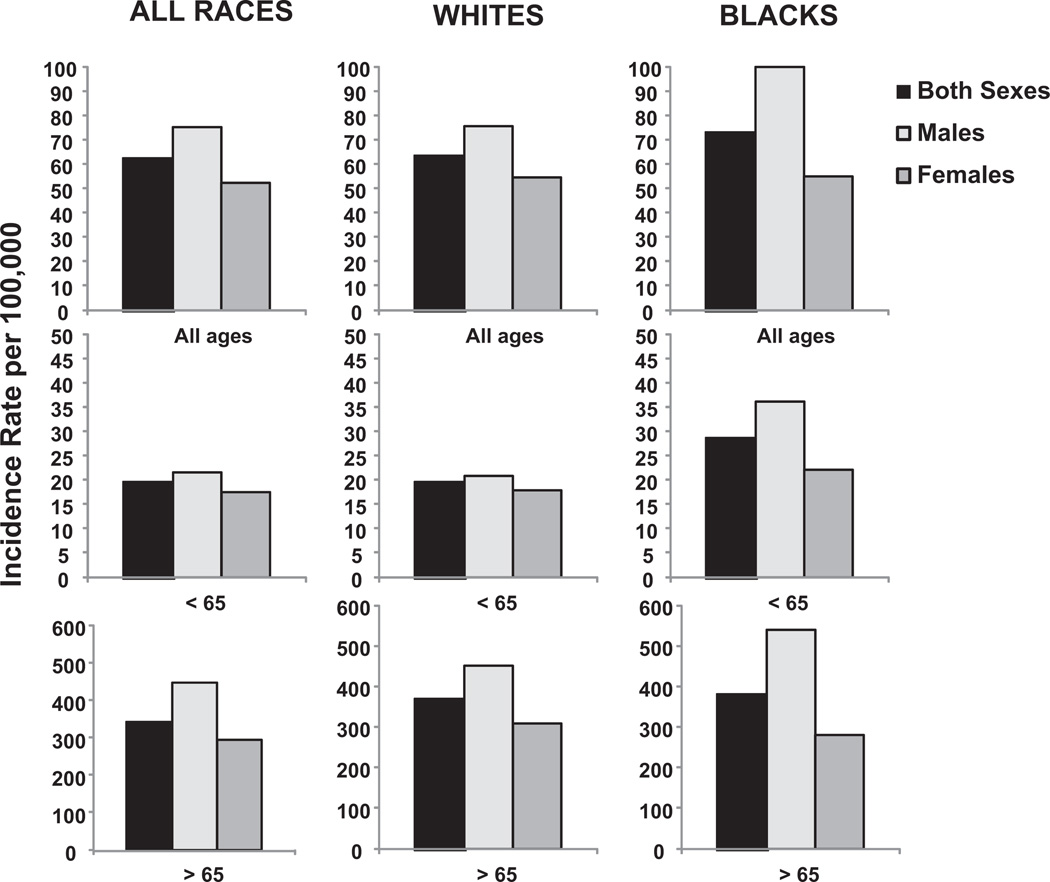
US age-adjusted lung cancer incidence by gender, age, and race. (A) Separated by age <65 years and age ≥65 years. (B) Separated by age from <1 to 851 years. Rates are per 100,000 and are age-adjusted to the 2000 US standard population. (Data from Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2008. Bethesda (MD): National Cancer Institute; 2010. Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.)
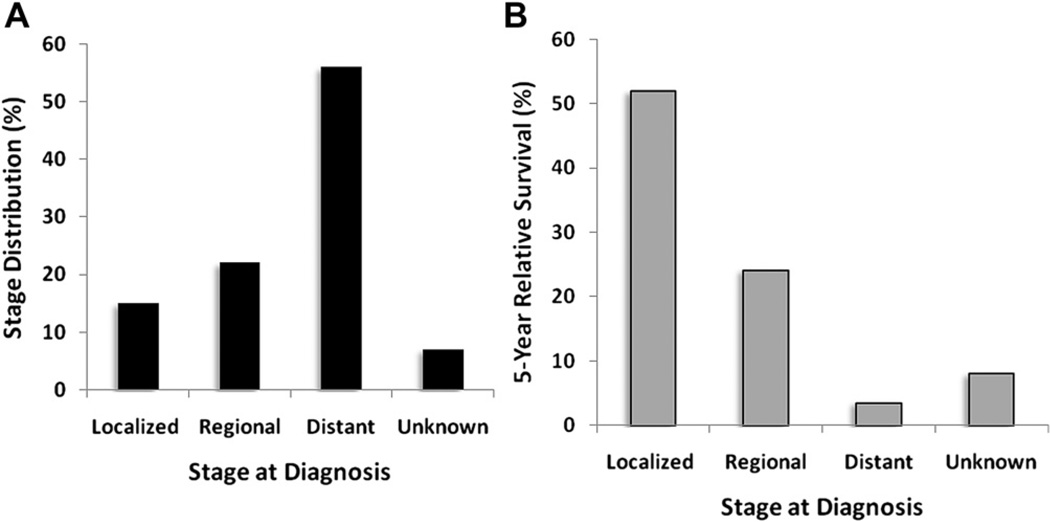
Lung cancer arises from the cells of the respiratory epithelium and can be divided into two broad categories. Small cell lung cancer (SCLC) is a highly malignant tumor derived from cells exhibiting neuroendocrine characteristics and accounts for 15% of lung cancer cases. Non–small cell lung cancer (NSCLC), which accounts for the remaining 85% of cases, is further divided into 3 major pathologic subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Adenocarcinoma by itself accounts for 38.5% of all lung cancer cases, with squamous cell carcinoma accounting for 20% and large cell carcinoma accounting for 2.9%.3,5 In the past several decades, the incidence of adenocarcinoma has increased greatly, and adenocarcinoma has replaced squamous cell carcinoma as the most prevalent type of NSCLC. The 5-year total survival rate for lung cancer in the United States from 2001 to 2007 was 15.6%. Patients with localized disease at diagnosis have a 5-year survival rate of 52%; however, the more than 52% of patients with distant metastasis at diagnosis have a dismal 5-year survival rate of 3.6%, which begs for the need for better screening methods to detect early-stage cancers (Fig. 6). (See article elsewhere in this issue by Mithun.)
Fig. 6.
Stage distribution and 5-year relative survival by stage at time of diagnosis for 2001 to 2007. (A) Stage distribution and (B) 5-year relative survival based on stage at diagnosis of lung cancer. Localized disease defined by confinement to primary site. Regional refers to spread to regional lymph nodes. Distant refers to when cancer has metastasized. Unknown includes unstaged cancers. Stage distribution is based on summary stage 2000 documentations. (Data from Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975– 2008. Bethesda (MD): National Cancer Institute; 2010. Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.)
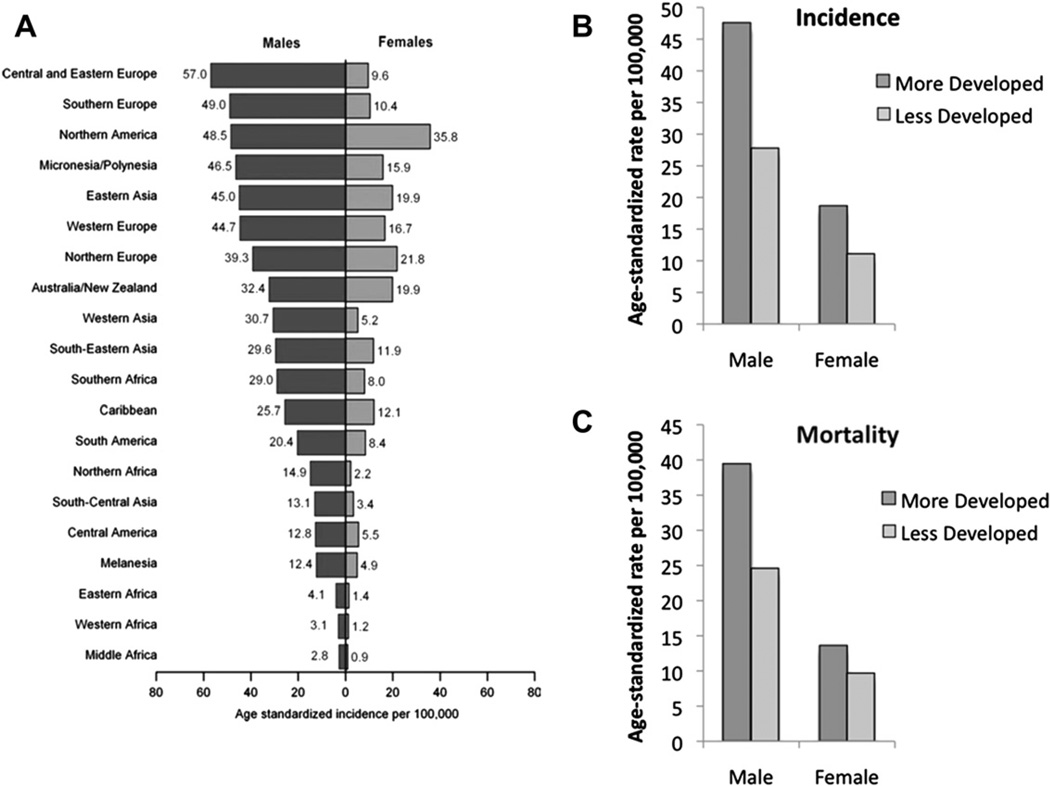
Lung cancer was the most commonly diagnosed cancer and the leading cause of cancer death in men in 2008 globally.2 For women, lung cancer was the fourth most commonly diagnosed cancer and the second leading cause of cancer death. Overall, lung cancer accounted for 13% or 1.6 million of total cancer cases and 18% or 1.4 million cancer-related deaths worldwide in 2008. Lung cancer incidence and mortality rates are highest in the United States and the developed countries. In contrast, lung cancer rates in underdeveloped geographic areas, including Central America and most of Africa, are lower, except the rates are slowly increasing (Fig. 7A). More developed countries have higher incidence and mortality rates from lung cancers in both genders than less developed countries (see Fig. 7B, C). The World Health Organization estimates that lung cancer deaths worldwide will continue to rise, largely as a result of an increase in global tobacco use, especially in Asia. Tobacco use is the principal risk factor for lung cancer, and a large proportion of all pulmonary carcinomas are attributable to the effects of cigarette smoking.6 Despite efforts to curb tobacco smoking, there are approximately 1.1 billion smokers worldwide, and if the current trends continue, that number would increase to 1.9 billion by 2025.7 As of 2008, 20.6% (46.0 million) of American adults smoke.8 Of these, 79.8% (36.7 million) smoke every day and 20.2% (9.3 million) smoke some days. During the past decade, there has been a 3.5% point decrease in the number of US adults who smoke (20.6% in 2008 and 24.1% in 1998).
Fig. 7.
Age-standardized lung cancer incidence and mortality rates by gender and world area. Lung cancer incidence by gender and world area (A). Incidence (B) and mortality rates (C) of lung cancer by gender for more developed and less developed areas in the world, 2008. Rates are standardized to the world standard population. (Adapted from Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61(2):69–90; with permission.)
Despite the availability of new diagnostic and genetic technologies, advancements in surgical techniques, and the development of new biologic treatments, the overall 5-year survival rate for lung cancer in the United States remains at a dismal 15.6%.9 The situation globally is even worse, with 5-year survival in Europe, China, and developing countries estimated at only 8.9%.
This introductory article to the current edition of Clinics in Chest Medicine dedicated to lung cancer focuses on modifiable risk factors, including tobacco smoking, occupational carcinogens, diet, and ionizing radiation. It also discusses briefly the molecular and genetic aspects of lung carcinogenesis.
ETIOLOGY OF LUNG CANCER
Tobacco Smoking
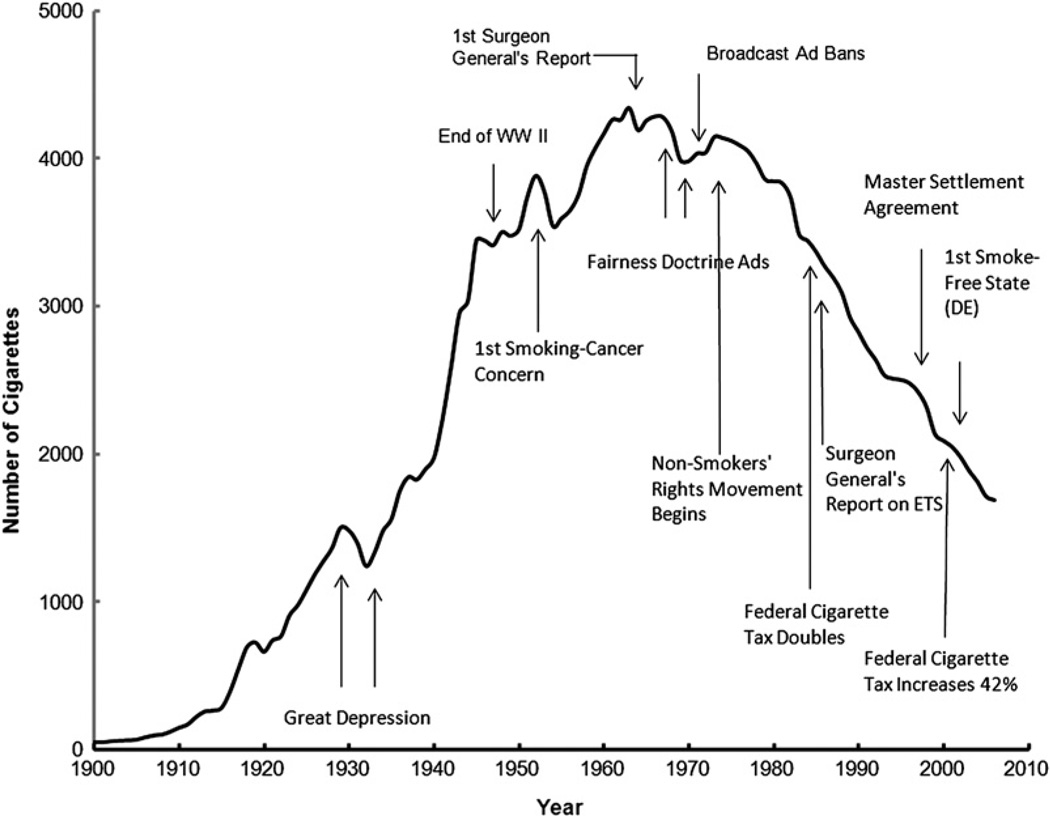
Tobacco has been part of the cultural and economic structure of this country since the time of Columbus. Originally chewed or smoked in pipes, tobacco became widely available in cigarette form after the development of cigarette wrapping machinery in the mid-1800s. Before World War I, cigarette use in the United States was modest. Wynder and Graham estimated that the average adult smoked fewer than 100 cigarettes per year in 1900.10 Fifty years later, this number rose to approximately 3500 cigarettes per person per year and reached a maximum of approximately 4400 cigarettes per person per year in the mid-1960s (Fig. 8).11 In 1964, the US Public Health Service published a landmark report from the Surgeon General on smoking and its effects on health.12 That seminal report stated the following important principal findings. (1) Cigarette smoking was associated with a 70% increase in the age-specific death rates of men and a lesser increase in the death rates of women. (2) Cigarette smoking was causally related to lung cancer in men. The magnitude of the effect of cigarette smoking far outweighed all other factors leading to lung cancer. The risk for lung cancer increased with the duration of smoking and the number of cigarettes smoked per day. The report estimated that the average male smoker had an approximately 9-fold to 10-fold risk for lung cancer, whereas heavy smokers had at least a 20-fold risk. (3) Cigarette smoking was believed more important than occupational exposures in the causation of lung cancer in the general population. (4) Cigarette smoking was the most important cause of chronic bronchitis in the United States. (5) Male cigarette smokers had a higher death rate from coronary artery disease than male nonsmokers.
Fig. 8.
The adult per capita cigarette consumption in the United States, 1900–2006, with historical highlights. (Adapted from Warner KE, Mendez D. Tobacco control policy in developed countries: yesterday, today, and tomorrow. Nicotine Tob Res 2010;12(9):876–87; with permission.)
The report concluded, “Cigarette smoking is a health hazard of sufficient importance in the United States to warrant appropriate remedial action.” Since the publication of the report, yearly per capita consumption of cigarettes has declined in the United States (see Fig. 8).11 It is estimated that 20.6% of all American adults over age 18 years continue to smoke, a figure that has only minimally decreased since approximately 1997, based on a recent Morbidity and Mortality Weekly Report report by Dube and colleagues.13 Of these smokers, 80.1% (36.3 million people) smoke every day and 19.9% (9 million) smoke some days. More men (23.5%) than women (17.9%) smoke. The decline in smoking rates is steeper for black men and white men than for white women and black women. The prevalence of smoking is 31.1% among persons below the federal poverty level. For adults older than 25 years, the prevalence of smoking was 28.5% among persons with less than a high school diploma compared with 5.6% among persons with a graduate degree.13 There were also regional differences in the United States, with the West having the lowest prevalence (16.4%) and higher prevalence observed in the South (21.8%) and the Midwest States (23.1%).8 More than 80% of adult smokers begin before the age 18 years. In 2009, 1 in 5 American high school students reported smoking cigarettes in the preceding 30 days.14 The smoking rate has declined but has slowed of late; the smoking prevalence increased from 27.5% in 1991 to 36.4% in1997, declined to 21.9% in 2003, and then declined less to 19.5% in 2009.15
One of the first descriptions of lung cancer was in 1912 by Adler16 in an extensive review of autopsy reports from hospitals in the United States and western Europe, which found 374 cases of primary lung cancer. This represented less than 0.5% of all cancer cases. He concluded, “primary malignant neoplasms of the lung are among the rarest forms of disease.” In 1920, lung cancer constituted only 1% of all malignancies in the United States. During the next several decades, researchers in the United States and abroad noted a significant increase in the incidence of carcinoma of the lung. In a series of 185,434 autopsy cases collected between 1897 and 1930, Hruby and Sweany17 noted that the incidence of lung cancer had increased disproportionately to the incidence of cancer in general.
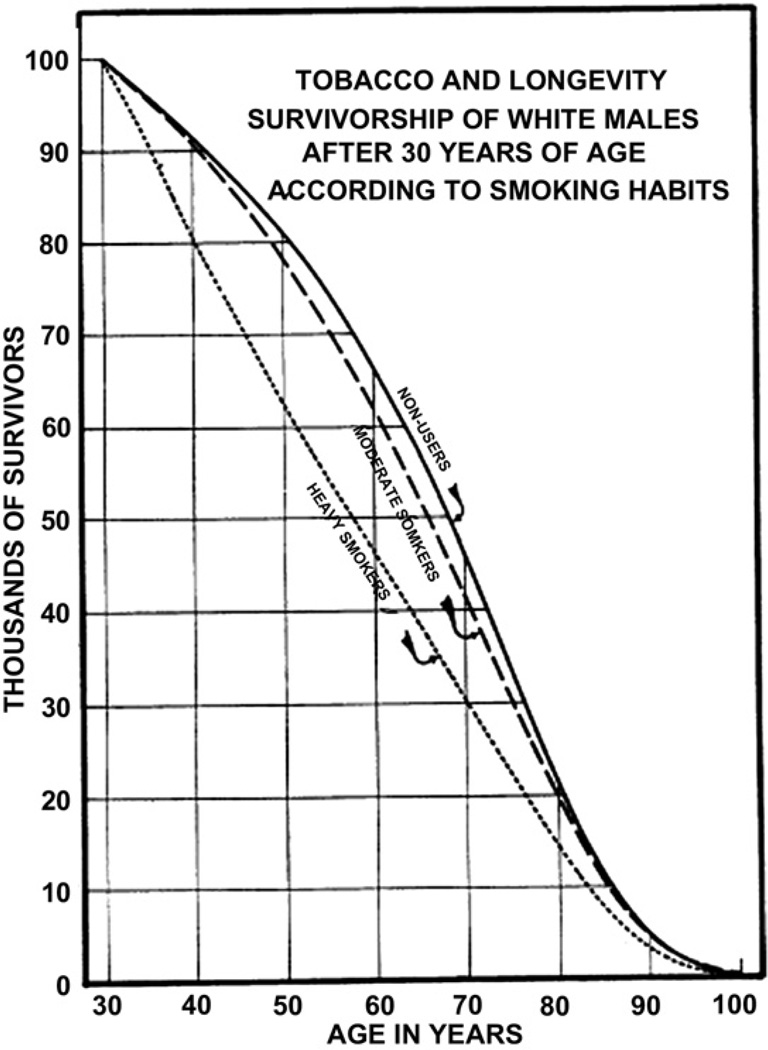
The first scientific report that associated cigarette smoking with an increased risk of premature death was in 1938, when Pear18 showed the degree of adverse effect on longevity increased with the amount of smoking (Fig. 9). The finding that tar applied to the skin of animals produced lung carcinoma raised concern that inhalation of tar products could be an important factor in the increase in lung cancer incidence. Observations in patients and experimental studies in animals have shown that tobacco tar liberated from the burning of tobacco was a carcinogenic agent.19 Other uncontrolled patient series highlighted the potential role of cigarette smoking in the increase in lung cancer incidence.20–23 In 1941, Ochsner and DeBakey stated in their review of lung carcinoma, “it is our definite conviction that the increase in the incidence of pulmonary carcinoma is due largely to the increase in smoking.”21
Fig. 9.
The survivorship lines of life tables for white men falling into 3 categories relative to the usage of tobacco. (A) Nonusers (solid line); (B) moderate smokers (dashed line); (C) heavy smokers (dotted line). (Adapted from Pear R. Tobacco smoking and longevity. Science 1938;87:216; with permission.)
In 1950, two large landmark epidemiologic studies established the role of tobacco smoking as a causal factor in bronchogenic carcinoma.24,25 In a case-control study in United Kingdom, Doll and Hill24 described an association between carcinoma of the lung and cigarette smoking and the effect of the amount of cigarette use on the development of lung cancer.18,24,26 In another case-control study in the United States, Wynder and Graham25 examined 605 cases of lung cancer in men compared with a general male hospital population without cancer. The American investigators found that 96.5% of lung cancers were in men who were moderate to heavy smokers for many years. The authors concluded, (1) the excessive and prolonged use of tobacco was an important factor in the induction of lung cancer; (2) lung cancer in a nonsmoker was rare (however, this is currently not the case [discussed later in section on never smokers]); and (3) there could be a lag period of 10 years or more between cessation of smoking and the clinical onset of carcinoma. Subsequently, the Surgeon General of the United States re-emphasized in 2004 the conclusions of the 1964 report that “cigarette smoking is the major cause of lung cancer.”27
Cigarette smoke is a complex aerosol composed of gaseous and particulate compounds. The smoke consists of mainstream smoke and sidestream smoke components. Mainstream smoke is produced by inhalation of air through the cigarette and is the primary source of smoke exposure for the smoker. Sidestream smoke is produced from smoldering of the cigarette between puffs and is the major source of environmental tobacco smoke (ETS). The primary determinant of tobacco addiction is nicotine, and tar is the total particulate matter of cigarette smoke after nicotine and water have been removed. Exposure to tar seems to be a major component of lung cancer risk. The Federal Trade Commission determines the nicotine and tar content of cigarettes by measurements made on standardized smoking machines. The composition of mainstream smoke, however, can vary greatly depending on the intensity of inhalation by a smoker. Although the use of filter tips decreases the amount of nicotine and tar in mainstream smoke, the effect of filter tips also varies because the compression of the tips by lips or fingers and the depth of inhalation of the smoker. There are more than 4000 chemical constituents of cigarette smoke: 95% of the weight of mainstream smoke comes from 400 to 500 gaseous compounds28; the rest of the weight is made up of more than 3500 particulate components.
Mainstream smoke contains many potential carcinogens, including polycyclic aromatic hydrocarbons (PAHs), aromatic amines, N-nitrosamines, and other organic and inorganic compounds, such as benzene, vinyl chloride, arsenic, and chromium. The PAHs and N-nitrosamines require metabolic activation to become carcinogenic. Metabolic detoxification of these compounds can also occur, and the balance between activation and detoxification likely affects individual cancer risk. Radioactive materials, such as radon and its decay products, bismuth, and polonium, are also present in tobacco smoke.
The International Agency for Research on Cancer (IARC) has identified at least 50 carcinogens in tobacco smoke.29,30 The agents that seem of particular concern in lung carcinoma are the tobacco-specific N-nitrosamines (TSNAs) formed by nitrosation of nicotine during tobacco processing and during smoking. Eight TSNAs have been described, including 4-(methylnitrosamino)-1(3-pyridyl)-1-butanone (NNK), which is known to induce adenocarcinoma of the lung in experimental animals. Other TSNAs have been linked to cancer of the esophagus, bladder, pancreas, oral cavity, and larynx. Of the TSNAs, NNK, which seems the most important inducer of lung cancer, has carcinogenic effects with both topical and systemic administration. TSNAs are directly delivered to the lung by inhalation of tobacco smoke. TSNAs are also absorbed systemically, and hematogenous delivery to the lung can occur by way of the pulmonary circulation.
The dosage of smoke constituents received depends not only on the cigarette itself but also on the duration and intensity of inhalation, the presence and competence of a filter, and the duration of cooling of the smoke before inhalation. The primary factor determining intensity of cigarette use is the nicotine dependence of the smoker, and although cigarettes now contain less nicotine and tar than in the past, smokers tend to smoke more intensively with higher puffs per minute and deeper inhalations to satisfy their nicotine need. Therefore, the measurements of tar and nicotine content made by smoking machines may significantly underestimate individual exposure. Low-yield filtered cigarettes might be a contributing factor to the increase in the incidence of adenocarcinoma of the lung.31 The nicotine-addicted smoker smokes low-yield cigarettes far more intensively than nonfiltered higher-yield cigarettes, and with deeper inhalation, higher-order bronchi in the peripheral lung are exposed to carcinogen-containing smoke as opposed to the major bronchi alone. These peripheral bronchi lack protective epithelium and are exposed to carcinogens, including TSNAs, which have been linked to the induction of adenocarcinoma.
Tobacco carcinogens, such as NNK, can bind to DNA and create DNA adducts, which are pieces of DNA covalently bonded to a cancer-causing chemical, such as PAH in cigarette smoke. Repair processes may remove these DNA adducts and restore normal DNA, or cells with damaged DNA may undergo apoptosis. Failure of the normal DNA repair mechanisms to remove DNA adducts, however, can lead to permanent mutations. NNKs can mediate an array of signaling pathway activation that includes modulation of critical oncogenes and tumor suppressor genes that ultimately can result in uncontrolled cellular proliferation and tumorigenesis.32
NNK is associated with DNA mutations resulting in the activation of K-ras oncogenes.33,34 K-ras oncogene activation has been detected in 24% of human lung adenocarcinomas35 and is present in adenocarcinoma of the lung in ex-smokers, suggesting that such mutations do not revert necessarily with the cessation of tobacco smoking.36 This may in part explain the persistent elevation in lung cancer risk in exsmokers even years after discontinuing cigarette use. In addition, a specific chemical constituent of tobacco smoke, benzo[a]pyrene metabolite, can damage various p53 tumor-suppressor gene loci that are known to be abnormal in approximately 60% of primary lung cancer cases.37 Related PAHs found in tobacco smoke are also capable of targeting other lung cancer mutational hotspots.38
One in 9 smokers eventually develops lung cancer.39 The relative risk of lung cancer in long-term smokers has been estimated as 10-fold to 30-fold compared with lifetime nonsmokers.40 The cumulative lung cancer risk among heavy smokers can be as high as 30% compared with a lifetime risk of less than 1% in nonsmokers. The lung cancer risk is proportional to the quantity of cigarette consumption, because factors, such as the number of packs per day smoked, the age of onset of smoking, the degree of inhalation, the tar and nicotine content of cigarettes, and use of unfiltered cigarettes, become important.41,42 There is no question that tobacco smoking remains the most important modifiable risk factor for lung cancer. It has been estimated that up to 20% of all cancer deaths worldwide could be prevented by the elimination of tobacco smoking.43 It is also clear that individual susceptibility is a factor in carcinogenesis. Although more than 80% of lung cancers occur in persons with tobacco exposure, fewer than 20% of smokers develop lung cancer. This variability in cancer susceptibility is likely affected by other environmental factors or by genetic predisposition.
Other Types of Smoking
Other forms of tobacco use, such as cigar smoking and pipe smoking, have been associated with increased risk for lung cancer. The risk seems weaker, however, than with cigarette smoking. Most cigars are composed of primarily of a single type of tobacco that is air cured and fermented but can vary in their size and shape to contain from 1 g to 20 g of tobacco. Smoking 5 cigars a day on average is equivalent to smoking 1 pack a day of cigarettes. A large prospective study of more than 130,000 men over 12 years showed that cigar smokers have a relative risk of lung cancer of 5.1 compared with non–cigar smokers.44 Another study showed a relative risk of 2.1 for lung cancer compared with nonsmokers, with men who smoked 5 or more cigars a day having the greatest risk.45 The increased risk for lung cancer as a result of pipe smoking is comparable to cigar smoking.46,47 A large cohort study showed that active pipe smoking was associated with a relative risk for lung cancer of 5.0.48 Cigar and pipe smokers have a greater risk for lung cancer than lifelong nonsmokers or former smokers.49
The effects of inhaling smoke from recreational drugs, such as marijuana and cocaine, are less studied than the effects of tobacco smoke. Meta-plastic histologic and molecular changes similar to premalignant alterations have been described in the bronchial epithelium in habitual smokers of marijuana or cocaine.50,51 A clear association has not been fully established, however, between such inhalant drug use and lung cancer. A case-control study showed that there is an 8% increased risk for lung cancer for each joint-year of marijuana smoking after adjusting for tobacco cigarette smoking.52 Similarly, there is a 7% increased risk for lung cancer for each pack-year of cigarette smoking after adjusting for marijuana smoking. The relationship between cocaine smoking and lung cancer is not well studied.
Never Smokers
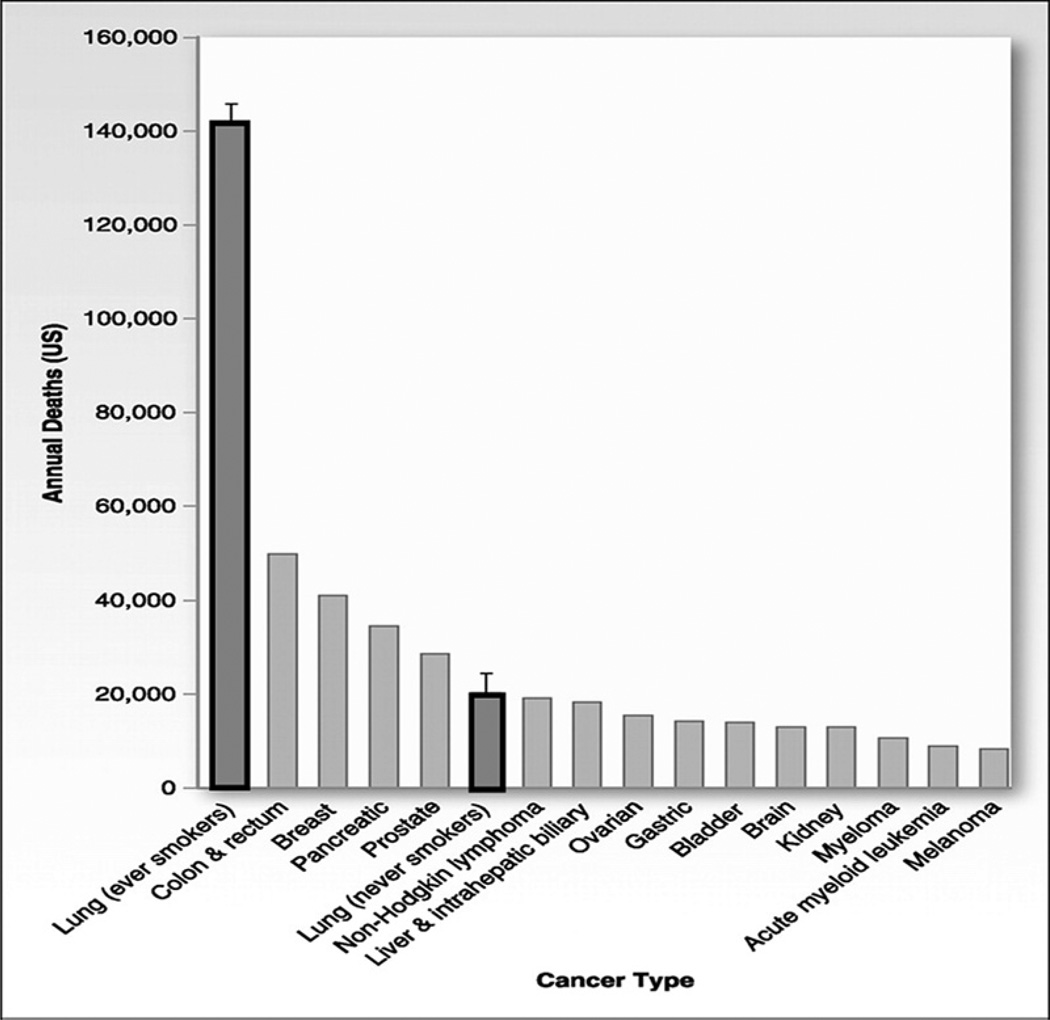
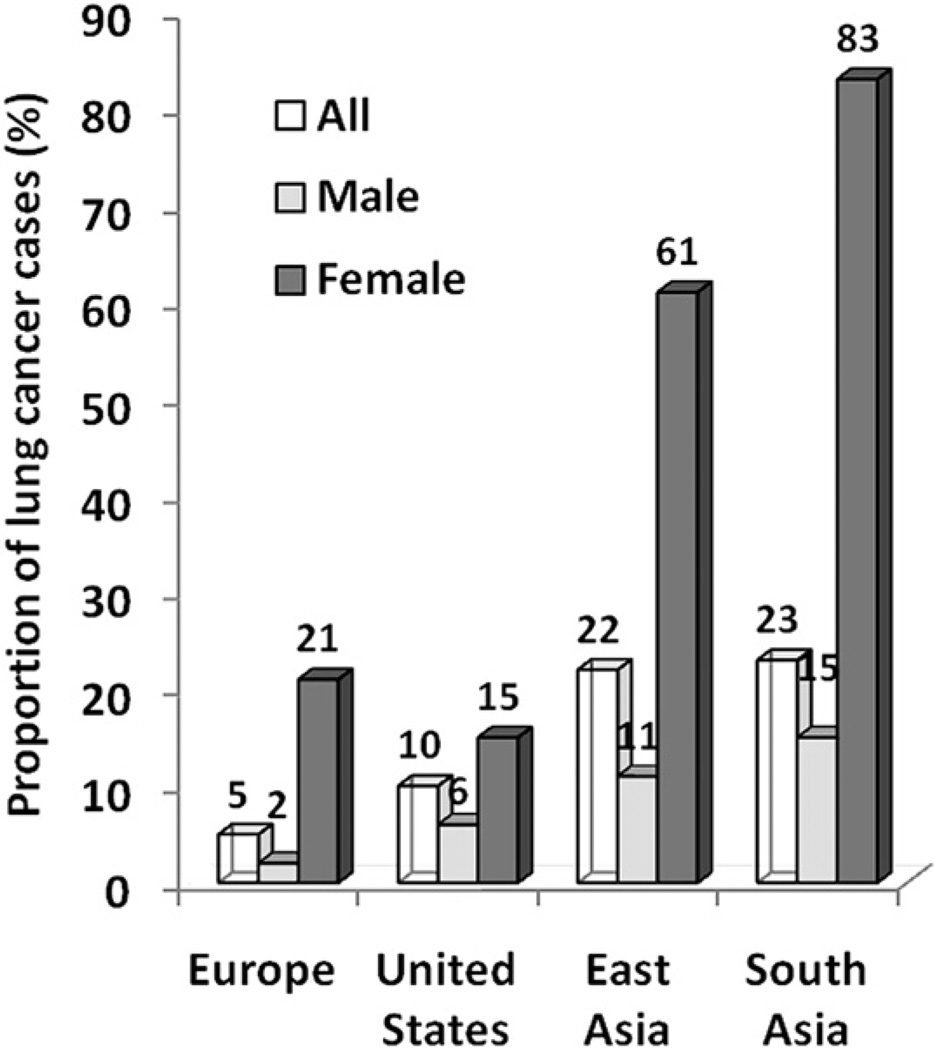
The term, never smokers, refers to persons who have smoked fewer than 100 cigarettes in their lifetime, including lifetime nonsmokers. Most studies that track the trend of lung cancer rates often include both smokers and never smokers, and few studies independently study the trends over time for never smokers because of the limited longitudinal collection and the limited reliability of smoking information in population-based registries. From what is available, however, the overall global statistics estimate that 15% of lung cancers in men and up to 53% in women are not attributable to smoking, with never smokers accounting for 25% of all lung cancer cases worldwide.53 If lung cancer in never smokers were considered separately, it would rank as the seventh most common cause of cancer death worldwide before cervical, pancreatic, and prostate cancer (Fig. 10).54 In countries in South Asia, up to 80% of women with lung cancer are never smokers (Fig. 11).55 In the United States, one study estimated that 19% of lung cancer in women and 9% of lung cancer in men occurs in never smokers.56 The age-adjusted rate for lung cancer in never smokers (ages 40–79 years) ranged from 11.2 to 13.7 per 100,000 person-years for men and from 15.2 to 20.8 per 100,000 person-years for women. The rates are 12 to 30 times higher in current smokers of the same age group.
Fig. 10.
Common causes of cancer deaths in the United States with focus on never smokers. Total lung cancer deaths, estimated at 161,840 in 2008, have been split into ever smokers and never smokers. Error bars reflect that the number of lung cancer deaths in never smokers, including cases attributable to secondhand smoke exposure and cases not attributable to tobacco, are estimated to total 16,000 to 24,000 per year. (Adapted from Rudin CM, Avila-Tang E, Samet JM. Lung cancer in never smokers: a call to action. Clin Cancer Res 2009;15(18):5622–5; with permission.)
Fig. 11.
Geographic and gender variations of lung cancers in never smokers. Systematic compilation of published study involving 18 reports with 82,0237 cases. A marked gender bias was observed whereby lung cancer in never smokers seems to affect women more frequently than men, irrespective of geography. The proportion of female lung cancer cases in never smokers is particularly high in East Asia and South Asia. (Adapted from Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer 2007;7(10):778–90; with permission.)
The incidence of lung cancer in never smokers seems to have a geographic variation. For example, a series following 12,000 patients with lung cancer in California found a dramatic increase in bronchoalveolar carcinoma in never smokers from 19% during 1995 to 1999 to 26% during 1999 to 2003.57 The percentage of other types of lung cancer in never smokers also increased from 8.6% to 9.4%. Another study in the United States found a small but statistically significant increase in the mortality rate in women with non– smoking-associated lung cancers from 12.3% in the years 1959 to 1972 to 14.7% in the years 1982 to 2000.41 A corresponding increase did not occur in men. In Japan, the percentage of never smoker NSCLC increased from 16% to 33% over a 30-year period ending in 2004.58 A European case-control study, however, comparing data from 1950 and 1990, showed no significant change in the percentage of never smokers in male lung cancer patients and a decrease in the percentage of never smokers among female lung cancer patients.59 Similarly, an analysis of 13 American cohorts and 22 cancer registry studies found no substantial change in the rate of lung cancer in women never smokers.60 Two major epidemiologic trends seem to be emerging in lung cancer in never smokers: (1) women are more frequently affected than men and (2) it is more prevalent in certain parts of the world, such as Asia.
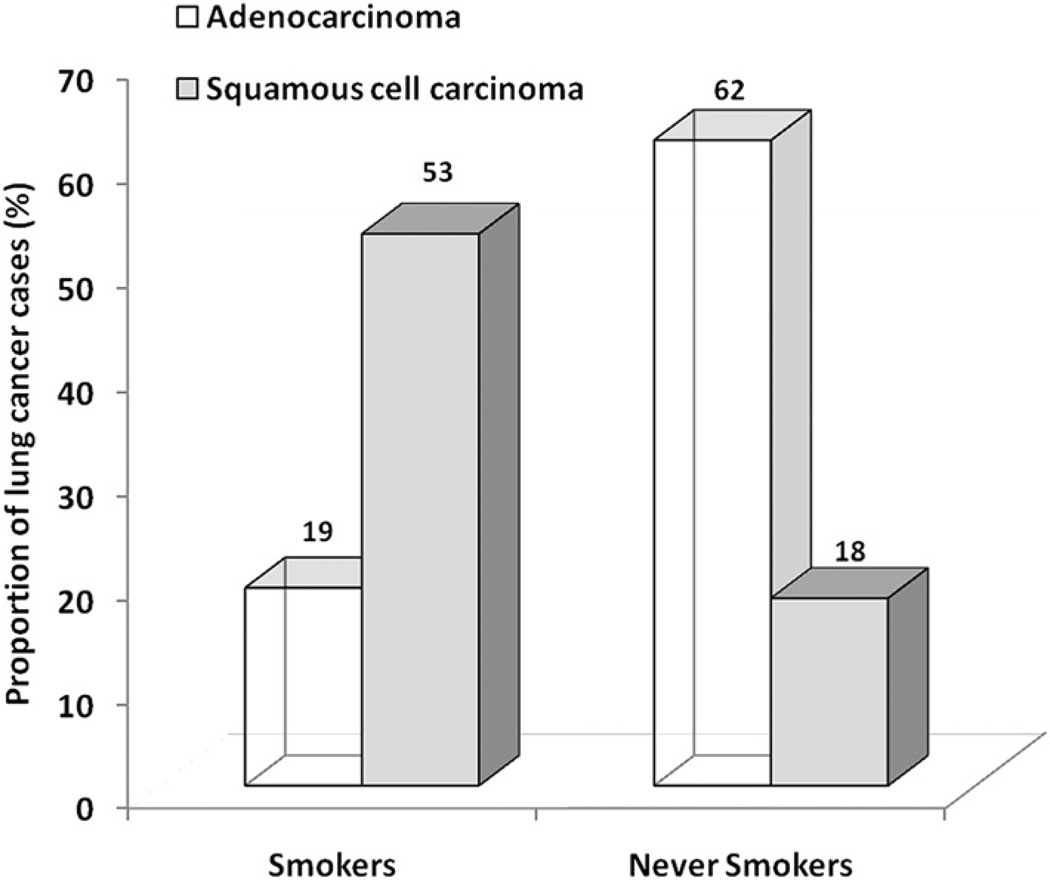
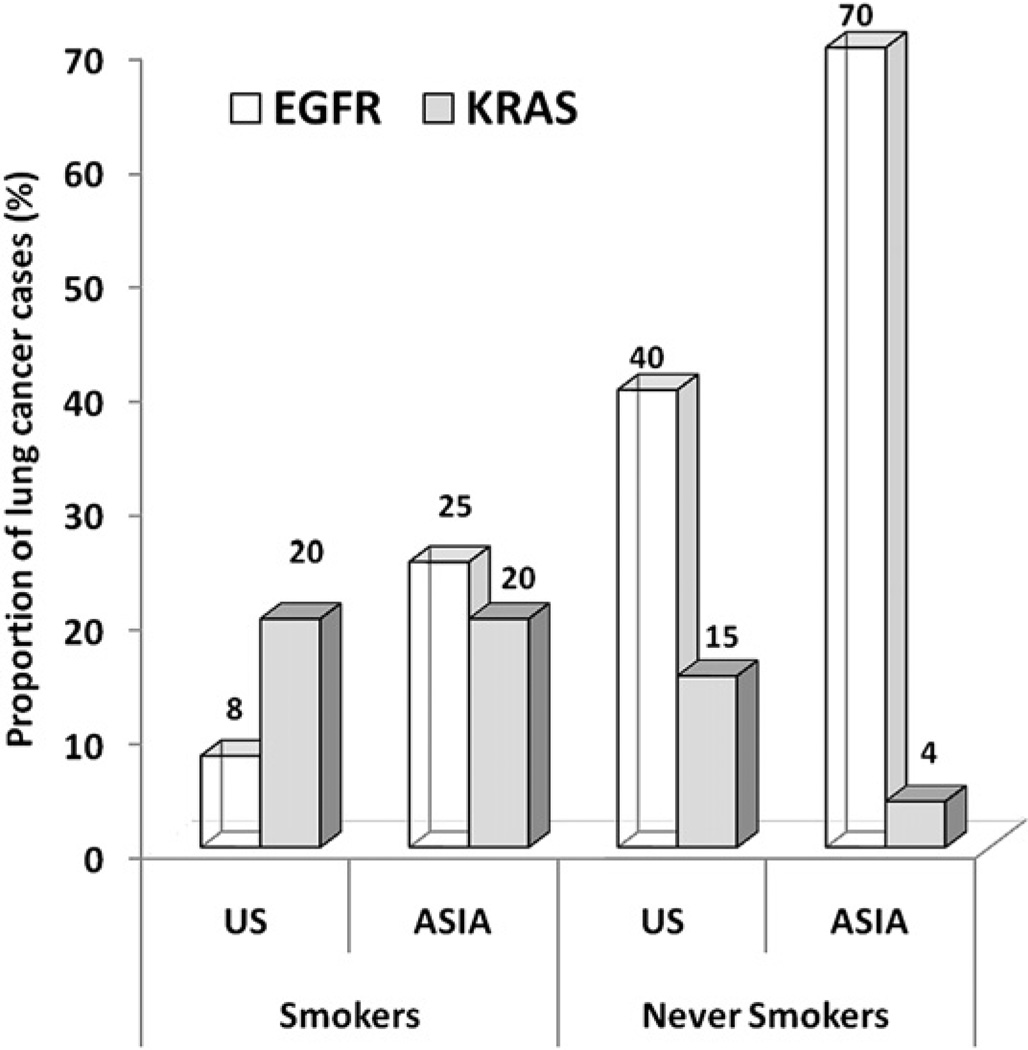
Although all histologic types of lung cancer are associated with cigarette smoking, in smokers the association is stronger for SCLC and for squamous cell carcinoma. In contrast, adenocarcinoma of the lung is more common in never smokers (62% vs 18%, based on 5144 cases55) compared with smokers (19% vs 53% based on 21,853 cases61) (Fig. 12). Adenocarcinoma, however, is becoming more common even among smokers.62,63 This finding may be attributable to the deeper inhalation of lowered tar-containing and nicotine-containing cigarettes, leading to a more peripheral distribution of cigarette smoke in the lung.64 Adenocarcinoma is becoming a common lung cancer type in young patients, however, especially never smokers.65 Other series have also shown the common prevalence of adenocarcinoma in never smokers.56,66 Although there has been no predominant causal factor that can fully explain lung cancer in never smokers, the risk factors considered important for never smokers include secondhand smoke; radon exposure; environmental exposures, such as indoor air pollution, asbestos, and arsenic; history of lung disease; and genetic factors.67 A population-based case-control study in Canada found that occupational exposures, history of lung disease, and family history of early-onset cancer were important risk factors for lung cancer among never smokers.68 In this study, potential environmental sources of increased risk included exposure to solvents, paints, or thinners; welding equipment; and smoke, soot, or exhaust. This finding is particularly important because there are few data on occupational exposures and lung cancer among never smokers. Other studies have also shown an association between lung cancer in never smokers and a family history of lung cancer, a finding that suggests a role for genetic factors.69–71 For example, a case-control study following 2400 relatives of 316 never smokers with lung cancer cases showed a 25% excess risk for cancer in first-degree relatives of lung cancer cases.70 Specific genetic factors in these studies have not been identified. Some studies, however, suggest the role of the epidermal growth factor receptor gene (EGFR) pathway, the human repair gene (hMSH2), and various cytochrome P450 and glutathione-S-transferase enzymes.72–75 No unique susceptibility gene has been identified that distinguishes lung cancers in never smokers from smokers. Recent data have shown, however, an increased frequency of EGFR mutations in lung adenocarcinomas of never smokers, especially in Asian cohorts (Fig. 13).76–79
Fig. 12.
Different histologic features of lung cancer in never smokers. Histologic distribution of lung cancers in never smokers compared with smokers. Cases of bronchioloalveolar carcinoma included with adenocarcinoma. Histologic subtypes were classified as adenocarcinoma, squamous cell carcinoma, or others. Ratio of the number of adenocarcinoma to squamous cell carcinoma was 0.4:1 in smokers, whereas it was 3.4:1 in never smokers. (Adapted from Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer 2007;7(10):778–90; with permission.)
Fig. 13.
Differential frequencies of EGFR and K-ras mutations reported in lung adenocarcinomas in East Asia compared with the United States in never smokers and ever smokers. Activating mutations in both genes are found predominantly in adenocarcinomas, and occur in nonoverlapping cohorts. (Adapted from Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res 2009;15(18): 5646–61; with permission.)
Lung cancers among never smokers in Asia (Hong Kong, Singapore, and Japan) are diagnosed at an earlier age than in smokers.66,80 These findings have not been reproduced in the United States or Europe.56,81 It has been suggested that the investigation threshold in symptomatic never smokers is higher, leading to diagnosis at later stages in never smokers.66 Despite this potential delayed diagnosis and later presentation of lung cancer in never smokers, the survival rate for never smokers is better than for smokers, independent of stage of disease, treatment received, and presence of comorbidities.57,81,82 A multivariate analyses of lung adenocarcioma found that the never smoking status was an independent predictor of improved survival (23% 5-year survival rate for never smokers and 16% for current smokers).81 Such findings have suggested that the cancer in never smokers may display a distinct biologic and natural history. There are also epidemiologic, clinicopathologic, and molecular differences between lung cancers in never smokers and smokers, differences that have led some investigators to suggest that lung cancer in never smokers may be a different disease. Microarray gene-profiling studies have found that lung adenocarcinomas are heterogeneous, and the profiles of cancer in smokers and never smokers are different.83,84 In 2010, the first genome-wide association study (GWAS) reported genetic variations in chromosome 13q31.3 that altered the expression of glypican 5 (GPC5), a heparin sulfate proteoglycan with many known functions involving cell growth and differentiation and tissue responses.85 Another GWAS focusing on lung adenocarcinomas in female Han Chinese never smokers in Taiwan identified genetic variation in the CLPTM1L-TERT locus of chromosome 5p15.33 as associated with risk for lung cancer in this population.86 This 5p15.33 chromosome contains two well-known genes, telomerase reverse transcriptase (TERT) and cleft lip and palate transmembrane 1-like (CLPTM1L), both of which have been implicated in carcinogenesis.
Genetic Factors
There is a genetic component to the pathogenesis of lung cancer, whether it relates to host susceptibility to lung cancer, with or without exposure to cigarette smoke to the development of certain types of lung cancer, or to an individual’s responsiveness to biologic therapies. A lung cancer risk prediction analysis developed by Spitz and colleagues87,88 incorporated multiple variables, such as smoking history, exposure to environmental tobacco smoke, occupational exposures to dusts and to asbestos, and family history of cancer. They showed the influence of a family history of cancer on the risk for lung cancer in never smokers, former smokers, and current smokers (Table 1). Cassidy and colleagues89 also highlighted the significantly increased risk for lung cancer specifically for persons with a family history of early-onset lung cancer (<60 years of age) (Table 2).
Table 1.
Multivariable logistic model for lung cancer by smoking status
| Risk Factor | P Value | OR (95% CI) |
|---|---|---|
| Never smoker | ||
| ETS (yes vs no) | .0042 | 1.80 (1.20–2.89) |
| Family history (≥2 vs <2)a | <.001 | 2.00 (1.39–2.90) |
| Former smoker | ||
| Emphysema (yes vs no) | <.001 | 2.65 (1.95–3.60) |
| Dust exposure (yes vs no) | <.001 | 1.59 (1.29–1.97) |
| Family History (≥2 vs <2)a | <.001 | 1.59 (1.28–1.98) |
| Age stopped smoking | ||
| <42 years | Reference | |
| 42–54 years | .1110 | 1.24 (0.95–1.61) |
| ≥54 years | .0018 (P for trend = .017) | 1.50 (1.16–1.94) |
| Current smoker | ||
| Emphysema (yes) | <.001 | 2.13 (1.58–2.88) |
| Pack-years | ||
| <28 | Reference | |
| 28–41.9 | .1932 | 1.25 (0.89–1.74) |
| 42–57.4 | .0241 | 1.45 (1.05–2.01) |
| ≥57.5 | <.001 (P for trend <.001) | 1.85 (1.35–2.53) |
| Dust exposure (yes vs no) | .0075 | 1.36 (1.09–1.70) |
| Asbestos exposure (yes vs no) | .0127 | 1.51 (1.09–2.08) |
| Family historyb | ||
| 0 | Reference | |
| ≥1 | .0021 | 1.47 (1.15–1.88) |
Number of first-degree relatives with any cancer.
Number of first-degree relatives with a smoking-related cancers, such as lung cancers, cancers, renal cancer, cancers of upper digestive tract, esophagus, pancreas, bladder, and cervix.
Data from Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007; 99(9):715–26.
Table 2.
Liverpool lung project—multivariable risk model lung cancer
| Risk Factor | P Value | OR (95% CI) |
|---|---|---|
| Smoking duration | <.001 | |
| Never | 1.00 Reference | |
| 1–20 years | 2.16 (1.21–3.85) | |
| 21–40 years | 4.27 (2.62–6.94) | |
| 41–60 years | 12.27 (7.41–20.30) | |
| >60 years | 15.25 (5.71–40.65) | |
| Prior diagnosis of pneumonia | .002 | |
| No | 1.00 Reference | |
| Yes | 1.83 (1.26–2.64) | |
| Occupational exposure to asbestos | <.001 | |
| No | 1.00 Reference | |
| Yes | 1.89 (1.35–2.62) | |
| Prior diagnosis of malignant tumor | .005 | |
| No | 1.00 Reference | |
| Yes | 1.96 (1.22–3.14) | |
| Family history of lung cancer | .01 | |
| No | 1.00 Reference | |
| Early onset (<60 years) | 2.02 (1.18–3.45) | |
| Late onset (≥60 years) | 1.18 (0.79–1.76) |
Data from Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98(2):270–6.
Recently, Schwartz and colleagues90 reviewed the molecular epidemiology of lung cancer, focusing on host susceptibility genetic markers to lung carcinogens. (See the article by Larsen and Minna elsewhere in this issue.) The susceptibility genetic factors include high-penetrance, low-frequency genes; low-penetrance, high-frequency genes; and acquired epigenetic polymorphisms. Takemiya and colleagues91 and Yamanaka and colleagues92 showed the association of lung cancer with rare mendelian cancer syndromes, such as Bloom and Werner syndromes. Studies on familial aggregation have supported the hypothesis that there is a hereditary component to the risk for lung cancer. These familial association approaches have been used to discover high-penetrance, low-frequency genes. A meta-analysis involving 32 studies showed a 2-fold increased risk for lung cancer in persons with a family history of lung cancer with an increased risk also present in nonsmokers.93 Bailey-Wilson and colleagues,94 using family linkage approaches, reported the first association of familial lung cancer to the region on chromosome 6q23–25 (146cM– 164cM). The addition of smoking history to the effect of this inheritance was associated with a 3-fold increase risk for lung cancer.
There have also been many studies on candidate susceptibility genes that are of low penetrance and high frequency. The approach has been to target genes known to be involved in the absorption, metabolism, and accumulation of tobacco or other carcinogens in lung tissue. For example, genetic polymorphisms encoding enzymes involved in the activation and conjugation of tobacco smoke compounds, such as PAHs, nitrosamines, and aromatic amines, have been widely studied. Metabolism of these compounds occurs through either phase I enzymes (oxidation, reduction, and hydrolysis) or phase II (conjugation) enzymes. Some of the frequently studied enzymes in this system include CYP1A1, the glutathione S-transferases (GST), microsomal epoxide hydrolase 1 (mEH/EPHX1), myeloperoxidase (MPO), and reduced form of nicotinamide adenine dinucleotide phosphate quinine oxidoreductase 1 (NQO1). Polymorphisms in CYP1A1 and their association with lung cancer risks have been conflicting. A meta-analysis involving 16 studies by Le Marchand and colleagues95 showed no significant risk associated with the CYP1A1 Ile462Val allele; however, in pooled analysis, a significant 55% increased risk for squamous cell carcinoma in whites was observed, especially in women and nonsmokers. GST gene products help conjugate electrophilic compounds to the antioxidant glutathione. GSTM1 in its null form occurs in 50% of the population, and studies by Benhamou and colleagues96 showed a 17% increased lung cancer risk in persons who were GSTM1 null. A more recent and larger meta-analysis involving more than 53,000 case-controls by Ye and colleagues97 showed an 18% increase risk for lung cancer among persons who were GSTMI null, but this significant association was not present when the analysis was limited to larger studies only. Amos and colleagues98 performed a GWAS scan of tagged single nucleotide polymorphisms in histologically confirmed NSCLC in an effort to identify common low-penetrance alleles that influence lung cancer risk. They identified a susceptibility locus for lung cancer at chromosome 15q25.1, a region that contains the nicotinic acetylcholine receptor genes.
Results from the many candidate gene polymorphism studies focusing on a single polymorphism in one gene have been mixed. This has led to studies to look at gene-gene interactions, which require even a larger study population. For example, Zhou and colleagues99 studied the interaction between variants in genes coding for NAT2 (which activates arylamine cigarette smoke metabolites and deactivates aromatic amines) and mEH (which activates PAHs and deactivates various epoxides). They found significant interactions between NAT2 variants associated with certain acetylation pheynotype and mEH variants associated with certain activity level with the risk of lung cancer. For example, a 2-fold increase risk for lung cancer was observed in 120 pack-year smokers who had the NAT2 slow-acetylation and mEH high-activity genotype. Alternatively, in nonsmokers, a 50% decrease risk for lung cancer was observed among persons with the combined NAT2 slow-acetylation and mEH high-activity genotype. Susceptibility to carcinogenic agents may also be affected by individual differences in mutagen sensitivity. Spitz and colleagues100 reviewed the phenotypic studies of DNA repair capacity and lung cancer risks.
Polymorphisms in genes involved in DNA repair enzymes active in base excision repair (XRCC1 and OGG1), nucleotide excision repair (ERCC1, XPD, and XPA), and double-strand break repair (XRCC3), and different mismatch repair pathways have also been studied as they relate to lung cancer risks. Chronic inflammation in response to repetitive tobacco exposure has been theorized as involved in lung tumorigenesis. Genes encoding for the interleukins (IL-1, IL-6, and IL-8). The cyclooxygenase enzymes (eg, COX-2) involved in inflammation, or the metalloproteases (MMP-1, MMP-2, MMP-3, and MMP-12) involved in repair during inflammation have been associated with lung cancer risk. Several cell cycle–related genes have been implicated in lung cancer susceptibility, including the tumor suppressor genes p53 and p73, mouse double minute 2 (MDM2), and the apoptosis genes encoding FAS and FASL.
Wu and colleagues101 showed that the presence of mutagen sensitivity is associated with an increased risk for lung cancer. Spitz and colleagues100 noted that the combined risk for lung cancer was greater in individuals with mutagen sensitivity who smoked than in persons with either smoking or mutagen sensitivity characteristics alone. DNA adducts can be measured as biomarkers to represent the degree of carcinogenesis. Several of the lung cancer susceptibility genes (discussed previously) have been associated with increased levels of DNA adducts. Acquired or epigenetic changes to DNA chromosome can also lead to increased lung cancer susceptibility. These events include changes, such as DNA methylation, histone deacetylation, and phosphorylation, all of which can affect gene expression. Despite many genetic association studies, the specific genes responsible for the enhanced risk for lung cancer remain poorly understood. Work is under way to pool findings to achieve greater study sample sizes in collaborative efforts, such as the Genetic Susceptibility to Environmental Carcinogens and the International Lung Cancer Consortium.
Lung cancer susceptibility is determined at least in part by host genetic factors. Persons with genetic susceptibility might therefore be at higher risk if they smoke tobacco. As technology advances, it may be possible to target subgroups identified as genetically high risk for lung cancer for specific interventions, including intensive efforts at smoking cessation, screening, and prevention programs.
Gender
Lung cancer surpassed breast cancer as the leading cause of cancer deaths in women in the late 1980s, and now almost twice as many women die of lung cancer than breast cancer.1 Since 1950 there has been more than a 600% increase in the lung cancer mortality rate in women. In the United States, the cigarette smoking rate for women increased during the period from 1930 to 1960, and this increase was followed two decades later by an increase in lung cancer in women starting in 1960.102,103 Cigarette smoking peaked during World War II among men born in the 1920s. The smoking rate in women peaked approximately a decade later among those who were born in the 1930s. Lung cancer deaths are expected to keep falling in both genders because older men and women and their younger counterparts smoke less. Smoking prevalence is higher among men (23.1%) than women (18.3%); however, this difference is narrowing.9 Fortunately, the lung cancer death rate in women is beginning to plateau, with an annual increase of 0.2% in 2005.104 Lung cancer death rates for women fell for the first time in four decades amid continued declines in the overall cancer death rate (see Fig. 4).4 There has been a drop of 2.5% in lung cancer deaths among men and a 0.9% decline in lung cancer deaths in women. Even though the overall age-adjusted lung cancer incidence is still higher in men than women, this difference is decreasing due to a continued decrease in the male incidence of lung cancer. Cigarette smoking remains the most important factor for the development of lung cancer in women with some suggesting up to 80% of cases in women are related to smoking.105 Alternatively, for never smokers (discussed previously), the age-adjusted incidence rate of lung cancer is higher for women than men based on the compilation of several prospective cohort studies (14.4–20.8 per 100,000 person-years for women compared with 4.8–13.7 per 100,000 person-years for men).56
Whether women are more or less susceptible than men to the carcinogenic effects of cigarette smoke is controversial. The American Cancer Society Cancer Prevention Study II, which followed 1 to 2 million subjects between 1982 and 1988, reported an overall risk for lung cancer in women smokers of 11.94, compared with an overall risk of 22.36 in male smokers, after taking into account the intensity of smoking.106 Recent analysis of the SEER data from 1997 to 2006 showed that the lung cancer mortality rate is 74.08 per 100,000 man-years compared with 40.81 per 100,000 woman-years.107 Other studies have suggested, however, that women may be actually more vulnerable to carcinogens in tobacco smoke than men.108–111 A study using the American Health Foundation data found that the odds ratio (OR) for the major lung cancer types has been consistently higher for women than for men at every level of exposure to cigarette smoke.111 The dose-response ORs for lung cancer in women were 1.2-fold to 1.7-fold higher than in men. A Canadian case-control study of male–female differences in lung cancer covering the period 1981 to 1985 showed that with a history of 40 pack-years of cigarette smoking relative to lifelong nonsmoking, the OR for women developing lung cancer was 27.9 versus 9.6 in men.110 In both these studies, the increase in lung cancer risk held for all major histologic types.
The observed gender differences in susceptibility may be related to gender-related differences in nicotine metabolism and in metabolic activation or detoxification of lung carcinogens. Such gender differences in clearance of plasma nicotine by cytochrome P450 enzymes have been reported. For example, several reports have commented on gender differences in lung cancer observed at the molecular level. Ryberg and colleagues112 noted that women with lung cancer have higher levels of DNA adducts than men. Such patients might be anticipated more susceptible to carcinogens, which might explain why women seem to develop lung cancer with lower-intensity cigarette exposure. Furthermore, hormonal factors may also play a role in susceptibility. A case-control study showed that estrogen replacement therapy was significantly associated with an increased risk for adenocarcinoma (OR 1.7), whereas the combination of cigarette smoking and estrogen replacement increased that risk substantially (OR 32.4).113 Conversely, early menopause (age 40 years or younger) was associated with a decreased risk for adenocarcinoma (OR 0.3). More recent large randomized studies suggest that the use of hormonal therapies, such as estrogen and progestin, is associated with an increased risk for lung cancer in women.114 For example, the Vitamins and Lifestyle study followed perimenopausal women for 6 years and found the risk for lung cancer was increased in those who used estrogen and progestin.114 The observed risk was proportional to the duration of hormone exposure, with approximately 50% increased risk for those who used hormone replacement therapy for 10 years or longer. Two studies as part of the Women’s Health Initiative found a statistically nonsignificant trend toward increased incidence of NSCLC and an increased number of deaths from lung cancer in women taking hormone therapy compared with those taking placebo.115,116
A second issue is whether cigarette smoking may be associated with a higher risk for nonmalignant lung disease in women than in men. Neither of two large population studies, the British Doctors Study in the United Kingdom117,118 or the Lung Health Study in the United States,119 found gender differences in mortality from smoking-related chronic obstructive pulmonary disease (COPD). Other studies, however, including a study by Chen and colleagues,120 suggest that cigarette smoking may be more harmful to the pulmonary function in women than in men. In this study, changes in forced expiratory volume in the first second of expiration (FEV1) and maximal midexpiratory flow rate increased with increasing pack-years more rapidly in women smokers than in their male counterparts. These changes were independent of age, height, and weight. Beck and colleagues121 in a study of 4690 white subjects found that for a given level of smoking, women had more changes in FEV1 and maximal expiratory flow at 25% and 50% of vital capacity at a younger age (15–24 years) than men (40–45 years). Because smokers with spirometric evidence of airway obstruction are at higher risk for lung cancer, the suggestion that women have increased susceptibility to smoking-induced airway disease may be important in the consideration of their risk for lung cancer.121
Finally, it also seems that lung cancer is more common in nonsmoking women than in nonsmoking men. In an early study of tobacco smoking, Wynder and Graham10noted that a greater percentage of cancers in nonsmokers occurred in women than men. The number of women in that study was relatively small, however, and few women had at that time smoked for the duration of decades. Since then, it has become clear that women never smokers are more likely than male never smokers to develop lung cancer. In a case-control study by Zang and Wynder111 of 1889 lung cancer subjects and 2070 control subjects, the proportion of never smoking lung cancer patients was more than twice as high for women than for men. The reasons for this finding are not clear, but speculation has been raised regarding the potential of women having greater susceptibility to nontobacco environmental carcinogens or increased exposure to ETS or the existence of gender-linked differences in the metabolism of nontobacco environmental carcinogens.
Race and Ethnicity
Race is a complex variable that often has a strong socioeconomic association. Racial differences in disease states can shed light, however, on the specific issues of a particular subpopulation. Menck122 showed that the incidence of lung cancer is substantially higher among blacks and Native Hawaiians and other Polynesians and lower among Japanese Americans and Hispanics than among whites in the United States. These differences initially have been attributed to the variations in cigarette smoking pattern among the different ethnic and racial groups. Recent smoking data show that among the different groups, Asians (9.9%) had the lowest smoking prevalence in the United States, whereas American Indians and Alaska Natives (32.4%) had significantly higher prevalence than the other groups.8 Smoking prevalence among whites (22%) and blacks (21.3%) were significantly higher than among Hispanics (15.8%). The Department of Health and Human Services reported, however, that the age-adjusted prevalence of cigarette smoking was similar among blacks and whites (30.1% and 27.3%, respectively). In addition, only 8% of black smokers smoked at least 25 cigarettes per day compared with 28% of white smokers. Native Hawaiians also had higher rates of lung cancer than whites and Asians despite having similar smoking habits. Haiman and colleagues123 reported in their Multiethnic Cohort Study that among participants who smoked no more than 30 cigarettes per day, black Americans and Native Hawaiians had significantly greater risk for lung cancer than did whites. The relative risk for lung cancer among subjects smoking less than 20 cigarettes per day were 0.21 to 0.39 for Japanese Americans and Latinos, and 0.45 to 0.57 for whites as compared with black Americans. The differences in lung cancer risks were not significant, however, among all racial groups who exceeded 30 cigarettes per day of smoking. Recent SEER report based on data from 2004 to 2008 showed that black men, but not black women, in the United States had a higher age-adjusted incidence of lung cancer than their white counterparts at all age groups (Fig. 14).
Fig. 14.
Age-adjusted lung cancer incidence by gender, age, and race. Rates are per 100,000 and are age adjusted to the 2000 US standard population. Note the different scale, which highlights the predominant incidence of lung cancer in the population age >65 years for both genders and races. (Data from Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61(4):212–36; and Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2008. Bethesda (MD): National Cancer Institute; 2010. Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.)
Smokers with a history of early-onset lung cancer in a first-degree relative have a higher risk for lung cancer with increasing age than smokers without such a family history. Coté and colleagues124 showed in a case-control study that first-degree relatives of black persons with early-onset lung cancer have a greater risk for lung cancer than their white counterparts (25.1% vs 17.1%, respectively). These cumulative differences in risk for lung cancer among blacks and whites are further amplified by increasing cigarette smoking exposure. The explanation for these observed racial or ethnic variations in risk for lung cancer is not known. Black Americans also have higher mortality rates from lung cancer than white Americans.8 This difference in mortality rates has been attributed not only to the higher incidence rates but also to the poorer survival of black patients with lung cancer than white patients. For example, from 1995 to 2000, the 5-year survival rate was 14.3% lower in black Americans compared with white Americans. The reasons for these racial differences are not known. Brooks and colleagues125 hypothesized a potential role for greater use of menthol cigarettes among black Americans than among white Americans (69% vs 22%) or the deeper inhalation of menthol cigarettes compared with nonmenthol cigarettes. No evidence, however, supports this hypothesis.
Age
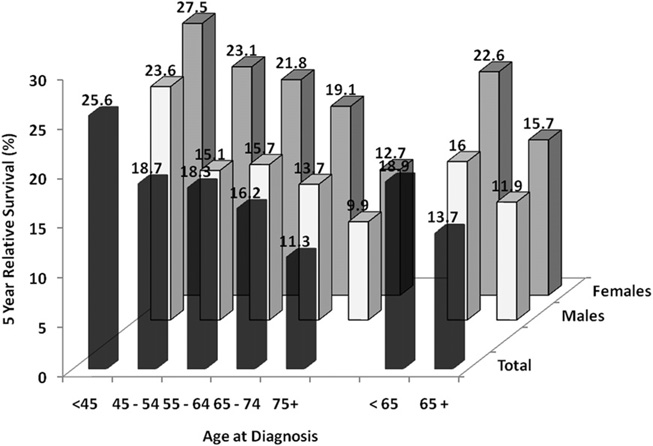
The average age of most populations in developed nations is increasing, and cancer is a disease of the elderly. Although smoking prevalence is lowest among persons aged 65 years and older (9.3%) compared with persons aged 18 to 24 years (21.4%), 25 to 44 years (23.7%) and 45 to 64 years (22.6%),8 (see Fig. 14), more than 65% of patients with lung cancer are older than 65. Specifically, 31.1% of patients with lung cancer are between 65 and 74 years, 29% between 75 and 84 years, and 8.3% are 85 years old and older.3 The mean age at the time of diagnosis is over 70. This difference between lower current smoking prevalence and the higher cancer rate in the elderly population likely reflects heavy smoking history in current elderly population. In the past decade, the incidence and mortality from lung cancer have decreased among persons aged 50 years and younger but have increased among persons aged 70 years and older.126 The 5-year survival rate for lung cancer decreases incrementally with age for both genders (Fig. 15). “Older patients” are usually considered those older than 70 years with the “very elderly” those 80 years or older. Patients older than 80 years constitute 14% of all patients with lung cancer in the United States but account for almost a quarter of all lung cancer deaths. It has been estimated that the number of lung cancer patients aged 85 years and older will quadruple by 2050. Few studies have examined management of the elderly population with lung cancer. Recent reviews concluded that elderly patients, specially the functionally fit elderly, with lung cancer can benefit from many of the treatments used for younger patients, including surgery for early-stage disease and single-agent chemotherapy for advanced disease.127,128
Fig. 15.
Five-year relative survival (%) from lung cancer based on age at diagnosis. Based on data from 2001 to 2008 covering SEER 17 areas. (Data from Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2008. Bethesda (MD): National Cancer Institute; 2010. Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.
Diet and Obesity
It has been suggested that diet is responsible for approximately 30% of all cancers.129 Many reports suggest that dietary factors contribute to the risk for lung cancers.130 For example, low serum concentrations of antioxidants, such as vitamins A, C, and E, have been associated with the development of lung cancer.131,132 Vitamin A has both an animal (retinol) and a vegetable (carotenoid) source; the vegetable component only has been shown to have protective effects against lung cancer. In particular, β-carotene, a prominent carotenoid, has been shown to have the greatest protective effect against lung cancer.133 Vitamins C and E (α-tocopherol) have also been shown to have some protective effect.134,135
One of the most widely cited reports of the effect of diet on the development of cancer was a prospective survey of approximately 2000 men aged 40 to 55 years employed by the Western Electric Company where detailed dietary histories were recorded in 1957 and followed for more than 19 years.136 In this study, β-carotene intake was inversely related to lung cancer incidence, suggesting that vitamin A and β-carotene may have a protective effect against lung cancer. Byers and colleagues137 evaluated 27 such studies published before 1994 and concluded that persons in the lowest quartile of carotene intake had an approximately 50% to 100% increase in lung cancer risk compared with persons in the highest quartile of carotene intake. In response to these positive observations, three large-scale intervention trials have been conducted to try to determine the relationship between vitamin supplementations and lung cancer. Unfortunately, these studies showed that vitamin supplementation did not reduce lung cancer risk and in some circumstances increased the incidence of lung cancer. The Alpha-Tocopherol, Beta Carotene Cancer Prevention (ATBC) Study was a randomized, double-blind, placebo-controlled trial designed to determine whether daily supplementation of α-tocopherol, β-carotene, or both could reduce the incidence of cancers, including lung cancer.138 The study enrolled 29,133 male smokers aged 50 to 60 years in Finland. Unexpectedly, a higher than expected mortality, primarily due to lung cancer and heart disease, was observed in the group receiving β-carotene. Omenn and colleagues139,140 then reported results of the Beta-Carotene and Retinol Efficacy Trial (CARET), also a randomized, double-blind, placebo-controlled study. The study was intended to determine whether dietary supplementation with β-carotene, vitamin A, or both would decrease the incidence of lung cancer. It enrolled 18,314 men and women considered at increased risk for lung cancer. The CARET study was stopped 21 months early because of “clear evidence of no benefit and substantial evidence of harm” in the group that received β-carotene and retinol palmitate, especially women.139,141 The group that received both vitamin A and β-carotene had a 17% increase in mortality and a 28% increase in the number of lung cancers compared with placebo. A third randomized, double-blind, placebo-controlled trial, the Physicians’ Health Study, evaluated the effect of β-carotene in 22,071 male physicians142; 11% of the participants were current smokers and 39% former smokers at the onset of the trial. Over 12 years of follow-up, neither benefit nor harm in terms of malignancy or cardiovascular disease was demonstrated. The dose of β-carotene in this trial was lower than in both the ATBC trial and the CARET study.
Because of the findings of the ATBC and CARET trials, the use of supplemental β-carotene and vitamin A is discouraged. There have also been suggestions that low dietary intake of certain minerals, including magnesium, zinc, copper, and iron, is associated with increased lung cancer risk; however, later prospective cohort studies observed no significant associations between total mineral intake and lung cancer risk.143,144 The role of dietary supplementation in cancer chemoprevention is currently unsettled. These studies should serve as a reminder, however, that indiscreet and excessive intake of vitamins or other chemicals can be potentially harmful.
A diet rich in fruits and vegetables has been linked to decreased cancer incidence as suggested by a large cohort study in the Netherlands, with the protective effects stronger in current than in former smokers.145 In this study, no specific type of vegetable or fruit was identified as particularly responsible for the effect. Consumption of vegetables described as cruciferous, such as broccoli and cabbage, which are rich in isothiocyanates, has some protective effect against lung cancer.146 When study participants were stratified according to their GSTM1 and GSTT1 gene status, which are genes important at eliminating isothiocanates, the protective effect of the cruciferous vegetable consumption was best seen in subjects with the null gene.146 Overall, it has been shown that low or no intake of fruits or vegetables has been associated with up to 3-fold risk for lung cancer.147 It has been also further suggested that consuming fruits or vegetables raw rather than cooked is associated with a further reduction in risk for lung cancer because important carotenoids can be destroyed with cooking.148 A large prospective study (the NIH-AARP Diet and Health Study) showed no relation between total intake of fruit and vegetables with lung cancer risk.149 The study did show, however, that higher consumption of several botanic groups, such as rosaceae (apples, peaches, and strawberries), convolvulaceas (sweet potatoes and yams), and umbelliferae (carrots), was significantly inversely associated with lung cancer risk in men and in former smokers.149 Flavonoid plant metabolites have properties described as antioxidant and anti-proliferative. Flavonoids can be found in foods, such as berries, citrus fruits, tea, dark chocolate, and red wine. A prospective study showed the risk for lung cancer was lower in men with the highest total flavonoid intake compared with those with the lowest intake.150
Certain dietary items, including red meat, dairy products, saturated fats, and lipids, have been suggested as increasing the risk for lung cancer.151–154 Other foods found to have an adverse effect on lung cancer include items that contain nitrosodimethylamines and nitrites, such as those found in salami and salted and smoked meat products.155,156 Despite the negative large-scale chemoprevention studies of vitamin supplementation and because of the large body of epidemiologic literature pointing to the benefits of fruits and vegetables, health authorities continue to recommend a balanced dietary intake of fruits and vegetables, including those containing β-carotene.
Because of the current obesity epidemic, discussion of dietary factors cannot be complete without mention of the role of excessive weight in lung cancer. In 2005, 23.25% of the world’s adult population (937 million people) was overweight and 9.8% (396 million) was obese with a body mass index (BMI) of greater than 30 kg/m2.157 These numbers are greater in industrialized countries where more than one-fifth of the adult population is obese. In the United States, 35.1% of adults aer classified as obese.158 Excessive body weight has been associated with increased risk for endometrial, breast, and colorectal cancer but not for lung cancer. A meta-analysis by Renehan and colleagues159 reported that there was an inverse association between BMI and lung cancer risk and obesity may even have a protective role. In the absence of cigarette smoking, however, the association between BMI and lung cancer was not significant. It has been proposed that the observed BMI and cancer association may be related to residual strong confounding effects of smoking itself.160 For example, smokers tend to have lower mean BMI than age-matched and gender-matched nonsmokers.161 Smokers have a lower BMI than nonsmokers, and they gain weight when they quit smoking. It has been suggested also that leanness was associated with increased lung cancer risk, but the studies were small and did not clearly exclude the confounding effects of smoking or pre-existing diseases.162 More recent studies by Kabat and colleagues,163 after adjusting for pack-years of smoking and other relevant covariates in a female cohort, showed that there was evidence for inverse associations of BMI and lung cancer risk in current and former smokers; whereas in never smokers, BMI was positively associated with lung cancer. A different study showed that waist circumference was positively associated with lung cancer risk in the smokers.164 Recent prospective studies in Chinese men showed an inverse relationship between BMI and lung cancer mortality after adjustment for potential obvious confounders, such as smoking.165,166 These studies did not have information on exposure to cooking fumes that have been reported to play a role in lung cancer in the Chinese population.167
Other Lung Diseases and Airways Obstruction
Some nonmalignant diseases have been associated with an increased risk for lung cancer, the strongest association being with COPD. Tobacco smoking is the primary cause of both lung cancer and COPD. A study of women never smokers with lung cancer showed a statistically significant association between the presence of airflow obstruction and the development of lung cancer.168 There is other evidence that airflow obstruction is a risk for lung cancer.169,170 This conclusion is supported by the Lung Health Study in which 5887 male and female smokers with spirometric evidence of mild to moderate COPD were monitored over a 5-year period with or without smoking cessation counseling or bronchodilator therapy.171 Lung cancer was the most common cause of death, accounting for 38% of all deaths and lung cancer deaths exceeded deaths from cardiovascular disease by nearly 50%. More recent studies in large cohorts have shown that COPD is significantly associated with an increased risk for lung cancer, especially in men.172,173 Because COPD affects an estimated 40% to 70% of patients with lung cancer, a coexisting disease of lung cancer and COPD likely reflects a common smoking exposure. Potential confounders by age, gender, and smoking history or the effects of lung cancer on spirometry could have resulted in the overdiagnosis of COPD in patients with lung cancer. A recent study evaluated 602 patients with lung cancer and found that 50% of them had prebronchodilator pulmonary function test results consistent with a diagnosis of COPD with Global Initiative for Chronic Obstructive Lung Disease stage 2 and higher, independent of age, gender, and smoking history, with an OR of 11.6.174 The prevalence of COPD in newly diagnosed lung cancer was 6-fold greater than matched smokers, suggesting that COPD itself is an important independent risk factor with potential relationship to the pathogenesis of lung cancer.
COPD is characterized by chronic inflammation that responds to corticosteroids, and chronic inflammation itself has been suggested as associated with lung cancer. A Dutch study found that the likelihood of developing lung cancer was increased if C-reactive protein, a measure of generalized inflammation, was greater than 3 mg/L compared with patients with lower levels (<1 mg/L).175 A large retrospective study of patients with COPD patients found that the risk for lung cancer was lower among patients who took high-dose inhaled corticosteroids compared with patients taking lower doses or none at all.176 These results suggest that inhaled corticosteroids may have a chemoproventive role in lung cancer among patients with COPD. A study by Yang and colleagues177 tested whether α1-antitrypsin deficiency carriers have a higher risk for lung cancer, after adjusting for the effects of tobacco smoke exposure and COPD. Using a multiple logistic regression analysis, they found a significantly increased lung cancer risk (approximately 2-fold increased risk) among α1-antitrypsin deficiency carriers from two parallel case-control cohorts.
Interstitial fibrosis has also been associated with an increase in lung cancer risk. Hubbard and colleagues178 evaluated 890 patients with cryptogenic fibrosing alveolitis (idiopathic pulmonary fibrosis) and 5884 control subjects and found that the incidence of lung cancer in patients with fibrosis was markedly increased, even after adjustment for smoking. Patients with such fibrosis had an OR for lung cancer of 8.25 compared with control subjects. Other fibrosing diseases, including asbestosis and scleroderma-related lung disease, also seem to have an increased association with lung cancer (asbestos-related disease is discussed later). The association of scleroderma with lung cancer, however, is weaker. A British study followed patients with idiopathic pulmonary fibrosis and found the incidence of lung cancer markedly increased compared with the general population.179 Although the mechanisms by which pulmonary interstitial disease may predispose to malignancy are not clear, various hypotheses have been raised, including malignant transformation related to chronic inflammation, epithelial hyperplasia, impaired clearance of carcinogens, and infections.
Infections
Infection as a causative factor in lung cancer has been evoked but remains debatable. For example, oncogenic viruses have been proposed as a cause of lung cancer. Early studies on sheep pulmonary adenomatosis caused by the Jaagsiekte sheep retrovirus show pathologic similarities to human bronchioloalveolar carcinoma; however, there is not enough evidence to link these two diseases and prove the involvement of viruses in the development of human bronchioloalveolar carcinoma.180 More recent findings have suggested a potential role for human papillomavirus (HPV), known to cause carcinoma in other tissues. The possible involvement of HPV in bronchial squamous cell lesions was first suggested by Syrjänen,181 who described epithelial changes in bronchial carcinomas that closely resemble those of established HPV condylomatous lesions in the female genital tract.181 HPV DNA within squamous cell carcinoma lung cancer tissues has been detected. There is inconsistency, however, in the reported prevalence of infection by HPV in patients with lung cancer in different countries with racial and geographic variations. High incidence of HPV DNA in lung cancer has been reported in Asian cohorts, especially in nonsmokers; alternatively, studies in Western Europe failed to show an etiologic role of HPV in lung cancer.182–184 HPV serotypes 16 and 18 are associated with lung cancer more than any other serotypes. E6 and E7 oncogenes from these HPV serotypes have been shown to immortalize human tracheal epithelial cells, which themselves are highly prone to genetic damage.185 Currently, studies testing lung cancer specimens for HPV have yielded mixed results, and such variability of the frequency of HPV-positive lung cancer may be due to genetic susceptibility; methodologic approaches to detect HPV, such as those that involve the use of polymerase chain reaction (PCR); in situ hybridization and immunohistochemistry; and environmental and high-risk behavior variables. It would be interesting to see if HPV-directed vaccine for cervical cancer has any impact on the incidence of lung cancer.
Epstein-Barr virus, associated with Burkitt lymphoma and nasopharygeal carcinoma, has been strongly associated with lymphoepithelioma-like carcinoma, a rare form of lung cancer, in Asian patients, but this association has not been observed in the Western population.186 Other viruses suggested as etiologic for lung cancer include BK virus, JC virus, the human cytomegalovirus, simian virus 40, and measles virus; however, the results have remained inconclusive.187–190 More recently, DNA from Torque teno virus, a new virus, has been detected at high levels in idiopathic pulmonary fibrosis patients with lung cancer, and although suggestive that Torque teno virus infection might be associated with the development of lung cancer in idiopathic pulmonary fibrosis, more studies are needed to confirm these findings and determine their clinical significance.191
It has also been suggested that Chlamydia pneumonia, a common cause of acute respiratory infection, especially in cigarette smoke –exposed individuals, might be involved in lung cancer carcinogenesis.192 Identification of C pneumoniae as etiologically related to lung cancer, whether independent of tobacco smoking or as a cofactor, could have profound implications, particularly in the area of lung cancer prevention. Using serology to define Chlamydia infection, multiple epidemiologic studies have reported higher lung cancer risk associated with positive serology compared with those without such evidence of infection.192 Although there were concerns about measurement differences, the results were consistent and suggested a potentially novel association of this organism with lung cancer. Although Chlamydia is not a known oncogenic pathogen, some investigators have hypothesized that the inflammation resulting from the infection can lead to reactive oxygen species that can cause DNA damage, cell injury, and repair, increasing the risk of mutations, which can confer selective advantages that lead to cancer. Such infection can also act synergistically with cigarette smoking to increase the risk of lung cancer. Similar to the concerns related to the evidence for various viruses as causes of lung cancer, however, further investigations are needed to solidify the evidence for a causal role of Chlamydia in lung cancer.
Some studies have reported association of pulmonary tuberculosis with lung cancer.193,194 A cohort study from Taiwan showed an increased risk for lung cancer in tuberculosis patients with hazard ratio of 3.3 after adjusting for confounding factors, such as COPD and smoking-related cancers other than lung cancer. The effect of tuberculosis was even greater when combined with COPD or with other smoking-related cancers.194 Other investigators speculate that the tuberculosis-related inflammation and scarring contribute to lung cancer pathogenesis.193
Lung cancer has become a new challenge in HIV-infected individuals. AIDS-related mortality has dramatically fallen since the advent of highly active antiretroviral treatments; however, this has been accompanied by an increase in the proportion of deaths attributable to non–AIDS-defining tumors, especially lung cancer.195,196 The increased risk of lung cancer relative to the general population of the same age seems due in part to the higher prevalence of smoking among HIV-infected patients. In a study of 2840 HIV-infected patients, HIV was associated with a hazard ratio of 3.6 for lung cancer after controlling for smoking status.197 Although smoking is a key risk factor for lung cancer in HIV-infected patients, several other factors may contribute to the higher incidence of lung cancer. These include greater prevalence of co-infection with oncogenic viruses, such as human herpesvirus 8, HPV, and Epstein-Barr virus, and the potential direct effects of the HIV virus and the consequences of long-term immunosuppression.198 For example, the HIV tat protein can transactivate cellular genes or proto-oncogenes, whereas other HIV genes inhibit tumor suppressor genes.199 HIV-infected cancer patients have a worse prognosis than similarly staged non–HIVinfected patients with the same cancer.200 They are also more likely to have more advanced disease at diagnosis.201 Studies have reported that HIV-infected patients were younger, were more likely to be smokers, and had significantly reduced median survival.201,202 Taken together, evidence suggests that infection may play a role in lung cancer; however, definite proof of a causal relationship is currently lacking.
Environmental Tobacco Smoke
Secondhand smoke, also referred to as ETS, can contribute to an increased risk for lung cancer with a dose-dependent relationship between the degree of exposure and the relative risk. One study showed that household exposure of 25 or more smoker-years before adulthood doubled the risk for lung cancer; exposure of less than 25 smoker-years did not increase risk. The investigators estimated that at least 17% of lung cancers in nonsmokers are attributable to exposure to high levels of ETS during childhood and adolescence.203 The Surgeon General reported in the early 1970s on the health consequences of smoking and raised concerns about hazards relating to such environmental smoke exposure.12 Because nonsmokers exposed to ETS have an increased rate of smoke-related problems, including upper respiratory symptoms and eye irritation, and because there is an increased frequency of respiratory illnesses in children, it was suggested that the acknowledged carcinogenic effect of active tobacco smoking might also be present in those involuntarily exposed. Other reports showed an increased risk for lung cancer in nonsmoking women married to men who smoke.204,205 A summary analysis of a large number of epidemiologic studies on the risk for lung cancer in nonsmokers found an excess risk for lung cancer of 24% in nonsmokers who lived with a smoker.206 This should be interpreted from the perspective that the background risk for lung cancer in a nonsmoker is low and contrasted with the 1000-fold increase of lung cancer risk in lifelong active smokers. Another study in nonsmoking women found that tobacco use by the spouse was associated with a 30% excess risk for all types of lung cancer.207 These studies showed a dose-response relationship of the risk for lung cancer with both the number of cigarettes smoked by the spouse and the duration of exposure.206,207
In 1986, the National Research Council commissioned a review of the effects of ETS as a potential causal agent of lung cancer in nonsmokers exposed to household cigarette smoke.208 Review of all the available evidence yielded an overall OR of 1.34 in lung cancer risk associated with ETS. In nonsmokers this translates into an approximately 30% increase of risk for lung cancer. At the same time, the US Department of Health and Human Services in a report on the health consequences of involuntary tobacco smoke exposure concluded that involuntary smoking is a cause of disease, including lung cancer, in healthy nonsmokers.209 The US Environmental Protection Agency210 and the IARC211 both currently classify ETS as containing lung carcinogens. Cardenas and colleagues212 examined lung cancer mortality and ETS within the context of the American Cancer Society Cancer Prevention Study. These investigators performed a prospective comparative evaluation of 133,835 never smokers with smoking spouses versus 154,000 never smokers with nonsmoking spouses. They concluded that the relative risk for lung cancer in women with smoking husbands was 1.2, which represented an increase in lung cancer incidence of 20%. The relative risk in nonsmoking men with smoking wives was somewhat less but still elevated at 1.1. These figures are consistent with data from prior studies evaluating lung cancer risk from ETS. Pooled data from 8 such studies in the United States from 1981 to 1991 found the relative risk for lung cancer in nonsmokers living with smokers to be 1.23.213
ETS consists of both mainstream (exhaled) smoke and sidestream smoke. Various carcinogens have been identified in ETS, including benzene, benzo[a]pyrene, and NNK. Hecht and colleagues214 reported that male nonsmokers exposed to sidestream smoke generated by machine smoking of cigarettes had measurable carcinogenic metabolites in their urine. In the third National Health and Nutrition Examination Survey conducted between 1988 and 1991, Pirkle and colleagues215 reported that a surprising 88% of nontobacco users had detectable levels of serum cotinine, a metabolite of nicotine, presumably from exposure to ETS. The presence of ETS is pervasive and harmful. Therefore, efforts on public smoking limitations will be of great benefit in this regard. With 19.8% of the American adult population still smoking, however, ETS will continue to be a major public health issue until cigarette smoking altogether is eliminated.216 The exact number of cases of lung cancer due to involuntary smoking is difficult to calculate. Beckett, however, estimates that the number of lung cancer deaths in the United States attributable to ETS is comparable to the annual number caused by asbestos or radon.217
Biomass and Wood-smoke Exposure
In many parts of the world, wood is burned for cooking and heating purposes. Approximately 3 billion people worldwide rely on solid fuels as their primary source of domestic energy for cooking and heating. In China, for example, incomplete combustion of coal in homes has been linked with lung cancer.218 A case-control study followed a group of residents living in underground dwellings who burn coal and unprocessed biomass, such as crop residues, wood, sticks, and twigs, for heating and cooking. They found that the OR for lung cancer associated with such coal use compared with that for biomass in the house was 1.29, after adjusting for smoking.219 The IARC has recently classified indoor emissions from household coal combustion as a human carcinogen and emissions from biomass fuel primarily from wood as probable human carcinogen. A study using data from this consortium found that compared with nonsolid fuel users, predominant coal users (OR 1.64; in particular, coal users in Asia with OR 4.93), and predominant wood users in North American and European countries (OR of 1.21) exhibited higher risk for lung cancer.220 A European cohort showed similar association of solid fuel use for heating and cooking with lung cancer risk; OR of lung cancer in lifetime users of solid fuel was 1.80; switching to nonsolid fuels resulted in lowered risk.221 It has been suggested that the lung cancer that arise from wood smoke may behave differently from lung cancer due to tobacco smoke. Wood smoke exposure was found an important factor in predicting favorable response of NSCLC to tyrosine kinase inhibitor therapy.
Environmental Air Pollution
Air pollution has become a worldwide problem given the current staggering rate of globalization and industrialization. Wilkins222 chronicled the most impressive example of the severe adverse effect of air pollution: the 1952 “great smog” of London, during which thousands of persons died in a week from heavy pollution. This led to the implementation of the Clean Air Act 4 years later. The effects of low levels of air pollution exposure over a longer period of time are harder to measure, especially the long-term and accumulative effects on lung cancer risks. Air pollution is worsening in developing countries; the highest concentrations of suspected particulates, sulfur dioxide, and smoke have been recorded in large cities of these countries. Outdoor air pollution has long been thought to increase the risk for lung cancers. Advances in analytic methods used to detect specific pollutants have helped investigators study the effects of such airborne particulates. Early studies by Pershagen223 involving urban-rural comparisons have shown that an “urban factor” is associated with a 10% to 40% increase in lung cancer deaths. A case-control study in Sweden by Nyberg and colleagues224 showed a relative risk for lung cancer of 1.44 for persons exposed to more than 29.3 µg/m3 of nitric oxide (as a measure of traffic air pollutant) over 21 to 30 years compared with exposures to lower than 12.8 µg/m3 of nitric oxide. Two large United States cohort studies by Dockery225 and Pope and colleagues226 suggest that there is an excess risk for lung cancer of approximately 19% per 10 µg/m3 increment in the long-term average exposure to fine particulates after adjustments for multiple confounding factors. Pope and coworkers226 found as part of the Cancer Prevention Study II that fine particulate and sulfur oxide-related pollution were associated with 8% increased risk for lung cancer mortality for each 10 µg/m3 elevation in long-term average ambient concentration of fine particles less than 2.5 µm in diameter. Despite these studies, it is still difficult to pinpoint the carcinogenic role played by single constituents of air pollution.
Various potential carcinogenic components are thought to be emitted from different sources of fossil fuel combustion products. Cohen227 and Pope and coworkers226 suggested that there is a range gradient of relative risk for lung cancer associated with exposure to combustion products, from 7.0 to 22.0 in cigarette smokers, to 2.5 to 10.0 in coke oven workers, to 1.0 to 1.6 in residents of areas with high levels of air pollution, to 1.0 to 1.5 in nonsmokers exposed to environmental tobacco smoke. Diesel exhaust composed of a complex mixture of gases and fine particles represents an important component of air pollution. Some of these gaseous components, such as benzene, formaldehyde, and 1,3-butadiene, are suspected of causing or known to cause cancer in humans. Studies in the late 1980s concluded that there was a weak association between diesel engine exhaust exposure and cancer. Later work, however, that included two large independent meta-analyses by Lipsett and Campleman228 and Bhatia and colleagues,229 provided strong support that occupational exposures to diesel exhaust, especially in persons in the trucking industry, is associated with an approximately 30% to 50% increase in the relative risk for lung cancer. Data linking gasoline engine exhaust and lung cancer are less compelling.
Occupational Carcinogens
Several workplace substances have been suggested to be or have been proved carcinogens in the lung. The IARC has identified arsenic, asbestos, beryllium, cadmium, chloromethyl ethers, chromium, nickel, radon, silica, and vinyl chloride as carcinogens. The occupations associated with exposure to these agents are shown in Tables 3 and 4. In 2000, it was estimated that 10% of lung cancer deaths among men (88,000 deaths) and 5% among women (14,300 deaths) worldwide could be attributable to exposure to 8 occupational lung carcinogens, namely asbestos, arsenic, beryllium, cadmium, chromium, nickel, silica, and diesel fumes.230,231 Steeland and colleagues232,233 estimated that approximately 6800 to 17,000 lung cancers were a result of exposure to chemicals in the workplace.
Table 3.
Occupational carcinogens and associated occupational exposures
| Known Carcinogen | Occupational Exposure |
|---|---|
| Arsenic | Copper, lead, or zinc ore smelting Manufacture of insecticides Mining |
| Asbestos | Asbestos mining Asbestos textile production Brake lining work Cement production Construction work Insulation work Shipyard work |
| Beryllium | Ceramic manufacture Electronic and aerospace equipment manufacture Mining |
| Chloromethyl ethers | Chemical manufacturing |
| Chromium | Chromate production Chromium electroplating Leather tanning Pigment production |
| Nickel | Nickel mining, refining, electroplating Production of stainless and heat-resistant steel Polycyclic aromatics Aluminum production Hydrocarbon compounds Coke production Ferrochromium alloy production Nickel-containing ore smelting Roofing |
| Radon | Mining |
| Silica | Ceramics and glass industry Foundry industry Granite industry Metal ore smelting Mining and quarrying |
Table 4.
Suspected occupational carcinogens and associated occupational exposures
| Suspected Carcinogen | Occupational Exposures |
|---|---|
| Acrylonitrile | Textile manufacture Plastics, petrochemical manufacture |
| Cadmium | Electroplating Pigment production Plastics industry |
| Formaldehyde | Formaldehyde resin production Synthetic fibers Insulation work Insulation production |
| Vinyl chloride | Plastic production Polyvinyl chloride production |
Asbestos
Asbestos is the most widely known and most common occupational cause of lung cancer. Asbestos is a class of naturally occurring fibrous minerals consisting primarily of 2 types: (1) serpentine (chrysotile) and (2) amphibole (amosite, crocidolite, and tremolites). Asbestos has been used commercially since the late 1800s, and its fire-retardant qualities and strength have made it useful in construction and insulating materials. As early as the 1940s in Germany, asbestos was noted as a lung carcinogen.234 The wide recognition of its carcinogenicity, however, dates to reports in the United Kingdom in the 1950s, including that by Doll.235 Asbestos exposure can also result in pleural and pulmonary manifestations. Asbestos-related pleural disease can present as effusion, pleurisy, or both. Chronic pleural involvement is seen as areas of pleural thickening (pleural plaques) that usually involve the parietal pleura and that are often calcified. The presence of pleural plaques does not herald the development of mesothelioma and has not been proved a marker of increased risk for lung cancer. Inhalation of asbestos fibers can result in parenchymal lung disease, specifically interstitial lung disease, known as asbestosis. All the major types of asbestos can cause interstitial lung disease, although amphibole fibers may be more fibrogenic than chrysotile fibers. The presentation of asbestosis is essentially identical to that of nonspecific interstitial lung disease as well as idiopathic pulmonary fibrosis. Asbestosis develops above a threshold fiber dose of approximately 25 to 105 fibers per mL per year, where the threshold dose is usually reached only in workers, including asbestos insulators, miners, millers, and textile workers, who have heavy occupational exposure.236 The development of interstitial fibrosis usually requires prolonged exposure over months to years. Such fibrotic disease can follow shorter, more intense exposure, as occurs in shipyard workers. The latency period from exposure to presentation of disease is inversely proportional to exposure level. In the United States, the national rate of lung cancer in the form of mesothelioma is 13.8 per million population per year, with higher rates, exceeding 20 per million per year, in Maine, New Jersey, Pennsylvania, Washington, Wyoming, and West Virginia (Fig. 16).237
Fig. 16.
Malignant mesothelioma death rate per 1 million population by state in the United States from 1999 to 2005. Map of the United States indicates the malignant mesothelioma death rate per 1million population for each state during 1999–2005. The state death rate was greater than the national rate of 13.8 per million population per year in 26 states; in 6 states (Maine, New Jersey, Pennsylvania, Washington, Wyoming, and West Virginia), the rate exceeded 20 per million per year. (Adapted from Bang KM, Mazurek JM, Storey E, et al. Malignantmesotheliomamortality—UnitedStates, 1999–2005. MMWR 2009;58(15):393–6.)
The distinction between asbestos exposure and asbestosis is important because of the controversy as to which represents the actual risk factor for lung cancer. Two reviews discussing the extensive available epidemiologic data illustrate this controversy. Jones and colleagues238 in their extensive literature review highlight several important points. First, it is widely recognized that lung fibrosis of many causes, including idiopathic pulmonary fibrosis and interstitial disease associated with connective tissue disease, is associated with an increased risk for lung cancer. Second, in animal experiments asbestos-exposed animals that developed lung cancer only when they also developed pulmonary fibrosis. Third, pleural plaques, a marker for asbestos exposure, have not proved a reliable marker for increased risk for lung cancer. These investigators conclude that the issue of whether asbestosis is a necessary precursor to asbestos-attributable lung cancer cannot be definitively settled. Their assessment, however, was that the available data strongly support that hypothesis. In contrast, in an extensive assessment of the medical literature Egilman and Reinert conclude, “asbestos meets accepted criteria for causation of lung cancer in the absence of clinical or histologic parenchymal asbestosis.”239 Their evaluation of pathologic and epidemiologic studies resulted in the conclusion that asbestos can act as a carcinogen independent of the presence of asbestosis.
The question of whether asbestos exposure alone or asbestosis per se represents the risk factor for lung cancer remains an area of debate. A review of 9 epidemiologic studies by Hessel and colleagues240 in 2005 concluded that because of the relative insensitivity of chest radiographs and the uncertain specificity of histologic findings or CT, it is unlikely that epidemiologic studies alone can determine whether asbestos-related lung cancer arises only in the presence of pulmonary fibrosis. Recent studies have found that asbestos exposure was associated with a relative risk for lung cancer of 3.5 after adjusting for age, smoking, and vitamin intake.241 This risk for lung cancer associated with asbestos exposure is dose-dependent but varied with the type of asbestos fiber exposure. The risk for lung cancer seems higher for workers exposed to amphibole fibers than for those exposed to chrysotile fibers, after adjusting for similar exposure level. The presence of interstitial fibrosis, such as in the form of asbestosis in persons exposed to asbestos, is associated with increased likelihood for lung cancer than patients with asbestos exposure without the associated fibrosis.241 The study concluded that asbestosis was a better predictor of excess lung cancer risk than measures of asbestos exposure. Another study found excess risk for lung cancer was restricted to asbestos workers with radiographic evidence of asbestosis even though exposures to asbestos were similar to workers without such evidence.242
From a public health perspective, however, the issue is important because of concerns about lung cancer risk related to asbestos in the general environment. Jones and colleagues238 noted that all persons living in industrialized countries have accumulated asbestos fibers in their lungs; in adults, the number of fibers is estimated in the millions. Asbestosis, however, requires prolonged and intense exposure to asbestos and does not occur at the level of everyday asbestos exposure. The risk for lung cancer from nonoccupational asbestos exposure in the general environment is extremely low. Moreover, Hughes and Weill242 point out that if, as postulated, asbestosis is a necessary prerequisite to the development of cancer, the extrapolation of risk for lung cancer related to occupational asbestos exposure to the risk from exposure to asbestos in the general environment would substantially overestimate that risk. Another controversy in the area of asbestos and lung cancer is whether all types of fibers are carcinogenic. Epidemiologic and experimental data suggest that amphibole fibers are more carcinogenic than chrysotile.243 In the United States, chrysotile has been by far the most commonly used type of asbestos. Thus, although all fibers may be carcinogenic, public concern about low-level asbestos exposure and lung cancer should be appropriately tempered.
Tobacco smoking clearly potentiates the observed carcinogenic effect of asbestos; however, the magnitude of the combined effect is not clear. It is also unclear whether the interaction of these two agents results in an additive or synergistic increase in the risk for lung cancer. When considering the addition of cigarette smoke exposure to asbestos, the risk for lung cancer is further increased. One report found that the risk for death from lung cancer in asbestos workers increased by 16-fold if the asbestos workers smoked more than 1 pack per day and by 9-fold if they smoked less than 1 pack per day compared with asbestos workers without significant smoking history.244 The relative risk for lung cancer with asbestos exposure alone is 6-fold, with cigarette smoking alone 11-fold, but with exposure to both asbestos and cigarette smoke, the increase may be as high as 59-fold. The nature of the interaction in terms of relative risk, however, is again not clear. What is clear is that most lung cancers occur in asbestos-exposed workers who smoke. Smoking cessation should, therefore, be the most important goal of cancer prevention programs in this population, with particular targeting of the subgroup of workers with asbestosis. It is unclear if asbestosis is a marker for heavier exposure to asbestos or if the inflammation and fibrotic changes in the pathogenesis of asbestosis itself is mediating the cancer process. With recognition of the many health risks related to asbestos, its use has precipitously declined in the United States since the 1970s (Fig. 17).237
Fig. 17.
Asbestos use and permissible exposure limits in the United States from 1900 to 2007. The amount of asbestos use, in thousands of metric tons, and the Occupational Safety and Health Administration permissible asbestos exposure limits in the United States during 1900–2007 are shown. Asbestos use increased from 1000 metric tons in 1900 to a peak of 803,000 metric tons in 1973, then decreased to approximately 1700 metric tons in 2007. Permissible asbestos exposure limits were 12 fibers per cubic centimeter (f/cc) in 1971, 5 f/cc in 1972, 2 f/cc in 1976, 0.2 f/cc in 1986, and 0.1 f/cc in 1994. Arrows indicate year when the Occupational Safety and Health Administration permissible exposure limits were put in place. (Adapted from Bang KM, Mazurek JM, Storey E, et al. Malignant mesothelioma mortality—United States, 1999–2005. MMWR 2009;58(15):393–6.)
Radon
Mining is the oldest occupation associated with lung cancer. An illness described as a wasting pulmonary disease of miners and metal smelters has been associated with early mortality. The cause of this illness at the time, known as miners’ phthisis, was variably attributed to dust or metal exposure, tuberculosis, or even inbreeding among mining communities. Early autopsy study performed on miners exposed to ores in the Central European mines documented that the process was actually neoplastic. Frank245 points out that these same mines produced the material from which Marie Curie later isolated radium. Although the etiologic factors causing the increased lung cancer risk were originally speculated as dust-related pneumoconioses, arsenic, or cobalt, the actual carcinogens have been identified as radioactive materials, primarily radon and its decay products.
Radon (radon 222) is a naturally occurring decay product of radium 226, itself a decay product of uranium 238. Uranium and radium are ubiquitous in soil and rock, although in highly variable concentration. At usual temperatures, radon is released as a radium decay product as an inert radioactive gas. Radon itself decays with a half-life of 3.82 days into a series of radioisotopes, known as radon decay products (or radon daughters), that have half-lives measured in seconds to minutes. These products include polonium 218 and polonium 214, which emit alpha particles. α-Radiation is highly damaging to tissues including the respiratory epithelium. Inhalation of these radon decay products and subsequent alpha particle emission in the lung may cause damage to cells and genetic material. Ultimately, radon decay produces lead 210, which has a half-life of 22 years. Radon is a well-established carcinogen with extensive data available both as an occupational hazards as well as exposures experienced by the general population. Evidence from epidemiologic studies of underground miners shows a linear relationship between radon exposure and lung cancer risk.246,247 It has been found that uranium miners in Germany exposed to radon and the decay products have an increased risk for lung cancer, especially 15 to 24 years after exposure and in miners younger than 55 years.248 Pooled original data from 11 cohort studies of radon-exposed underground miners showed that almost 40% of all lung cancer deaths may be due to radon progeny exposure, with 70% of lung cancer deaths in never-smokers and 39% in smokers. Moreover, this report concludes that 10% of all lung cancer deaths might be due to indoor radon, with 11% of lung cancer deaths in smokers and 30% of lung cancers in never smokers.249
There is an increased risk for lung cancer in smoking miners compared with nonsmoking miners, with both potentially acting in an additive and potentially a synergistic and multiplicative fashion.250,251 Smokers and nonsmoking residents of smoking households are at increased risk for lung cancer even when radon levels are low. The combination of exposure to the two carcinogens is worse than exposure to either alone. The numbers of lung cancer cases reported in nonsmoking miners is small because miners have a high prevalence of cigarette smoking. In a study of underground uranium miners from Colorado, however, nonsmoking miners had a higher relative risk for lung cancer compared with all miners.252 Such work emphasizes the potential importance of radon as a carcinogen in the nonsmoking population at large. Uranium mining has now ceased in the United States. Radon exposure, however, continues to be an occupational concern in nonuranium mining and underground work as well as in uranium and nonuranium mines around the world. In the United States, occupational exposure to radon is legislatively controlled. Individual exposure records are mandated for all workers in areas where the concentration of radon exceeds 0.3 work level, with an annual cumulative exposure limit of 4 work-level month. The Biological Effects of Ionizing Radiation IV study estimated that a 40-year exposure at this level would increase a person’s lifetime risk for lung cancer 2-fold.253 This is at best an approximate estimate, however. Continued longitudinal evaluation of occupationally exposed persons is needed to improve understanding of the carcinogenic effects of radon.
In recent years, the possible risk for lung cancer in the general population associated with radon exposure has been a concern. The National Council on Radiation Protection and Measurements has identified radon and its decay products as the largest component of environmental radiation to persons living in the United States. These findings in conjunction with extrapolation of data collected in groups with high occupational radon exposure have escalated concern about the risks of lung cancer related to domestic radon. Radon is a ubiquitous indoor air pollutant in homes, and it has been projected that radon is the second leading cause of lung cancer after smoking. The concentration of radon gas in an environment varies depending on two factors: the richness of the source of radium and the degree to which the air around that source is ventilated. Therefore, certain sites may be more likely to have a high radon concentration, with the prototypical situation being underground mines with poorly ventilated passageways. In a 1991 survey of homes in the United States, Samet and colleagues254 reported a mean indoor radon level of approximately 1.25 pCi/L. In this survey, the range of indoor radon levels was broad. Most homes had levels only slightly higher than outdoor environmental levels, but a few had levels ranging in excess of 100 pCi/L. The primary factor determining radon gas concentration in homes is the concentration of radium in the soil and rock beneath those structures. Building materials, well water, and natural gas are less common sources, usually contributing only minimally to indoor radon concentrations. Indoor-to-outdoor air exchange may also affect radon concentration within the home.
Broad concern for the public health implications of domestic radon exposure has been heightened by the documented carcinogenic effect of radon in miners as described. The potential for mutagenic and carcinogenic effects of low-level α radiation, however, has been an area of controversy. Several studies examining lung cancer risks from domestic exposure have been performed. In a meta-analysis of 8 such studies that included 4263 patients with lung cancer and 6612 controls, Lubin and Boice255 concluded that greater residential exposure levels were associated with an increased overall relative risk for lung cancer of 1.14. This conclusion is consistent with extrapolation of risk from studies performed in miners as well as with actual calculated risks in miners with low cumulative radon exposure. This meta-analysis did not demonstrate any greater increase in lung cancer risk than what would be extrapolated from radon exposure in miners. Using miner-based risk models, it is now estimated that domestic radon may account for 7000 to 36,000 lung cancer deaths in the United States per year. There are studies that dispute this projection, however, or demonstrate no increased risk even with high indoor domestic radon levels.256–258 Cohen227,259 concluded that use of a theoretic linear no-threshold relationship to extrapolate known risks in miners with high radon exposure levels to risk in persons with residential radon exposure grossly overestimates lung cancer risk. Cohen pointed out that the effects of low-dose, low-rate radiation have never been adequately evaluated, and he contested the assumptions inherent in extrapolation of high radon exposure in miners to domestic situations. Hei and colleagues260 shed some insight into the effects of a single alpha particle hit to a cell as this is most relevant to radon exposure in the general population where the probability of multiple hits on a single cell is low given the level of environmental radon. They found that a majority of cells transversed by a single alpha particle survive such radon exposure. Up to 10% of cells can survive even up to 8 alpha particles traversals. Moreover, in the cells that survive such alpha particle exposure, the frequency of gene mutation after a single traversal was enhanced 2-fold, more with additional traversals by alpha particles. Therefore, small numbers of bronchial epithelial cells can be at significant risk for radiation-induced mutation in the setting of environmental radon levels. Assuming that genetic mutation may be an early step in induction of cancer, these data suggest that environmental and indoor radon exposure does indeed constitute a significant public health problem in its potential contribution to the development of lung cancer.
Other occupational carcinogens
Other lung carcinogens have been identified, relating to a wide array of occupations (see Tables 3 and 4). Worldwide, there are estimated 152,000 deaths from lung cancer and approximately 1.6 million disability-adjusted life years from lung cancer due to exposure to occupational exposures to carcinogens.230 Steenland and colleagues233 from the National Institute for Occupational Safety and Health estimated that approximately 9000 to 10,000 men and 900 to 1900 women per year in the United States develop lung cancer from exposure to occupational carcinogens. Although more than half of these are related to asbestos, a substantial number is attributable to other exposures. Furthermore, because these figures apply only to known carcinogens, they likely underestimate the actual number of lung cancer cases related to occupational exposures and represent another area in which prevention may play an important role.
PREVENTION OF LUNG CANCER
Multiple genetic, cellular, and local tissue alterations are involved in a chronic process that leads to lung carcinogenesis. The transformation of normal cells to preneoplastic cells to actual malignant cells involves changes that include DNA damages, genetic and epigenetic changes and the progression to unyielded proliferation of cells and invasion outside the boundaries of local tissues that is characterized as metastases. Exposure to various carcinogens alters normal cells long before clinically detectable invasive malignant tumors occur. Overwhelmingly, the major risk factor for lung cancer is cigarette smoking with a relative risk of 20 to 25 and an attributable risk of 85% to 90%.261 The remaining risk factors contributing to lung cancer include environmental tobacco smoke, occupational exposure to asbestos and radon progenies, and diet. The incidence of lung cancer and therefore its related mortality can be reduced by early detection, treatment of disease, chemoprevention, and smoking avoidance and cessation.261 Of these, only smoking prevention and cessation programs aimed at reducing smoking rates have been shown to reduce lung cancer risk.
Prevention of smoking initiation prevents the sequence of events leading to cancer. Despite intensive antismoking campaigns and widespread public awareness of the risks associated with smoking, there seems to be a committed smoking cohort that includes a 19.8% of the population of the United States.216 There is no question that smoking cessation can decrease the risk for lung cancer. Peto and colleagues59 reported two large case-control studies from approximately 1950 and from 1990 in the United Kingdom. In 1990, cessation of smoking had nearly halved what would have been the anticipated number of lung cancer cases. Lung cancer risk also seemed related to age at smoking cessation. For men who had stopped smoking at ages 60, 50, 40, and 30 years, the cumulative risks of lung cancer by age 70 years were 10%, 6%, 3%, and 2%, respectively. Jemal and colleagues,262 in an evaluation of data collected by the National Center for Health Statistics in the United States, however, identified a slowing in the rate of decrease of the birth cohort trend in lung cancer mortality for whites born after 1950, which they interpreted as a reflection of the impact of increasing teenage smoking. Although there has been debate as to whether age at initiation of smoking is an independent risk factor for lung cancer, their report supports data reported by Wiencke263 that patients in the youngest quartile of age at smoking initiation (7–15 years of age) have the highest levels of DNA adducts. Therefore, because a large percentage of persons in the United States and an increasing number of persons worldwide continue to smoke, efforts to prevent smoking initiation, particularly in children and teenagers, are of paramount importance. Furthermore, smoking cessation as the other method of primary prevention needs to be continually reinforced.
Smokers who quit for more than 15 years have an 80% to 90% reduction in their risk for lung cancer compared with persons who continue to smoke. Smokers who stop smoking even well into middle age avoid most of their subsequent risk for lung cancer, and stopping before middle age avoids more than 90% of the risk attributable to tobacco.59 It is this primary prevention that should be the main focus of every society to reduce the risks for lung cancer. (See discussion of the prevention of lung cancer by smoking cessation by Hurt and colleagues elsewhere in this issue.) Lung cancer risk among former smokers has been shown to decrease with increasing duration of smoking abstinence.264 Studies have observed a 50% or greater lung cancer risk reduction in the first decade of smoking abstinence for former smokers compared with current smokers.265,266 In the Women’s Health Study of women ages 55 to 69 years, there was a beneficial effect of smoking cessation among recent and distant former smokers. There are some investigators who argue that lung cancers may be triggered by smoking cessation.267,268 The process of lung cancer development, however, takes place over many years. With the median interval of cessation to diagnosis of 2.7 years, the majority of former smokers with lung cancer likely harbored cancer at the time of smoking cessation.269 A study described spontaneous smoking cessation before lung cancer diagnosis and challenged the widely believed notion that cessation was due to disease symptoms26 but instead spontaneous smoking cessation represent a presenting symptom of lung cancer itself. They speculate that lung cancers may produce factors that block or emulate the effects of nicotine.269
The risk for lung adenocarcinoma, however, remained elevated for up to 30 years for both former heavier and former lighter smokers, highlighting the importance of emphasis on smoking prevention for all smokers.270 Chemoprevention has been advocated as an approach to reduce lung cancers with the idea of treating in the early steps in lung carcinogenesis. Chemoprevention, a termed coined initially by Sporn and colleagues271 in 1976, consists of the use of specific natural or non-natural agents, dietary or pharmacologic, to interfere with the development of cancer cells by preventing the DNA damage that initiates carcinogenesis or by halting the progression of premalignant cells.272 Kelley and McCrory261 and Dragnev and colleageus273 extensively reviewed the studies related to lung cancer chemoprevention strategies. Chemoprevention strategies can be used for primary prevention of lung cancer in persons with known high-risk factors, for secondary prevention in persons with disease precursors, or for tertiary prevention in persons with a prior cancer that had been treated with curative intent. Chemoprevention has been used with some success in breast cancer (tamoxifen), prostate cancer (finasteride), and colon cancer (celocoxib); however, no agents have been validated as effective chemoprevention for lung cancer.274,275 There have been various large chemoprevention of lung cancer trials testing for various agents, such as retinol (vitamin A), β-carotene, N-acetylcysteine, and selenium; however, none of these trials has shown evidence for efficacy.261 As discussed previously, there is evidence to suggest that the use of β-carotene and isotretinoin for lung cancer chemoprevention in high-risk persons may increase their risk for lung cancer, especially in those who continue to smoke.273 Currently there are chemoprevention trials being conducted involving agents, such as COX inhibitors, prostacyclin analogues, leukotriene modifiers, green tea, and broccoli sprout extracts, with future trials planned using peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists or mammalian target of rapamycin inhibitors. For a successful lung cancer chemoprevention trial, incorporation of a highly defined risk population based on known lung cancer epidemiology and reliable biomarkers is needed. Until these chemopreventative agents are shown to be efficacious, smoking cessation and tobacco control are the main forms of prevention.
SUMMARY
A vast majority of lung cancer deaths are attributable to cigarette smoking, and curbing the rates of cigarette smoking is imperative. Understanding the epidemiology and causal factors of lung cancer can provide additional foundation for disease prevention. The role of tobacco as an etiologic factor in lung cancer has been convincingly established. Likewise, ionizing radiation and certain occupational exposures have been recognized as carcinogenic. At present, the 5-year survival rate for lung cancer is only 15.6%. This is in stark contrast to the 5-year survival rates for the other leading causes of cancer death in the United States, including cancers of the colon (66%), skin (melanoma 93%), breast (90%), and prostate (100%).1 The absolute number of lung cancer cases continues to be alarming, with the continued rise of lung cancer in women a particularly disturbing feature. The challenge in the future will be to modify the impact of these identified external sources of risk while continuing to expand knowledge of the genetic and molecular basis of carcinogenesis. Early diagnosis of lung cancer is imperative because the 5-year survival rate for treated stage I lung cancer is substantially better than for stages II to IV. The issue of benefit related to lung cancer screening is being actively revisited. The American Cancer Society does not currently recommend routine screening for lung cancer. The American College of Chest Physicians guidelines for lung cancer screening only recommend it for persons who are part of a clinical trial. Prior trials from the 1970s and 1980s demonstrating no reduction in cancer mortality despite screening by chest radiograph effectively eliminated such testing. Petty276 points out that groups at high risk, specifically heavy smokers with spirometric and clinical evidence of airflow obstruction, can be easily identified. Many clinicians think that screening with chest radiography and sputum cytology in such groups might be justifiable. Recent National Lung Screening Trial study shows that using low-dose CT scans to screen for lung cancer resulted in a 20% reduction in deaths from the disease.277 As promising as the National Lung Screening Trial results are, official guidelines on CT scan for lung cancer screening are not available pending careful evaluation of the new data to determine who should or should not consider undergo screen for early lung cancer detection. It is hoped that ongoing trials evaluating chest radiography, chest CT scanning, and sputum cytology will clarify this important controversial issue.
At present, with approximately a quarter of the American population still smoking cigarettes, continued efforts must be directed at smoking cessation and at preventing persons from becoming addicted to smoking. Although work in the field of lung cancer treatment continues to be and remains important, the dismal survival rate associated with this disease demands that the medical profession contribute to efforts aimed at limiting its primary cause. If tobacco smoking could be eliminated, or at least severely curtailed, and if some of the other known exposure risks of lung cancer are addressed, only then may lung cancer be able to be returned to its designation by Adler16 at the turn of the twentieth century as “among the rarest forms of disease.”
REFERENCES
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2008. Bethesda (MD): National Cancer Institute; 2010. Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 4.Kohler B, Ward E, McCarthy B, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:1–23. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin DM, Pisani P, Lopez AD, et al. At least one in seven cases of cancer is caused by smoking. Global estimates for 1985. Int J Cancer. 1994;59(4):494–504. doi: 10.1002/ijc.2910590411. [DOI] [PubMed] [Google Scholar]
- 7.Guindon GE, Boisclair D. Past, current and future trend in tobacco. Washington, DC: International Bank for Reconstruction and Development. The World Bank; 2009. pp. 13–16. [Google Scholar]
- 8.Dube S, McClave A, James C, et al. Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2009. MMWR. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 9.Minna J, Schiller J. Harrison’s principles of internal medicine. 17th edition. New York: McGraw-Hill; 2008. [Google Scholar]
- 10.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc. 1950;143(4):329–336. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 11.Warner KE, Mendez D. Tobacco control policy in developed countries: yesterday, today, and tomorrow. Nicotine Tob Res. 2010;12(9):876–887. doi: 10.1093/ntr/ntq125. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Public Health Service, office of the Surgeon General: The health consequences of smoking. National Clearinghouse for Smoking Health. 1972 [Google Scholar]
- 13.Dube S, Asman K, Malarcher A, et al. Cigarette smoking among adults and trends in smoking cessation—United States 2008. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. 2009 [Google Scholar]
- 14.Eaton D, Kann L, Kinchen S, et al. Youth Risk Behavior Surveillance – United States, 2009. Department of Health and Human Services. MMWR. 2010;59:1–36. [PubMed] [Google Scholar]
- 15.Office on Smoking and Health DoAaSH, National Center for Chronic Disease Prevention and Health Promotion, CDC. Cigarette use among high school students—United States, 1991–2007. MMWR Morb Mortal Wkly Rep. 2008;57:689–991. [Google Scholar]
- 16.Adler I. Primary malignant growth of the lung and bronchi. New York: Longman, Green, Company; 1912. [Google Scholar]
- 17.Hruby A, Sweany H. Primary carcinoma of the lung. Arch Intern Med. 1933;52:497. [Google Scholar]
- 18.Pear R. Tobacco smoking and longevity. Science. 1938;87(2253):216–217. doi: 10.1126/science.87.2253.216. [DOI] [PubMed] [Google Scholar]
- 19.Robert PN. Angel H Roffo: The forgotten father of experimental tobacco carcinogenesis. Bulletin of the World Health Organization. 2006;84:494–496. doi: 10.2471/blt.06.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tylecote F. Cancer of the lung. Lancet. 1927;2:256. [Google Scholar]
- 21.Ochsner A, DeBakey M. Primary pulmonary malignancy: treatment of total pneumonectomy. Analysis of seventy-nine collected cases and presentation of seven personal cases. Surg Gynecol Obstet. 1939;68:435. [PMC free article] [PubMed] [Google Scholar]
- 22.Levin ML, Goldstein H, Gerhardt PR. Cancer and tobacco smoking; a preliminary report. J Am Med Assoc. 1950;143(4):336–338. doi: 10.1001/jama.1950.02910390008002. [DOI] [PubMed] [Google Scholar]
- 23.McNally W. The tar in cigarette smoke and its possible effects. Am J Cancer. 1932;16:1502. [Google Scholar]
- 24.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2(4682):739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynder EL, Graham EA. Etiologic factors in bronchiogenic carcinoma with special reference to industrial exposures; report of eight hundred fifty-seven proved cases. A M A Arch Ind Hyg Occup Med. 1951;4(3):221–235. [PubMed] [Google Scholar]
- 26.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services PHS, Centers for Dsiease Control and Prevention. vol. 7829. Washington, DC: CDC Publication; 2004. The health consequences of smoking: a report of the Surgeon General. [PubMed] [Google Scholar]
- 28.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50(4):307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 29.Smith CJ, Perfetti TA, Rumple MA, et al. “IARC group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38(4):371–383. doi: 10.1016/s0278-6915(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Perfetti TA, Mullens MA, et al. “IARC group 2B Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38(9):825–848. doi: 10.1016/s0278-6915(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 31.Wynder EL, Hoffmann D. Smoking and lung cancer: scientific challenges and opportunities. Cancer Res. 1994;54(20):5284–5295. [PubMed] [Google Scholar]
- 32.Akopyan G, Bonavida B. Understanding tobacco smoke carcinogen NNK and lung tumorigenesis. Int J Oncol. 2006;29(4):745–752. [PubMed] [Google Scholar]
- 33.Hoffmann D, Djordjevic MV, Rivenson A, et al. A study of tobacco carcinogenesis. LI. Relative potencies of tobacco-specific N-nitrosamines as inducers of lung tumours in A/J mice. Cancer Lett. 1993;71(1–3):25–30. doi: 10.1016/0304-3835(93)90092-n. [DOI] [PubMed] [Google Scholar]
- 34.Belinsky SA, Devereux TR, Maronpot RR, et al. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res. 1989;49(19):5305–5311. [PubMed] [Google Scholar]
- 35.Rodenhuis S, Slebos RJ. Clinical significance of ras oncogene activation in human lung cancer. Cancer Res. 1992;52(Suppl 9):2665s–2669s. [PubMed] [Google Scholar]
- 36.Westra WH, Slebos RJ, Offerhaus GJ, et al. K-ras oncogene activation in lung adenocarcinomas from former smokers. Evidence that K-ras mutations are an early and irreversible event in the development of adenocarcinoma of the lung. Cancer. 1993;72(2):432–438. doi: 10.1002/1097-0142(19930715)72:2<432::aid-cncr2820720219>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Denissenko MF, Pao A, Tang M, et al. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 38.Smith LE, Denissenko MF, Bennett WP, et al. Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 2000;92(10):803–811. doi: 10.1093/jnci/92.10.803. [DOI] [PubMed] [Google Scholar]
- 39.Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294(10):1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 40.Mattson ME, Pollack ES, Cullen JW. What are the odds that smoking will kill you? Am J Public Health. 1987;77(4):425–431. doi: 10.2105/ajph.77.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris JE, Thun MJ, Mondul AM, et al. Cigarette tar yields in relation to mortality from lung cancer in the cancer prevention study II prospective cohort, 1982–8. BMJ. 2004;328(7431):72. doi: 10.1136/bmj.37936.585382.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeb LA, Ernster VL, Warner KE, et al. Smoking and lung cancer: an overview. Cancer Res. 1984;44(12 Pt 1):5940–5958. [PubMed] [Google Scholar]
- 43.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97(1):72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro JA, Jacobs EJ, Thun MJ. Cigar smoking in men and risk of death from tobacco-related cancers. J Natl Cancer Inst. 2000;92(4):333–337. doi: 10.1093/jnci/92.4.333. [DOI] [PubMed] [Google Scholar]
- 45.Iribarren C, Tekawa IS, Sidney S, et al. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med. 1999;340(23):1773–1780. doi: 10.1056/NEJM199906103402301. [DOI] [PubMed] [Google Scholar]
- 46.Boffetta P, Pershagen G, Jöckel KH, et al. Cigar and pipe smoking and lung cancer risk: a multicenter study from Europe. J Natl Cancer Inst. 1999;91(8):697–701. doi: 10.1093/jnci/91.8.697. [DOI] [PubMed] [Google Scholar]
- 47.Boffetta P, Nyberg F, Agudo A, et al. Risk of lung cancer from exposure to environmental tobacco smoke from cigars, cigarillos and pipes. Int J Cancer. 1999;83(6):805–806. doi: 10.1002/(sici)1097-0215(19991210)83:6<805::aid-ijc18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 48.Henley SJ, Thun MJ, Chao A, et al. Association between exclusive pipe smoking and mortality from cancer and other diseases. J Natl Cancer Inst. 2004;96(11):853–861. doi: 10.1093/jnci/djh144. [DOI] [PubMed] [Google Scholar]
- 49.Wald NJ, Watt HC. Prospective study of effect of switching from cigarettes to pipes or cigars on mortality from three smoking related diseases. BMJ. 1997;314(7098):1860–1863. doi: 10.1136/bmj.314.7098.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fligiel SE, Roth MD, Kleerup EC, et al. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112(2):319–326. doi: 10.1378/chest.112.2.319. [DOI] [PubMed] [Google Scholar]
- 51.Barsky SH, Roth MD, Kleerup EC, et al. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst. 1998;90(16):1198–1205. doi: 10.1093/jnci/90.16.1198. [DOI] [PubMed] [Google Scholar]
- 52.Aldington S, Harwood M, Cox B, et al. Cannabis use and risk of lung cancer: a case-control study. Eur Respir J. 2008;31(2):280–286. doi: 10.1183/09031936.00065707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 54.Rudin CM, Avila-Tang E, Samet JM. Lung cancer in never smokers: a call to action. Clin Cancer Res. 2009;15(18):5622–5625. doi: 10.1158/1078-0432.CCR-09-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 56.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zell JA, Ou SH, Ziogas A, et al. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J Clin Oncol. 2005;23(33):8396–8405. doi: 10.1200/JCO.2005.03.0312. [DOI] [PubMed] [Google Scholar]
- 58.Yano T, Miura N, Takenaka T, et al. Never-smoking nonsmall cell lung cancer as a separate entity: clinicopathologic features and survival. Cancer. 2008;113(5):1012–1018. doi: 10.1002/cncr.23679. [DOI] [PubMed] [Google Scholar]
- 59.Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11(5):533–538. doi: 10.1111/j.1440-1843.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 62.Kenfield SA, Wei EK, Stampfer MJ, et al. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17(3):198–204. doi: 10.1136/tc.2007.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bryant A, Cerfolio RJ. Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest. 2007;132(1):185–192. doi: 10.1378/chest.07-0442. [DOI] [PubMed] [Google Scholar]
- 64.Gray N. The consequences of the unregulated cigarette. Tob Control. 2006;15(5):405–408. doi: 10.1136/tc.2006.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu NS, Spitz MR, Kemp BL, et al. Adenocarcinoma of the lung in young patients: the M.D. Anderson experience. Cancer. 2000;88(8):1837–1841. doi: 10.1002/(sici)1097-0142(20000415)88:8<1837::aid-cncr12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 66.Toh CK, Gao F, Lim WT, et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol. 2006;24(15):2245–2251. doi: 10.1200/JCO.2005.04.8033. [DOI] [PubMed] [Google Scholar]
- 67.Yang P. Lung cancer in never smokers. Semin Respir Crit Care Med. 2011;32(1):10–21. doi: 10.1055/s-0031-1272865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenner DR, Hung RJ, Tsao MS, et al. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorlova OY, Zhang Y, Schabath MB, et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int J Cancer. 2006;118(7):1798–1804. doi: 10.1002/ijc.21561. [DOI] [PubMed] [Google Scholar]
- 70.Gorlova OY, Weng SF, Zhang Y, et al. Aggregation of cancer among relatives of never-smoking lung cancer patients. Int J Cancer. 2007;121(1):111–118. doi: 10.1002/ijc.22615. [DOI] [PubMed] [Google Scholar]
- 71.Wu PF, Lee CH, Wang MJ, et al. Cancer aggregation and complex segregation analysis of families with female non-smoking lung cancer probands in Taiwan. Eur J Cancer. 2004;40(2):260–266. doi: 10.1016/j.ejca.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 72.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37(12):1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 73.Wenzlaff AS, Cote ML, Bock CH, et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26(12):2207–2212. doi: 10.1093/carcin/bgi191. [DOI] [PubMed] [Google Scholar]
- 74.Wenzlaff AS, Cote ML, Bock CH, et al. GSTM1, GSTT1 and GSTP1 polymorphisms, environmental tobacco smoke exposure and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26(2):395–401. doi: 10.1093/carcin/bgh326. [DOI] [PubMed] [Google Scholar]
- 75.Jung CY, Choi JE, Park JM, et al. Polymorphisms in the hMSH2 gene and the risk of primary lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(4):762–768. doi: 10.1158/1055-9965.EPI-05-0834. [DOI] [PubMed] [Google Scholar]
- 76.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 78.Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64(24):8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 79.Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res. 2009;15(18):5646–5661. doi: 10.1158/1078-0432.CCR-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimizu H, Tominaga S, Nishimura M, et al. Comparison of clinico-epidemiological features of lung cancer patients with and without a history of smoking. Jpn J Clin Oncol. 1984;14(4):595–600. [PubMed] [Google Scholar]
- 81.Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126(2):347–351. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 82.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest. 2004;125(1):27–37. doi: 10.1378/chest.125.1.27. [DOI] [PubMed] [Google Scholar]
- 83.Takeuchi T, Tomida S, Yatabe Y, et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J Clin Oncol. 2006;24(11):1679–1688. doi: 10.1200/JCO.2005.03.8224. [DOI] [PubMed] [Google Scholar]
- 84.Lam DC, Girard L, Suen WS, et al. Establishment and expression profiling of new lung cancer cell lines from Chinese smokers and lifetime never-smokers. J Thorac Oncol. 2006;1(9):932–942. [PubMed] [Google Scholar]
- 85.Li Y, Sheu CC, Ye Y, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010;11(4):321–330. doi: 10.1016/S1470-2045(10)70042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsiung CA, Lan Q, Hong YC, et al. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. 2010;6(8):1–9. doi: 10.1371/journal.pgen.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99(9):715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 88.Spitz MR, Etzel CJ, Dong Q, et al. An expanded risk prediction model for lung cancer. Cancer Prev Res (Phila) 2008;1(4):250–254. doi: 10.1158/1940-6207.CAPR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98(2):270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwartz AG, Prysak GM, Bock CH, et al. The molecular epidemiology of lung cancer. Carcinogenesis. 2007;28(3):507–518. doi: 10.1093/carcin/bgl253. [DOI] [PubMed] [Google Scholar]
- 91.Takemiya M, Shiraishi S, Teramoto T, et al. Bloom’s syndrome with porokeratosis of Mibelli and multiple cancers of the skin, lung and colon. Clin Genet. 1987;31(1):35–44. doi: 10.1111/j.1399-0004.1987.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 92.Yamanaka A, Hirai T, Ohtake Y, et al. Lung cancer associated with Werner’s syndrome: a case report and review of the literature. Jpn J Clin Oncol. 1997;27(6):415–418. doi: 10.1093/jjco/27.6.415. [DOI] [PubMed] [Google Scholar]
- 93.Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer. 2005;93(7):825–833. doi: 10.1038/sj.bjc.6602769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bailey-Wilson JE, Amos CI, Pinney SM, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet. 2004;75(3):460–474. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Marchand L, Guo C, Benhamou S, et al. Pooled analysis of the CYP1A1 exon 7 polymorphism and lung cancer (United States) Cancer Causes Control. 2003;14(4):339–346. doi: 10.1023/a:1023956201228. [DOI] [PubMed] [Google Scholar]
- 96.Benhamou S, Lee WJ, Alexandrie AK, et al. Meta-and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23(8):1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 97.Ye Z, Song H, Higgins JP, et al. Five glutathione s-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of**130**studies. PLoS Med. 2006;3(4):e91. doi: 10.1371/journal.pmed.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40(5):616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou W, Liu G, Thurston SW, et al. Genetic polymorphisms in N-acetyltransferase-2 and microsomal epoxide hydrolase, cumulative cigarette smoking, and lung cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(1):15–21. [PubMed] [Google Scholar]
- 100.Spitz MR, Wei Q, Dong Q, et al. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12(8):689–698. [PubMed] [Google Scholar]
- 101.Wu X, Delclos GL, Annegers JF, et al. A case-control study of wood dust exposure, mutagen sensitivity, and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 1995;4(6):583–588. [PubMed] [Google Scholar]
- 102.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 103.Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest. 2005;128(1):370–381. doi: 10.1378/chest.128.1.370. [DOI] [PubMed] [Google Scholar]
- 104.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91(8):675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 106.Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst. 1993;85(6):457–464. doi: 10.1093/jnci/85.6.457. [DOI] [PubMed] [Google Scholar]
- 107.Cook MB, McGlynn KA, Devesa SS, et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brownson RC, Chang JC, Davis JR. Gender and histologic type variations in smoking-related risk of lung cancer. Epidemiology. 1992;3(1):61–64. doi: 10.1097/00001648-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 109.McDuffie HH, Klaassen DJ, Dosman JA. Female-male differences in patients with primary lung cancer. Cancer. 1987;59(10):1825–1830. doi: 10.1002/1097-0142(19870515)59:10<1825::aid-cncr2820591024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 110.Risch HA, Howe GR, Jain M, et al. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993;138(5):281–293. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 111.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88(3–4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 112.Ryberg D, Hewer A, Phillips DH, et al. Different susceptibility to smoking-induced DNA damage among male and female lung cancer patients. Cancer Res. 1994;54(22):5801–5803. [PubMed] [Google Scholar]
- 113.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86(11):869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 114.Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol. 2010;28(9):1540–1546. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 116.Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374(9697):1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doll R, Peto R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J. 1976;2(6051):1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doll R, Gray R, Hafner B, et al. Mortality in relation to smoking: 22 years’ observations on female British doctors. Br Med J. 1980;280(6219):967–971. doi: 10.1136/bmj.280.6219.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tashkin DP, Altose MD, Bleecker ER, et al. The lung health study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am Rev Respir Dis. 1992;145(2 Pt 1):301–310. doi: 10.1164/ajrccm/145.2_Pt_1.301. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y, Horne SL, Dosman JA. Increased susceptibility to lung dysfunction in female smokers. Am Rev Respir Dis. 1991;143(6):1224–1230. doi: 10.1164/ajrccm/143.6.1224. [DOI] [PubMed] [Google Scholar]
- 121.Beck GJ, Doyle CA, Schachter EN. Smoking and lung function. Am Rev Respir Dis. 1981;123(2):149–155. doi: 10.1164/arrd.1981.123.2.149. [DOI] [PubMed] [Google Scholar]
- 122.Menck H, Hnederson BE. Cancer incidence patterns in the Pacific Basin. Natl Cancer Mongr. 1982;62:101–109. [PubMed] [Google Scholar]
- 123.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 124.Coté ML, Kardia SL, Wenzlaff AS, et al. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA. 2005;293(24):3036–3042. doi: 10.1001/jama.293.24.3036. [DOI] [PubMed] [Google Scholar]
- 125.Brooks DR, Palmer JR, Strom BL, et al. Menthol cigarettes and risk of lung cancer. Am J Epidemiol. 2003;158(7):609–616. doi: 10.1093/aje/kwg182. [discussion: 617–20]. [DOI] [PubMed] [Google Scholar]
- 126.Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97(Suppl 12):3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 127.Blanchard EM, Arnaoutakis K, Hesketh PJ. Lung cancer in octogenarians. J Thorac Oncol. 2010;5(6):909–916. doi: 10.1097/jto.0b013e3181d89b48. [DOI] [PubMed] [Google Scholar]
- 128.Gridelli C, Langer C, Maione P, et al. Lung cancer in the elderly. J Clin Oncol. 2007;25(14):1898–1907. doi: 10.1200/JCO.2006.10.3085. [DOI] [PubMed] [Google Scholar]
- 129.Willett WC, Trichopoulos D. Nutrition and cancer: a summary of the evidence. Cancer Causes Control. 1996;7(1):178–180. doi: 10.1007/BF00115648. [DOI] [PubMed] [Google Scholar]
- 130.Ruano-Ravina A, Figueiras A, Barros-Dios JM. Diet and lung cancer: a new approach. Eur J Cancer Prev. 2000;9(6):395–400. doi: 10.1097/00008469-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 131.Boone CW, Kelloff GJ, Malone WE. Identification of candidate cancer chemopreventive agents and their evaluation in animal models and human clinical trials: a review. Cancer Res. 1990;50(1):2–9. [PubMed] [Google Scholar]
- 132.Woodson K, Tangrea JA, Barrett MJ, et al. Serum alpha-tocopherol and subsequent risk of lung cancer among male smokers. J Natl Cancer Inst. 1999;91(20):1738–1743. doi: 10.1093/jnci/91.20.1738. [DOI] [PubMed] [Google Scholar]
- 133.Buring JE, Hennekens CH. Beta-carotene and cancer chemoprevention. J Cell Biochem Suppl. 1995;22:226–230. doi: 10.1002/jcb.240590828. [DOI] [PubMed] [Google Scholar]
- 134.Stefani ED, Boffetta P, Deneo-Pellegrini H, et al. Dietary antioxidants and lung cancer risk: a case-control study in Uruguay. Nutr Cancer. 1999;34(1):100–110. doi: 10.1207/S15327914NC340114. [DOI] [PubMed] [Google Scholar]
- 135.Yong LC, Brown CC, Schatzkin A, et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am J Epidemiol. 1997;146(3):231–243. doi: 10.1093/oxfordjournals.aje.a009258. [DOI] [PubMed] [Google Scholar]
- 136.Shekelle RB, Lepper M, Liu S, et al. Dietary vitamin A and risk of cancer in the Western Electric study. Lancet. 1981;2(8257):1185–1190. doi: 10.1016/s0140-6736(81)91435-5. [DOI] [PubMed] [Google Scholar]
- 137.Byers TE, Graham S, Haughey BP. Diet and lung cancer risk: findings from the Western New York Diet Study. Am J Epidemiol. 1987;125(3):351–363. doi: 10.1093/oxfordjournals.aje.a114542. [DOI] [PubMed] [Google Scholar]
- 138.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 139.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 140.Omenn GS, Goodman GE, Thornquist MD. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 141.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 142.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 143.Mahabir S, Spitz MR, Barrera SL, et al. Dietary zinc, copper and selenium, and risk of lung cancer. Int J Cancer. 2007;120(5):1108–1115. doi: 10.1002/ijc.22451. [DOI] [PubMed] [Google Scholar]
- 144.Mahabir S, Forman MR, Dong YQ, et al. Mineral intake and lung cancer risk in the NIH-American Association of Retired Persons Diet and Health study. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1976–1983. doi: 10.1158/1055-9965.EPI-10-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voorrips LE, Goldbohm RA, Verhoeven DT, et al. Vegetable and fruit consumption and lung cancer risk in the Netherlands Cohort Study on diet and cancer. Cancer Causes Control. 2000;11(2):101–115. doi: 10.1023/a:1008906706084. [DOI] [PubMed] [Google Scholar]
- 146.Brennan P, Hsu CC, Moullan N, et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet. 2005;366(9496):1558–1560. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- 147.Fontham ET. Protective dietary factors and lung cancer. Int J Epidemiol. 1990;19(Suppl 1):S32–S42. doi: 10.1093/ije/19.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 148.Cooper DA, Eldridge AL, Peters JC. Dietary carotenoids and lung cancer: a review of recent research. Nutr Rev. 1999;57(5 Pt 1):133–145. doi: 10.1111/j.1753-4887.1999.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 149.Wright ME, Park Y, Subar AF, et al. Intakes of fruit, vegetables, and specific botanical groups in relation to lung cancer risk in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168(9):1024–1034. doi: 10.1093/aje/kwn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mursu J, Nurmi T, Tuomainen TP, et al. Intake of flavonoids and risk of cancer in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Int J Cancer. 2008;123(3):660–663. doi: 10.1002/ijc.23421. [DOI] [PubMed] [Google Scholar]
- 151.Swanson CA, Brown CC, Sinha R, et al. Dietary fats and lung cancer risk among women: the Missouri Women’s Health Study (United States) Cancer Causes Control. 1997;8(6):883–893. doi: 10.1023/a:1018468429744. [DOI] [PubMed] [Google Scholar]
- 152.Alavanja MC, Brownson RC, Benichou J. Estimating the effect of dietary fat on the risk of lung cancer in nonsmoking women. Lung Cancer. 1996;14(Suppl 1):S63–S74. doi: 10.1016/s0169-5002(96)90211-1. [DOI] [PubMed] [Google Scholar]
- 153.De Stefani E, Fontham ET, Chen V, et al. Fatty foods and the risk of lung cancer: a case-control study from Uruguay. Int J Cancer. 1997;71(5):760–766. doi: 10.1002/(sici)1097-0215(19970529)71:5<760::aid-ijc12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 154.Goodman MT, Kolonel LN, Yoshizawa CN, et al. The effect of dietary cholesterol and fat on the risk of lung cancer in Hawaii. Am J Epidemiol. 1988;128(6):1241–1255. doi: 10.1093/oxfordjournals.aje.a115078. [DOI] [PubMed] [Google Scholar]
- 155.Hecht SS. Approaches to cancer prevention based on an understanding of N-nitrosamine carcinogenesis. Proc Soc Exp Biol Med. 1997;216(2):181–191. doi: 10.3181/00379727-216-44168. [DOI] [PubMed] [Google Scholar]
- 156.De Stefani E, Deneo-Pellegrini H, Carzoglio JC, et al. Dietary nitrosodimethylamine and the risk of lung cancer: a case-control study from Uruguay. Cancer Epidemiol Biomarkers Prev. 1996;5(9):679–682. [PubMed] [Google Scholar]
- 157.Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 158.Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30(3):415–444. doi: 10.1016/j.ccm.2009.05.001. vii. [DOI] [PubMed] [Google Scholar]
- 159.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 160.Renehan AG, Soerjomataram I, Leitzmann MF. Interpreting the epidemiological evidence linking obesity and cancer: a framework for population-attributable risk estimations in Europe. Eur J Cancer. 2010;46(14):2581–2592. doi: 10.1016/j.ejca.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 161.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Henley SJ, Flanders WD, Manatunga A, et al. Leanness and lung cancer risk: fact or artifact? Epidemiology. 2002;13(3):268–276. doi: 10.1097/00001648-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 163.Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology. 2007;18(5):607–612. doi: 10.1097/ede.0b013e31812713d1. [DOI] [PubMed] [Google Scholar]
- 164.Kabat GC, Kim M, Hunt JR, et al. Body mass index and waist circumference in relation to lung cancer risk in the Women’s Health Initiative. Am J Epidemiol. 2008;168(2):158–169. doi: 10.1093/aje/kwn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang L, Yang G, Zhou M, et al. Body mass index and mortality from lung cancer in smokers and nonsmokers: a nationally representative prospective study of 220,000 men in China. Int J Cancer. 2009;125(9):2136–2143. doi: 10.1002/ijc.24527. [DOI] [PubMed] [Google Scholar]
- 166.Leung CC, Lam TH, Yew WW, et al. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40(1):174–182. doi: 10.1093/ije/dyq134. [DOI] [PubMed] [Google Scholar]
- 167.Hecht SS, Seow A, Wang M, et al. Elevated levels of volatile organic carcinogen and toxicant biomarkers in Chinese women who regularly cook at home. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1185–1192. doi: 10.1158/1055-9965.EPI-09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu AH, Fontham ET, Reynolds P, et al. Previous lung disease and risk of lung cancer among lifetime nonsmoking women in the United States. Am J Epidemiol. 1995;141(11):1023–1032. doi: 10.1093/oxfordjournals.aje.a117366. [DOI] [PubMed] [Google Scholar]
- 169.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986;105(4):503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 170.Tockman MS, Anthonisen NR, Wright EC, et al. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106(4):512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 171.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497–1505. [PubMed] [Google Scholar]
- 172.Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176(3):285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 173.Loganathan RS, Stover DE, Shi W, et al. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129(5):1305–1312. doi: 10.1378/chest.129.5.1305. [DOI] [PubMed] [Google Scholar]
- 174.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 175.Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24(33):5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 176.Parimon T, Chien JW, Bryson CL, et al. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(7):712–719. doi: 10.1164/rccm.200608-1125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang P, Sun Z, Krowka MJ, et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med. 2008;168(10):1097–1103. doi: 10.1001/archinte.168.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161(1):5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 179.Le Jeune I, Gribbin J, West J, et al. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101(12):2534–2540. doi: 10.1016/j.rmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 180.Mornex JF, Thivolet F, De las Heras M, et al. Pathology of human bronchioloalveolar carcinoma and its relationship to the ovine disease. Curr Top Microbiol Immunol. 2003;275:225–248. doi: 10.1007/978-3-642-55638-8_9. [DOI] [PubMed] [Google Scholar]
- 181.Syrjänen KJ. Bronchial squamous cell carcinomas associated with epithelial changes identical to condylomatous lesions of the uterine cervix. Lung. 1980;158(3):131–142. doi: 10.1007/BF02713715. [DOI] [PubMed] [Google Scholar]
- 182.Chen YC, Chen JH, Richard K, et al. Lung adenocarcinoma and human papillomavirus infection. Cancer. 2004;101(6):1428–1436. doi: 10.1002/cncr.20538. [DOI] [PubMed] [Google Scholar]
- 183.Syrjänen KJ. HPV infections and lung cancer. J Clin Pathol. 2002;55(12):885–891. doi: 10.1136/jcp.55.12.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rezazadeh A, Laber DA, Ghim SJ, et al. The role of human papilloma virus in lung cancer: a review of the evidence. Am J Med Sci. 2009;338(1):64–67. doi: 10.1097/MAJ.0b013e3181a393ba. [DOI] [PubMed] [Google Scholar]
- 185.Willey JC, Broussoud A, Sleemi A, et al. Immortalization of normal human bronchial epithelial cells by human papillomaviruses 16 or 18. Cancer Res. 1991;51(19):5370–5377. [PubMed] [Google Scholar]
- 186.Castro CY, Ostrowski ML, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol. 2001;32(8):863–872. doi: 10.1053/hupa.2001.26457. [DOI] [PubMed] [Google Scholar]
- 187.Brouchet L, Valmary S, Dahan M, et al. Detection of oncogenic virus genomes and gene products in lung carcinoma. Br J Cancer. 2005;92(4):743–746. doi: 10.1038/sj.bjc.6602409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Galateau-Salle F, Bidet P, Iwatsubo Y, et al. Detection of SV40-like DNA sequences in pleural mesothelioma, bronchopulmonary carcinoma and other pulmonary diseases. Dev Biol Stand. 1998;94:147–152. [PubMed] [Google Scholar]
- 189.Giuliani L, Jaxmar T, Casadio C, et al. Detection of oncogenic viruses SV40, BKV, JCV, HCMV, HPV and p53 codon 72 polymorphism in lung carcinoma. Lung Cancer. 2007;57(3):273–281. doi: 10.1016/j.lungcan.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 190.Sion-Vardy N, Lasarov I, Delgado B, et al. Measles virus: evidence for association with lung cancer. Exp Lung Res. 2009;35(8):701–712. doi: 10.3109/01902140902853176. [DOI] [PubMed] [Google Scholar]
- 191.Bando M, Takahashi M, Ohno S, et al. Torque teno virus DNA titre elevated in idiopathic pulmonary fibrosis with primary lung cancer. Respirology. 2008;13(2):263–269. doi: 10.1111/j.1440-1843.2007.01217.x. [DOI] [PubMed] [Google Scholar]
- 192.Littman AJ, Jackson LA, Vaughan TL. Chlamydia pneumoniae and lung cancer: epidemiologic evidence. Cancer Epidemiol Biomarkers Prev. 2005;14(4):773–778. doi: 10.1158/1055-9965.EPI-04-0599. [DOI] [PubMed] [Google Scholar]
- 193.Engels EA, Shen M, Chapman RS, et al. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. Int J Cancer. 2009;124(5):1183–1187. doi: 10.1002/ijc.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu YH, Liao CC, Hsu WH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol. 2011;6(1):32–37. doi: 10.1097/JTO.0b013e3181fb4fcc. [DOI] [PubMed] [Google Scholar]
- 195.Lavolé A, Wislez M, Antoine M, et al. Lung cancer, a new challenge in the HIV-infected population. Lung Cancer. 2006;51(1):1–11. doi: 10.1016/j.lungcan.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 196.Morris A, Crothers K, Beck JM, et al. An official ATS workshop report: emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc. 2011;8(1):17–26. doi: 10.1513/pats.2009-047WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kirk GD, Merlo C, O’Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mitsuyasu RT. Non-AIDS-defining malignancies in HIV. Top HIV Med. 2008;16(4):117–121. [PubMed] [Google Scholar]
- 199.el-Solh A, Kumar NM, Nair MP, et al. An RGD containing peptide from HIV-1 Tat-(65–80) modulates protooncogene expression in human bronchoalveolar carcinoma cell line, A549. Immunol Invest. 1997;26(3):351–370. doi: 10.3109/08820139709022692. [DOI] [PubMed] [Google Scholar]
- 200.Powles T, Thirwell C, Newsom-Davis T, et al. Does HIV adversely influence the outcome in advanced non-small-cell lung cancer in the era of HAART? Br J Cancer. 2003;89(3):457–459. doi: 10.1038/sj.bjc.6601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brock MV, Hooker CM, Engels EA, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43(1):47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 202.Engels EA, Brock MV, Chen J, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24(9):1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 203.Janerich DT, Thompson WD, Varela LR, et al. Lung cancer and exposure to tobacco smoke in the household. N Engl J Med. 1990;323(10):632–636. doi: 10.1056/NEJM199009063231003. [DOI] [PubMed] [Google Scholar]
- 204.Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. Br Med J (Clin Res Ed) 1981;282(6259):183–185. doi: 10.1136/bmj.282.6259.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Trichopoulos D, Kalandidi A, Sparros L, et al. Lung cancer and passive smoking. Int J Cancer. 1981;27(1):1–4. doi: 10.1002/ijc.2910270102. [DOI] [PubMed] [Google Scholar]
- 206.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315(7114):980–988. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fontham ET, Correa P, Reynolds P, et al. Environmental tobacco smoke and lung cancer in nonsmoking women. A multicenter study. JAMA. 1994;271(22):1752–1759. [PubMed] [Google Scholar]
- 208.National Research Council. Washington, DC: National Academy Press; 1986. Environmental tobacco smoke: measuring exposures and assessing health effects; p. 337. [PubMed] [Google Scholar]
- 209.US Department of Health and Services. Washington, DC: DHHS (CDC); 1986. Report of the Surgeon General: The Health Consequences of Involuntary Smoking. 87–8398. [Google Scholar]
- 210.US Environmental Protection Agency, Office of Air and Radiation and Office of Research and Development. Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. EPA 600-6-90-006F. 1992
- 211.Cancer IAfRo. Involuntary smoking. vol. 83. Lyon (France): International Agency for Research on Cancer Monographs; 2002. [Google Scholar]
- 212.Cardenas VM, Thun MJ, Austin H, et al. Environmental tobacco smoke and lung cancer mortality in the American Cancer Society’s Cancer Prevention Study. II. Cancer Causes Control. 1997;8(1):57–64. doi: 10.1023/a:1018483121625. [DOI] [PubMed] [Google Scholar]
- 213.Nyberg F, Pershagen G. Passive smoking and lung cancer. Accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1998;317(7154):347–348. [author reply: 348]. [PubMed] [Google Scholar]
- 214.Hecht SS, Carmella SG, Murphy SE, et al. A tobacco-specific lung carcinogen in the urine of men exposed to cigarette smoke. N Engl J Med. 1993;329(21):1543–1546. doi: 10.1056/NEJM199311183292105. [DOI] [PubMed] [Google Scholar]
- 215.Pirkle JL, Flegal KM, Bernert JT, et al. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275(16):1233–1240. [PubMed] [Google Scholar]
- 216.Thorne S, Malarcher A, Maurice M, et al. Cigarette smoking among adults x United States 2007. MMWR. 2008;57:1221–1226. [PubMed] [Google Scholar]
- 217.Beckett WS. Epidemiology and etiology of lung cancer. Clin Chest Med. 1993;14(1):1–15. [PubMed] [Google Scholar]
- 218.Luo RX, Wu B, Yi YN, et al. Indoor burning coal air pollution and lung cancer—a case-control study in Fuzhou, China. Lung Cancer. 1996;14(Suppl 1):S113–S119. doi: 10.1016/s0169-5002(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 219.Kleinerman RA, Wang Z, Wang L, et al. Lung cancer and indoor exposure to coal and biomass in rural China. J Occup Environ Med. 2002;44(4):338–344. doi: 10.1097/00043764-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 220.Hosgood HD, Boffetta P, Greenland S, et al. In-home coal and wood use and lung cancer risk: a pooled analysis of the International Lung Cancer Consortium. Environ Health Perspect. 2010;118(12):1743–1747. doi: 10.1289/ehp.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lissowska J, Bardin-Mikolajczak A, Fletcher T, et al. Lung cancer and indoor pollution from heating and cooking with solid fuels: the IARC international multicentre case-control study in Eastern/Central Europe and the United Kingdom. Am J Epidemiol. 2005;162(4):326–333. doi: 10.1093/aje/kwi204. [DOI] [PubMed] [Google Scholar]
- 222.Wilkins ET. Air pollution and the London fog of December, 1952. J R Sanit Inst. 1954;74(1):1–15. [discussion: 15–21]. [PubMed] [Google Scholar]
- 223.Pershagen G. Air pollution and cancer. IARC Sci Publ. 1990;(104):240–251. [PubMed] [Google Scholar]
- 224.Nyberg F, Gustavsson P, Järup L, et al. Urban air pollution and lung cancer in Stockholm. Epidemiology. 2000;11(5):487–495. doi: 10.1097/00001648-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 225.Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 226.Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cohen BL. How dangerous is low level radiation? Risk Anal. 1995;15(6):645–653. doi: 10.1111/j.1539-6924.1995.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 228.Lipsett M, Campleman S. Occupational exposure to diesel exhaust and lung cancer: a meta-analysis. Am J Public Health. 1999;89(7):1009–1017. doi: 10.2105/ajph.89.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bhatia R, Lopipero P, Smith AH. Diesel exhaust exposure and lung cancer. Epidemiology. 1998;9(1):84–91. [PubMed] [Google Scholar]
- 230.Driscoll T, Nelson DI, Steenland K, et al. The global burden of disease due to occupational carcinogens. Am J Ind Med. 2005;48(6):419–431. doi: 10.1002/ajim.20209. [DOI] [PubMed] [Google Scholar]
- 231.Fingerhut M, Nelson DI, Driscoll T, et al. The contribution of occupational risks to the global burden of disease: summary and next steps. Med Lav. 2006;97(2):313–321. [PubMed] [Google Scholar]
- 232.Steenland K, Burnett C, Lalich N, et al. Dying for work: The magnitude of US mortality from selected causes of death associated with occupation. Am J Ind Med. 2003;43(5):461–482. doi: 10.1002/ajim.10216. [DOI] [PubMed] [Google Scholar]
- 233.Steenland K, Loomis D, Shy C, et al. Review of occupational lung carcinogens. Am J Ind Med. 1996;29(5):474–490. doi: 10.1002/(SICI)1097-0274(199605)29:5<474::AID-AJIM6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 234.Hughes JM, Weill H. Potency versus importance in fiber pathogenicity. Am J Ind Med. 1994;25(4):609–610. doi: 10.1002/ajim.4700250416. [DOI] [PubMed] [Google Scholar]
- 235.Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12(2):81–86. doi: 10.1136/oem.12.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 237.Malignant Mesothelioma Mortality — United States, 1999–2005. MMWR. 2009;58(15):393–396. [PubMed] [Google Scholar]
- 238.Jones RN, Hughes JM, Weill H. Asbestos exposure, asbestosis, and asbestos-attributable lung cancer. Thorax. 1996;51(Suppl 2):S9–S15. doi: 10.1136/thx.51.suppl_2.s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Egilman D, Reinert A. Lung cancer and asbestos exposure: asbestosis is not necessary. Am J Ind Med. 1996;30(4):398–406. doi: 10.1002/(SICI)1097-0274(199610)30:4<398::AID-AJIM4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 240.Hessel PA, Gamble JF, McDonald JC. Asbestos, asbestosis, and lung cancer: a critical assessment of the epidemiological evidence. Thorax. 2005;60(5):433–436. doi: 10.1136/thx.2004.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Weiss W. Asbestosis: a marker for the increased risk of lung cancer among workers exposed to asbestos. Chest. 1999;115(2):536–549. doi: 10.1378/chest.115.2.536. [DOI] [PubMed] [Google Scholar]
- 242.Hughes JM, Weill H. Asbestosis as a precursor of asbestos related lung cancer: results of a prospective mortality study. Br J Ind Med. 1991;48(4):229–233. doi: 10.1136/oem.48.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Churg A, Wiggs B. Fiber size and number in amphibole asbestos-induced mesothelioma. Am J Pathol. 1984;115(3):437–442. [PMC free article] [PubMed] [Google Scholar]
- 244.Hammond EC, Selikoff IJ, Seidman H. Asbestos exposure, cigarette smoking and death rates. Ann N Y Acad Sci. 1979;330:473–490. doi: 10.1111/j.1749-6632.1979.tb18749.x. [DOI] [PubMed] [Google Scholar]
- 245.Frank AL. The epidemiology and etiology of lung cancer. Clin Chest Med. 1982;3(2):219–228. [PubMed] [Google Scholar]
- 246.Samet JM, Eradze GR. Radon and lung cancer risk: taking stock at the millenium. Environ Health Perspect. 2000;108(Suppl 4):635–641. doi: 10.1289/ehp.00108s4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Samet JM. Residential radon and lung cancer: end of the story? J Toxicol Environ Health A. 2006;69(7):527–531. doi: 10.1080/15287390500260879. [DOI] [PubMed] [Google Scholar]
- 248.Grosche B, Kreuzer M, Kreisheimer M, et al. Lung cancer risk among German male uranium miners: a cohort study, 1946–1998. Br J Cancer. 2006;95(9):1280–1287. doi: 10.1038/sj.bjc.6603403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lubin JH, Boice JD, Edling C, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst. 1995;87(11):817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- 250.Samet JM. Indoor radon and lung cancer: risky or not? J Natl Cancer Inst. 1994;86(24):1813–1814. doi: 10.1093/jnci/86.24.1813. [DOI] [PubMed] [Google Scholar]
- 251.Saccomanno G, Huth GC, Auerbach O, et al. Relationship of radioactive radon daughters and cigarette smoking in the genesis of lung cancer in uranium miners. Cancer. 1988;62(7):1402–1408. doi: 10.1002/1097-0142(19881001)62:7<1402::aid-cncr2820620727>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 252.Roscoe RJ, Steenland K, Halperin WE, et al. Lung cancer mortality among nonsmoking uranium miners exposed to radon daughters. JAMA. 1989;262(5):629–633. [PubMed] [Google Scholar]
- 253.Fabrikant JI. Radon and lung cancer: the BEIR IV Report. Health Phys. 1990;59(1):89–97. doi: 10.1097/00004032-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 254.Samet JM, Stolwijk J, Rose SL. Summary: International workshop on residential Rn epidemiology. Health Phys. 1991;60(2):223–227. doi: 10.1097/00004032-199102000-00010. [DOI] [PubMed] [Google Scholar]
- 255.Lubin JH, Boice JD. Lung cancer risk from residential radon: meta-analysis of eight epidemiologic studies. J Natl Cancer Inst. 1997;89(1):49–57. doi: 10.1093/jnci/89.1.49. [DOI] [PubMed] [Google Scholar]
- 256.Auvinen A, Mäkeläinen I, Hakama M, et al. Indoor radon exposure and risk of lung cancer: a nested case-control study in Finland. J Natl Cancer Inst. 1996;88(14):966–972. doi: 10.1093/jnci/88.14.966. [DOI] [PubMed] [Google Scholar]
- 257.Alavanja MC, Brownson RC, Lubin JH, et al. Residential radon exposure and lung cancer among nonsmoking women. J Natl Cancer Inst. 1994;86(24):1829–1837. doi: 10.1093/jnci/86.24.1829. [DOI] [PubMed] [Google Scholar]
- 258.Létourneau EG, Krewski D, Choi NW, et al. Case-control study of residential radon and lung cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol. 1994;140(4):310–322. doi: 10.1093/oxfordjournals.aje.a117253. [DOI] [PubMed] [Google Scholar]
- 259.Cohen BL. Dose-response relationship for radiation carcinogenesis in the low-dose region. Int Arch Occup Environ Health. 1994;66(2):71–75. doi: 10.1007/BF00383360. [DOI] [PubMed] [Google Scholar]
- 260.Hei TK, Piao CQ, Willey JC, et al. Malignant transformation of human bronchial epithelial cells by radon-simulated alpha-particles. Carcinogenesis. 1994;15(3):431–437. doi: 10.1093/carcin/15.3.431. [DOI] [PubMed] [Google Scholar]
- 261.Kelley MJ, McCrory DC. Prevention of lung cancer: summary of published evidence. Chest. 2003;123(Suppl 1):50S–59S. doi: 10.1378/chest.123.1_suppl.50s. [DOI] [PubMed] [Google Scholar]
- 262.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93(4):277–283. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 263.Wiencke JK. DNA adduct burden and tobacco carcinogenesis. Oncogene. 2002;21(48):7376–7391. doi: 10.1038/sj.onc.1205799. [DOI] [PubMed] [Google Scholar]
- 264.Department of Health and Human Services. Washington, DC: US DHHS. Public Health Service; 1990. The Health Benefits of Smoking Cessation: A Report of the Surgeon General. DHHS Pub. No. (CDC) 90–8416. [PubMed] [Google Scholar]
- 265.Speizer FE, Colditz GA, Hunter DJ, et al. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA) Cancer Causes Control. 1999;10(5):475–482. doi: 10.1023/a:1008931526525. [DOI] [PubMed] [Google Scholar]
- 266.Burns DM. Primary prevention, smoking, and smoking cessation: implications for future trends in lung cancer prevention. Cancer. 2000;89(Suppl 11):2506–2509. doi: 10.1002/1097-0142(20001201)89:11+<2506::aid-cncr33>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 267.Lange JH, Mastrangelo G, Fadda E, et al. Elevated lung cancer risk shortly after smoking cessation: is it due to a reduction of endotoxin exposure? Med Hypotheses. 2005;65(3):534–541. doi: 10.1016/j.mehy.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 268.Kumar A, Mallya KB, Kumar JC. Are lung cancers triggered by stopping smoking? Med Hypotheses. 2007;68(5):1176–1177. doi: 10.1016/j.mehy.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 269.Campling BG, Collins BN, Algazy KM, et al. Spontaneous smoking cessation before lung cancer diagnosis. J Thorac Oncol. 2011;6(3):517–524. doi: 10.1097/JTO.0b013e318208c7da. [DOI] [PubMed] [Google Scholar]
- 270.Ebbert JO, Yang P, Vachon CM, et al. Lung cancer risk reduction after smoking cessation: observations from a prospective cohort of women. J Clin Oncol. 2003;21(5):921–926. doi: 10.1200/JCO.2003.05.085. [DOI] [PubMed] [Google Scholar]
- 271.Sporn MB, Dunlop NM, Newton DL, et al. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35(6):1332–1338. [PubMed] [Google Scholar]
- 272.Kelloff GJ, Sigman CC, Greenwald P. Cancer chemoprevention: progress and promise. Eur J Cancer. 1999;35(14):2031–2038. doi: 10.1016/s0959-8049(99)00299-3. [DOI] [PubMed] [Google Scholar]
- 273.Dragnev KH, Stover D, Dmitrovsky E, et al. Lung cancer prevention: the guidelines. Chest. 2003;123(Suppl 1):60S–71S. doi: 10.1378/chest.123.1_suppl.60s. [DOI] [PubMed] [Google Scholar]
- 274.Keith RL. Chemoprevention of lung cancer. Proc Am Thorac Soc. 2009;6(2):187–193. doi: 10.1513/pats.200807-067LC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Omenn GS. Chemoprevention of lung cancer is proving difficult and frustrating, requiring new approaches. J Natl Cancer Inst. 2000;92(12):959–960. doi: 10.1093/jnci/92.12.959. [DOI] [PubMed] [Google Scholar]
- 276.Petty TL. The predictive value of spirometry. Identifying patients at risk for lung cancer in the primary care setting. Postgrad Med. 1997;101(3):128–130. 133–134, 140. doi: 10.3810/pgm.1997.03.176. [DOI] [PubMed] [Google Scholar]
- 277.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]