Abstract
Background
YKL-40 is a chitinase-like protein that, in cross-sectional clinical studies, has been associated with severe asthma and COPD in smokers.
Aim
To determine the longitudinal relation of circulating YKL-40 to levels and lung function decline in the general population.
Methods
We used longitudinal data from up to 12 surveys from the population-based TESAOD study which was conducted in Tucson, Arizona between 1972-1996. In cross-sectional analyses, we also used data from 3 Spanish centers of the multicenter ECRHS study (ECRHS-Sp). Serum YKL-40 was measured at baseline in TESAOD and in survey 2 in ECRHS-Sp using ELISAs. Multivariate linear regression was used to test associations of serum YKL-40 to concomitant lung function. In TESAOD, random coefficients models were used to test associations of serum YKL-40 to subsequent decline of lung function.
Results
Data on YKL-40 and lung function were available from 1088 TESAOD and 854 ECRHS-Sp adult participants (59% and 51% females; respectively). In adjusted multivariate meta-analyses, being in the highest YKL-40 quartile was associated cross-sectionally with significant deficits in FEV1 and FVC %predicted. In adjusted longitudinal analyses, TESAOD participants in the top YKL-40 quartile had an FEV1 decline that was 5 ml/yr (p=0.05) faster than subjects in the third quartile, 5 ml/yr (p=0.02) faster than subjects in the second quartile, and 10 ml/yr (p<0.001) faster than subjects in the lowest YKL-40 quartile. These longitudinal effects were particularly strong in smokers and absent in never smokers. After adjusting for covariates, as compared with the other three quartiles combined the top YKL-40 quartile was associated with a 9 ml/yr (p=0.001) faster FEV1 decline among smokers, while no significant effects were found among never smokers (2 ml/yr, p=0.35).
Conclusions
Circulating YKL-40 is associated with levels and decline of lung function in the general population and may be a biomarker of susceptibility to the long-term effects of cigarette smoking.
Keywords: YKL-40, lung function, smoking
Introduction
The need for integrating molecular biomarkers in risk prediction and clinical assessment of chronic lung diseases is widely acknowledged1,2. However, at the present time there is a lack of informative biomarkers that can be used to identify subjects susceptible to pulmonary disease and lung function deficits in response to specific environmental exposures.
YKL-40 is a glycoprotein that is encoded by the CHI3L1 gene and belongs to the 18 glycosylhydrolase family of chitinases and chitinase-like proteins, which have been studied in recent years as possible biomarkers of obstructive lung diseases3. In the first study on YKL-40 and asthma4, patients with severe disease were found to have higher YKL-40 levels in bronchial biopsies and serum than controls. Subsequent reports5-9 confirmed airway and systemic YKL-40 levels to be associated with markers of asthma severity, such as frequency of exacerbations. Recent studies have also assessed YKL-40 in chronic obstructive pulmonary disease (COPD). In cross-sectional case-control studies10-12, serum YKL-40 levels were found to increase with pack-years of cigarette smoking and to be higher in smokers with COPD than in smokers with no COPD and non-smoker controls. Similar associations with smoking and COPD status were also found when YKL-40 was assessed directly in the airways, either in bronchoalveolar lavage (BAL) or lung biopsies10,11.
Although the biological functions of YKL-40 are still incompletely understood, in vitro and in vivo studies indicate a possible role of this molecule in Th2 adaptive immunity, lung fibrosis and remodeling, and airway inflammation3. Indeed, data from several of the above studies4,6,9,10,12 showed inverse associations between YKL-40 and lung function. A cross-sectional relation of elevated YKL-40 levels from blood and/or airway samples to lower forced expiratory volume in one second (FEV1) has been established in patients with asthma4,6,9 and, although with conflicting results11, it has also been reported in patients with COPD10,12 and in a sample from the general population5. These studies were cross-sectional and for most part based on clinical populations. To date, no longitudinal data are available on the possible role of this biomarker in lung function decline. The goal of our study was to determine the relation of circulating YKL-40 to levels and decline of lung function in the general population.
Methods
Study populations and design
Study populations and study design are summarized in Figure E1. For this study, we used the Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD) and, in cross-sectional analyses, also the independent cohort of the European Community Respiratory Health Survey (ECRHS).
TESAOD is a population-based prospective cohort study of non-Hispanic white households initiated in Tucson, Arizona in 1972. Details of the enrollment process have been previously reported13. At baseline and in 12 follow-up surveys completed approximately every two years up to 1996, participants completed a standardized respiratory questionnaire and – with the only exception of survey 4 – spirometric lung function tests according to methods previously described14. For the present study, we used data from 1088 participants who were 21 to 70 years old at enrollment, had available serum samples with sufficient volume from the baseline survey, and had completed lung function tests at baseline and in at least one follow-up survey.
ECRHS is a study involving multiple centers mainly in Europe. Detailed methods of the study are describes elsewhere15. For the present study, we used data from participants from the Spanish centers of Barcelona, Galdakao, and Albacete (hereafter ECRHS-Sp). Briefly, in 1991-93 a random sample of individuals aged 20-44 years was enrolled. An enrichment sample of subjects who reported taking currently asthma medication or having had asthma attacks or shortness of breath at night during the last year were also recruited. Participants completed a detailed questionnaire and spirometric lung function tests at baseline and in a follow-up survey taken about 9 years later. A total of 854 ECRHS-Sp participants (637 from the random and 217 from the enriched sample) who completed questionnaire and lung function tests and had available serum samples of sufficient volume from survey 2 were included in this study.
YKL-40 measurements
Serum YKL-40 was measured using the same procedures and commercially available enzymelinked immunosorbent assays kit (Quantikine Human CHI3L1 immunoassay by R&D, Inc, Minneapolis, MN, USA, and Abingdon, UK) in both populations. Further details are provided in the online Supplement. Serum samples came from the baseline survey in TESAOD and the follow-up survey in ECRHS-Sp (no serum samples were available from the baseline survey in ECRHS-Sp).
Lung function
Consistent with previous studies, in TESAOD percent predicted values for lung function indices were computed using reference equations generated from the same population by Knudson and colleagues16. In ECRHS-Sp, reference equations by Hankinson et al17 were used. In both cohorts, spirometric patterns were defined as normal (FEV1/FVC ≥ 70% and forced vital capacity [FVC] ≥ 80% predicted), restrictive (FEV1/FVC ≥ 70% and FVC < 80% predicted), or obstructive (FEV1/FVC < 70% independent of FVC values).
Other exposure and phenotype variables
With the exception of lung function indices in TESAOD, which were modeled longitudinally, all other exposure and phenotype variables were assessed at the same survey in which YKL-40 measurements were completed, i.e., the baseline survey in TESAOD and the follow-up survey in ECRHS-Sp. A description of these variables is provided in the online supplement.
In both cohorts, physician-confirmed asthma was defined as a positive report that a physician told the participant that he or she had asthma. Asthma was defined as active if the participant reported to have had any asthma attacks in the past year or to be currently taking any asthma medications.
Statistical analyses
Because distribution of YKL-40 levels was skewed to the right, values were log-transformed when used on a continuous scale. YKL-40 values were categorized into quartiles and analyses were completed by contrasting the top quartile against the other three quartiles separately to study the effects of high YKL-40 on lung health. Results from analyses contrasting the top quartile (high YKL-40) against the other three quartiles combined (low-medium YKL-40) are shown in the online supplement.
In cross-sectional analyses, for each lung function index (FEV1, FVC, and FEV1/FVC ratio) linear regression models were fitted in TESAOD and ECRHS-Sp separately. If coefficients associated with having high YKL-40 were found not to be different between TESAOD and ECRHS-Sp by heterogeneity test, combined estimates were obtained by meta-analysis.
In TESAOD, YKL-40 was measured at baseline and could be studied prospectively in relation to decline of lung function. In order to study the effects of elevated YKL-40 on subsequent decline of lung function while adjusting for the intra-subject serial correlation of repeated observations, we used random coefficients models18 that included covariates and an interaction term between YKL-40 quartiles and years of follow-up to predict FEV1, FVC, and the ratio FEV1/FVC, respectively. This interaction term tested whether the decline of the specific lung function index differed between subjects in the top YKL-40 quartile and subjects in the other YKL-40 quartiles. An unstructured covariance structure was chosen for all models. To rule out that YKL-40 effects on subsequent decline of lung function were due to effects on initial levels of lung function, we also ran mixed models for each lung function index by excluding observations from survey 1 from the dependent variable and including % predicted values of the specific lung function index at the baseline survey among covariates.
A group variable based on the combination of YKL-40 (top quartile versus the other three quartiles combined) and smoking (ever versus never) was also generated and tested in random coefficients models in order to depict graphically expected levels and decline of FEV1 over the TESAOD study follow-up across the four groups.
Results
Demographic, behavioral, and clinical characteristics of TESAOD participants are summarized in Table I. Overall, the study participants had a mean age of 46 years and included 59% females, 60% ever smokers, and 11% asthmatics. The mean and median levels of serum YKL-40 were 46.5 and 34.0 ng/ml, respectively.
Table I.
Baseline characteristics of TESAOD participants at the time of blood collection.
| N | 1088 |
|
| |
| Females: N (%) | 639/1088 (58.7%) |
|
| |
| Age: mean; range in years | 46 (21 – 70) |
|
| |
| Body Mass Index: N (%) | |
| Under-weight (< 18.5 Kg/m2) | 21 (2.0%) |
| Normal-weight (≥18.5, < 25 Kg/m2) | 612 (58.2%) |
| Over-weight (≥ 25, < 30 Kg/m2) | 344 (32.7%) |
| Obese (≥ 30 Kg/m2) | 75 (7.1%) |
|
| |
| Smoking status: N (%) | |
| Never | 431 (39.7%) |
| Former | 268 (24.7%) |
| Current | 388 (35.7%) |
|
| |
| Smoke intensity in pack-years: N (%) | |
| 0 pack-years | 431 (39.7%) |
| >0; ≤10 pack-years | 210 (19.3%) |
| >10; ≤20 pack-years | 141 (13.0%) |
| >20; ≤40 pack-years | 170 (15.7%) |
| >40 packyears | 134 (12.3%) |
|
| |
| Physician-confirmed asthma: N (%) | |
| Never | 965 (88.8%) |
| Inactive | 52 (4.8%) |
| Active | 70 (6.4%) |
|
| |
| Atopy*: N (%) | 412/1075 (38.3%) |
|
| |
| Spirometric patterns: N (%) | |
| Normal | 846 (77.8%) |
| Restrictive | 114 (10.5%) |
| Obstructive | 128 (11.8%) |
|
| |
| FEV1 % predicted: mean; SD | 94.8% (18) |
|
| |
| FVC % predicted: mean; SD | 97.5% (17) |
|
| |
| FEV1/FVC ratio %: mean; SD | 80.2% (10) |
|
| |
| YKL40: mean; median; IQR in ng/ml | 46.5; 34.0; 23.5 – 54.3 |
defined as having a skin prick test wheal ≥ 2 mm larger than control for ≥ one of 5 tested allergens
Associations of serum YKL-40 with demographic factors and smoking behavior are summarized in Table E2. YKL-40 levels correlated strongly and significantly with age (Spearman correlation coefficient 0.41, p<0.001) and with pack-years (0.26, p<0.001).
Cross-sectional associations of YKL-40 with asthma and lung function in TESAOD
No cross-sectional significant associations were found between YKL-40 levels and physician confirmed asthma (Table E3). Among asthmatics, YKL-40 levels were not affected by disease activity, age at onset, or atopic status. However, serum YKL-40 levels were higher in asthmatics with lung function deficits (i.e., FEV1 % predicted < 80%) than in subjects with no asthma or asthmatics with no lung function deficits.
YKL-40 levels were higher in participants with spirometric restrictive and moderate/severe obstructive patterns than in subjects with normal spirometry (Figure E2a). Consistent with this observation, serum YKL-40 levels correlated significantly and inversely with concomitant FEV1 % predicted (Spearman correlation coefficient: −0.12; p<0.001), FVC % predicted (−0.13; p<0.001), and FEV1/FVC levels (−0.12; p<0.001).
Replication of cross-sectional associations in ECRHS-Sp
Characteristics of ECRHS-Sp participants are given in Table E1. As compared with TESAOD, the ECRHS-Sp study population was on average 5-years younger and had a narrower age range and a higher BMI. However, the two populations had relatively comparable rates of ever smoking, heavy smoking, and physician-confirmed asthma. Like in TESAOD, also in ECRHS-Sp serum YKL-40 levels correlated with age and pack-years (Table E2). In ECRHS-Sp, higher YKL-40 levels were also found in males and current smokers.
The findings of increased YKL-40 levels in asthmatics with lung function deficits and in subjects with abnormal spirometric patterns that were observed in TESAOD were replicated in ECRHSSp (Table E3 and Figure E2b). Similar to TESAOD, in ECRHS-Sp serum YKL-40 levels correlated inversely with FEV1 % predicted (Spearman correlation coefficient: −0.21; p<0.001), FVC % predicted (−0.19; p<0.001), and FEV1/FVC levels (−0.17; p<0.001). In analyses by YKL-40 quartiles, in both cohorts subjects in the top YKL-40 quartile had lower FEV1% predicted, FVC % predicted, and FEV1/FVC levels as compared with subjects in the other three quartiles (Tables II and E4). Although adjustment for potential confounders reduced the strength of these associations, in fully adjusted meta-analyses subjects in the highest YKL-40 quartile were still found to have significant deficits in both FEV1 and FVC % predicted.
Table II.
Cross-sectional associations between YKL-40 quartiles and lung function in TESAOD and ECRHS-Sp populations.
| β coefficients (95% CIs; p value) from regression models | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted | Fully adjusted* | |||||
|
| ||||||
| TESAOD (N = 1088) | ECRHS-Sp (N = 854) | Meta-analysis (N = 1942) | TESAOD (N = 1085) | ECRHS-Sp (N = 844) | Meta-analysis (N = 1929) | |
|
| ||||||
| FEV1 % predicted | ||||||
| 1st YKL-40 quartile | 6.8% (3.8, 9.8) | 8.6% (5.8, 11.3) | 7.8% (5.8, 9.8) | 2.6% (−0.5, 5.7) | 3.5% (0.8, 6.3) | 3.1% (1.0, 5.2) |
| 2nd YKL-40 quartile | 5.6% (2.6, 8.7) | 7.2% (4.4, 9.9) | 6.5% (4.4, 8.5) | 2.0% (−1.0, 5.1) | 4.0% (1.3, 6.6) | 3.1% (1.1, 5.2) |
| 3rd YKL-40 quartile | 5.0% (2.0, 8.0) | 4.5% (1.7, 7.2) | 4.7% (2.7, 6.7) | 1.6% (−1.4, 4.5) | 2.5% (−0.1, 5.1) | 2.1% (0.1, 4.0) |
| 4th YKL-40 quartile | Reference | Reference | Reference | Reference | Reference | Reference |
|
| ||||||
| FVC % predicted | ||||||
| 1st YKL-40 quartile | 6.1% (3.4, 8.9) | 7.1% (4.7, 9.5) | 6.7% (4.9, 8.5) | 3.4% (0.4, 6.4) | 3.9% (1.4, 6.4) | 3.7% (1.8, 5.6) |
| 2nd YKL-40 quartile | 4.9% (2.1, 7.7) | 5.3% (2.9, 7.7) | 5.1% (3.3, 6.9) | 2.5% (−0.4, 5.4) | 3.0% (0.6, 5.4) | 2.8% (1.0, 4.6) |
| 3rd YKL-40 quartile | 4.5% (1.8, 7.3) | 3.0% (0.6, 5.3) | 3.6% (1.9, 5.4) | 2.4% (−0.4, 5.2) | 1.6% (−0.7, 4.0) | 1.9% (0.1, 3.7) |
| 4th YKL-40 quartile | Reference | Reference | Reference | Reference | Reference | Reference |
|
| ||||||
| FEV1/FVC in % | ||||||
| 1st YKL-40 quartile | 3.6% (2.0, 5.2) | 3.1% (1.8, 4.5) | 3.3% (2.3, 4.3) | −0.2% (−1.8, 1.4) | 0% (−1.3, 1.3) | −0.1% (−1.1, 0.9) |
| 2nd YKL-40 quartile | 2.8% (1.2, 4.4) | 3.2% (1.8, 4.5) | 3.0% (2.0, 4.1) | −0.1% (−1.7, 1.4) | 1.0% (−0.3, 2.3) | 0.6% (−0.3, 1.5) |
| 3rd YKL-40 quartile | 2.3% (0.7, 3.9) | 2.4% (1.1, 3.8) | 2.4% (1.3, 3.4) | −0.2% (−1.7, 1.3) | 0.9% (−0.3, 2.1) | 0.5% (−0.4, 1.4) |
| 4th YKL-40 quartile | Reference | Reference | Reference | Reference | Reference | Reference |
coefficients correspond to unadjusted and adjusted differences in lung function parameters for subjects in the first, second, and third YKL-40 quartile as compared with subjects in the highest quartile, respectively. Results are from simple and multivariate regression models predicting FEV1 % predicted, FVC % predicted, and the FEV1/FVC ratio, respectively. Lung function indices are from the same survey when blood for YKL-40 measurements was collected (i.e., survey 1 in TESAOD and survey 2 in ECRHS-Sp).
adjusted for sex, age, smoking status, pack-years, BMI categories, and asthma for TESAOD, adjusted for sex, age, smoking status, pack-years, BMI categories, asthma, sample type (random versus enriched), and center for ECRHS-Sp
YKL-40 and decline of lung function in TESAOD
The TESAOD participants included in this study completed on average 6.8 lung function tests (SD: 3; range: 2 to 12) over a mean follow-up of 13.5 years (SD: 7, range: 1 to 23 years). A total of 7325 lung function observations were used in mixed models to determine the effects of YKL-40 on decline of FEV1, FVC, and FEV1/FVC ratio.
In mixed models adjusted for sex, age, height, BMI categories, smoking status, pack-years, and asthma, increasing rates of lung function decline were evident across YKL-40 quartiles (Table III), with participants in the top YKL-40 quartile at baseline having an FEV1 decline that was 5 ml/yr (p=0.046) faster than subjects in the third quartile, 5 ml/yr (p=0.02) faster than subjects in the second quartile, and 10 ml/yr (p<0.001) faster than subjects in the lowest YKL-40 quartile. As compared with the third, second, and first quartile, respectively, the corresponding estimates for the accelerated decline associated with the top YKL-40 quartile were 3 ml/yr (p=0.23), 5 ml/yr (p=0.04), and 11 ml/yr (p<0.001) for FVC; and 0.11 %/yr (p=0.009), 0.08 %/yr (p=0.07), and 0.10 %/yr (p=0.03) for the FEV1/FVC ratio (Table III). When the top YKL-40 quartile was contrasted against the other three quartiles combined (Table E5), after adjusting for all covariates the top YKL-40 quartile was found to be associated with a 7 ml/yr (p<0.001) faster FEV1 decline, a 7 ml/yr (p=0.003) faster FVC decline, and a 0.10 %/yr (p=0.008) faster FEV1/FVC decline during the study follow-up. Significant YKL-40 effects on decline of lung function were confirmed after further adjustment for levels of lung function at the baseline survey (data not shown). Although the cut-off of the top YKL-40 quartile was chosen a priori for statistical analyses, the effects of alternative YKL-40 cut-offs on FEV1 decline were tested in exploratory analyses and a trend from the top tertile to the top decile was found (Figure E3), despite differences in effects and sample size in some of the groups were quite small.
Table III.
Longitudinal associations between YKL-40 at baseline and lung function decline during follow up in the TESAOD cohort.
Coefficients (95% CIs) correspond to adjusted differences between subjects in the first, second, and third YKL-40 quartile as compared with subjects in the highest quartile (ref category) in rate of decline of FEV1, FVC, and FEV1/FVC ratio during the study follow-up in the total population, among never smokers, and ever smokers.
| Differences in rate of lung function decline between each of the other three quartiles and the highest YKL-40 quartile (positive coefficient indicates slower decline as compared with the highest YKL-40 quartile) | |||
|---|---|---|---|
|
| |||
| Total population N subjects=1085 N observations=7325 | Never smokers N subjects=431 N observations=3026 | Smokers N subjects=654 N observations=4299 | |
|
| |||
| Fully adjusted* coefficients (95% CI) | Fully adjusted** coefficients (95% CI) | Fully adjusted* coefficients (95% CI) | |
|
| |||
| Differences in FEV1 decline in ml/yr | |||
| 1st YKL-40 quartile | 10 (5, 14) ml/yr p < 0.001 | 5 (0, 10) ml/yr p = 0.06 | 13 (6, 19) ml/yr p < 0.001 |
| 2nd YKL-40 quartile | 5 (1, 10) ml/yr p = 0.02 | −1 (−6, 5) ml/yr p = 0.78 | 9 (3, 16) ml/yr p = 0.004 |
| 3rd YKL-40 quartile | 5 (0, 9) ml/yr p = 0.046 | 2 (−4, 8) ml/yr p = 0.46 | 6 (0, 13) ml/yr p = 0.049 |
| 4th YKL-40 quartile | Reference | Reference | Reference |
|
| |||
| Differences in FVC decline in ml/yr | |||
| 1st YKL-40 quartile | 11 (6, 16) ml/yr p < 0.001 | 7 (0, 14) ml/yr p = 0.04 | 14 (6, 21) ml/yr p < 0.001 |
| 2nd YKL-40 quartile | 5 (0, 11) ml/yr p = 0.04 | 0 (−7, 7) ml/yr p = 0.97 | 10 (2, 17) ml/yr p = 0.01 |
| 3rd YKL-40 quartile | 3 (−2, 9) ml/yr p = 0.23 | 0 (−7, 8) ml/yr p = 0.92 | 5 (−2, 13) ml/yr p = 0.15 |
| 4th YKL-40 quartile | Reference | Reference | Reference |
|
| |||
| Differences in FEV1/FVC decline in %/yr | |||
| 1st YKL-40 quartile | 0.10 (0.01, 0.18) %/yr p = 0.03 | 0.05 (− 0.06, 0.15) %/yr p = 0.41 | 0.11 (− 0.01, 0.24) %/yr p = 0.07 |
| 2nd YKL-40 quartile | 0.08 (−0.01, 0.16) %/yr p = 0.07 | 0.02 (− 0.09, 0.13) %/yr p = 0.74 | 0.11 (− 0.01, 0.23) %/yr p = 0.07 |
| 3rd YKL-40 quartile | 0.11 (0.03, 0.20) %/yr p = 0.009 | 0.10 (− 0.01, 0.21) %/yr p = 0.08 | 0.12 (0, 0.24) %/yr p = 0.051 |
| 4th YKL-40 quartile | Reference | Reference | Reference |
from mixed models adjusted for sex, age at enrollment, height, smoking status, pack-years, BMI categories, and asthma
from mixed models adjusted for sex, age at enrollment, height, BMI categories, and asthma
When analyses were stratified by smoking, the effects on subsequent decline of lung function across the four YKL-40 quartiles were found to be stronger among smokers than never smokers (Table III). In the former, after adjusting for covariates, being in the highest YKL-40 quartile at baseline was associated with a 13 ml/yr (p<0.001) faster FEV1 decline, a 14 ml/yr (p<0.001) faster FVC decline, and a 0.11 %/yr (p=0.07) faster FEV1/FVC decline as compared with subjects in the lowest quartile (Table III), and with a 9 ml/yr (p=0.001) faster FEV1 decline, a 9 ml/yr (p=0.003) faster FVC decline, and a 0.11 %/yr (p=0.03) faster FEV1/FVC decline as compared with subjects in the other three quartiles combined (Table E5). In contrast, among never smokers serum YKL-40 was not significantly associated with decline in any of the lung function indices (Tables III and E5). No interactive effects between YKL-40 and age at enrollment were found on subsequent decline of lung function (data not shown).
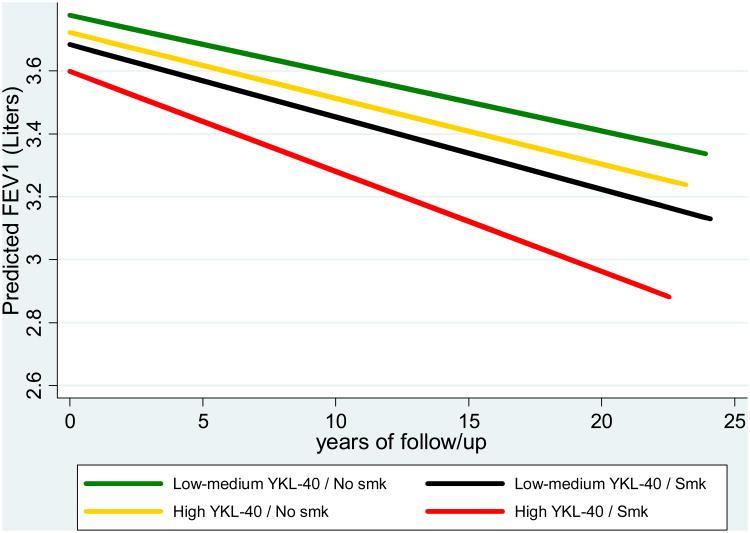
Consistent with the findings above, fully adjusted mixed models showed that smokers with high YKL-40 had a decline of lung function during the study follow-up that was significantly faster than that of any other group (i.e., smokers with low-medium YKL-40, non-smokers with high YKL-40, and non-smokers with low-medium YKL-40) (Figure 1).
Figure 1.
FEV1 levels at baseline and decline during the study follow-up as predicted from fully adjusted random coefficients models for TESAOD groups of subjects with low-medium YKL-40 (i.e., three bottom quartiles combined) who never smoked (green line; number of subjects = 333; number of observations = 2385), subjects with low-medium YKL-40 who smoked (yellow line; number of subjects = 481; number of observations = 3238), subjects with high YKL-40 (i.e., top quartile) who never smoked (black line; number of subjects = 98; number of observations = 641), and subjects with high YKL-40 who smoked (red line; number of subjects = 173; number of observations = 1061).
Depicted values represent predicted values for a 175-cms tall male who was 45 years old at baseline and had no asthma and normal weight.
- - “Low-medium YKL-40/Smk” steeper than “Low-medium YKL-40/No smk”; p = 0.008
- - “High YKL-40/Smk” steeper than “Low-medium YKL-40/No smk”; p < 0.001
- - “High YKL-40/Smk” steeper than “Low-medium YKL-40/Smk”; p < 0.001
- - “High YKL-40/Smk” steeper than “High YKL-40/No smk”; p = 0.001
Discussion
This is the first prospective study to address serum YKL-40 in relation to levels and decline of lung function. Our findings show that, cross-sectionally, serum YKL-40 is consistently associated with both FEV1 and FVC deficits in the general population and that these associations are partially independent of other known predictors of lung function, including asthma. Most importantly, they indicate that longitudinally subjects with elevated YKL-40 have a decline of lung function that is faster than that of subjects with low or medium YKL-40 and that these effects are synergistic with those of cigarette smoking. Both in cross-sectional and prospective analyses, the magnitude of YKL-40 associations was stronger with FEV1 and FVC values than with the FEV1/FVC ratio.
Subjects who both smoked and had elevated YKL-40 at baseline experienced the steepest decline of FEV1 and FVC during the study follow-up, with an estimated additional FEV1 decline of 9 ml/yr compared with smokers with low-medium YKL-40 and 13 ml/yr compared with smokers in the lowest YKL-40 quartile after adjusting for covariates that included pack-years as a quantitative assessment of smoking exposure. Although the impact of smoking on FEV1 decline is highly dependent on the number of cigarettes smoked per day, a systematic review estimated that continuing smoking was associated on average with an 11 ml/yr increase in FEV1 decline19. These observations suggest that the combined lung function deficits associated with smoking and high YKL-40 may be clinically important and that – in conjunction with other known determinants of risk prediction – elevated serum YKL-40 may prove helpful in identifying smokers who are susceptible to the effects of smoking on lung health. Although secondary analyses in our study suggested that YKL-40 cut-offs higher than the top quartile may be more powerful in predicting subsequent decline of lung function, specifically designed studies will be required to conclusively establish optimal YKL-40 cut-off levels for risk prediction.
The YKL-40 effects on decline of lung function were at least partly independent of asthma because they held true after adjustment for asthma. Indeed, in our two population-based cohorts – in which a substantial part of asthma cases are expected to be mild – we did not find an association between increased serum YKL-40 and asthma per se, whereas we did find YKL-40 to be elevated in those asthma cases who presented with concomitant lung function deficits. Previous studies4,6,7,9 have associated YKL-40 levels with several markers of asthma severity, including lung function deficits. Similarly in the original study by Chupp and colleagues4, after adjusting for covariates, subjects with moderate and severe asthma, but not those with mild asthma, had higher serum YKL-40 than controls. All these observations are consistent with the possibility that YKL-40 is a biomarker of severity of asthma. Whether YKL-40 is also associated with lung function deficits that may precede adult onset asthma20 and in turn it may be useful in risk prediction of asthma incidence remains to be determined.
Although our findings are based on observational data, several studies suggest that smoking exposure directly increases expression of YKL-40 and implicate YKL-40 as a potential mediator of the lung inflammatory response to cigarette smoke. First, murine models have demonstrated over-expression of breast regression protein 39 (BRP-39, the murine equivalent of YKL-40) in alveolar macrophages and airway epithelial cells following cigarette smoke exposure11,21. Second, stimulation of alveolar macrophages with TNF-α promoted release of YKL-40 and this effect was enhanced in smokers – particularly those with COPD. Increased production of inflammatory and fibrogenic mediators by alveolar macrophages stimulated with YKL-40 was also preferentially observed in cells obtained from smokers with COPD10. Finally BRP-39-/-mice showed a blunted inflammatory response following chronic exposure to cigarette smoking11, although they also had an enhanced alveolar destruction which might suggest a protective role of either BRP-39 or other aspects of the blunted inflammatory response in regard to alveolar destruction.
Yet, whether YKL-40 is causally or indirectly implicated in smoking-related damage to the lungs remains unknown. It also remains to be determined if the involved mechanisms are independent of other alternative pathways – such as allergen induced Th2 inflammation22 and bronchial smooth muscle remodeling23,24 – that have been proposed for effects on asthma severity. YKL-40 has been linked to severity and outcomes in a number of other diseases characterized by inflammation and tissue remodeling (reviewed by Lee et al3) and with all-cause mortality in the general population25,27. While this observation argues against YKL-40 being a lung specific biomarker, it suggests that this protein might be over-expressed in smokers with COPD who also have other inflammation-related comorbidities frequently linked to this disease28,29. This hypothesis was not addressed in our study.
As is the case for most large epidemiological biomarker studies, only one measurement at a single point in time was available for YKL-40 from each of our cohorts. Although previous studies have shown the within-subject coefficient of variation of serum YKL-40 levels over 3-4 years to be below 40%4,30, the informative value of YKL-40 temporal trends in risk prediction warrants future studies.
In conclusion, we found that serum YKL-40 is a biomarker linked to lung function deficit and decline in the general population, particularly in smokers. Being in the top YKL-40 quartile increased the rate of FEV1 decline by 13 ml per year among smokers, as compared with smokers in the lowest YKL-40 quartile. These findings implicate YKL-40 as a potential biomarker of susceptibility to the long-term effects of cigarette smoking on lung function decline and warrant further evaluation in the preventive and clinical setting.
Supplementary Material
Acknowledgments
This study was supported by awards HL107188 and HL095021 from the National Heart, Lung, and Blood Institute; and by FIS award PS09/01354 from the Instituto de Salud Carlos III. IL was the recipient of a fellowship by the Environmental Health Fund.
Footnotes
This article has an online data supplement
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Taylor DR. Using biomarkers in the assessment of airways disease. J Allergy Clin Immunol. 2011;128(5):927–34. doi: 10.1016/j.jaci.2011.03.051. quiz 35-6. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Donohue JF, Rennard SI. The future of chronic obstructive pulmonary disease treatment-- difficulties of and barriers to drug development. Lancet. 2011;378(9795):1027–37. doi: 10.1016/S0140-6736(11)61047-7. [DOI] [PubMed] [Google Scholar]
- 3.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357(20):2016–27. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 5.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–91. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang H, Fang Z, Sun Y, Li B, Shi Z, Chen J, et al. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur Respir J. 2010;35(4):757–60. doi: 10.1183/09031936.00034409. [DOI] [PubMed] [Google Scholar]
- 7.Goldman DL, Li X, Tsirilakis K, Andrade C, Casadevall A, Vicencio AG. Increased chitinase expression and fungal-specific antibodies in the bronchoalveolar lavage fluid of asthmatic children. Clin Exp Allergy. 2011 doi: 10.1111/j.1365-2222.2011.03886.x. [DOI] [PubMed] [Google Scholar]
- 8.Specjalski K, Jassem E. YKL-40 protein is a marker of asthma. J Asthma. 2011;48(8):767–72. doi: 10.3109/02770903.2011.611955. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka K, Matsumoto H, Niimi A, Muro S, Ito I, Takeda T, et al. Sputum YKL-40 Levels and Pathophysiology of Asthma and Chronic Obstructive Pulmonary Disease. Respiration. 2011 doi: 10.1159/000330840. [DOI] [PubMed] [Google Scholar]
- 10.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181(7):5167–73. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura H, Hartl D, Kang MJ, Dela Cruz CS, Koller B, Chupp GL, et al. Role of breast regression protein-39 in the pathogenesis of cigarette smoke-induced inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44(6):777–86. doi: 10.1165/rcmb.2010-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakazaki Y, Hoshino T, Takei S, Sawada M, Oda H, Takenaka S, et al. Overexpression of chitinase 3- like 1/YKL-40 in lung-specific IL-18-transgenic mice, smokers and COPD. PLoS One. 2011;6(9):e24177. doi: 10.1371/journal.pone.0024177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102(2):137–52. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- 14.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 15.The European Community Respiratory Health Survey II. Eur Respir J. 2002;20(5):1071–9. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- 16.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow- volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Brown H, Prescott R. Applied mixed models in medicine. Chichester, UK: John Wiley & Sons, LTD; 2001. [Google Scholar]
- 19.Lee PN, Fry JS. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med. 2010;8:84. doi: 10.1186/1741-7015-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anto JM, Sunyer J, Basagana X, Garcia-Esteban R, Cerveri I, de Marco R, et al. Risk factors of new- onset asthma in adults: a population-based international cohort study. Allergy. 2010;65(8):1021–30. doi: 10.1111/j.1398-9995.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 21.Nikota JK, Botelho FM, Bauer CM, Jordana M, Coyle AJ, Humbles AA, et al. Differential expression and function of breast regression protein 39 (BRP-39) in murine models of subacute cigarette smoke exposure and allergic airway inflammation. Respir Res. 2011;12:39. doi: 10.1186/1465-9921-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206(5):1149–66. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J. 2010;36(5):1174–84. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 24.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, et al. Role of YKL-40 in Bronchial Smooth Muscle Remodeling in Asthma. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201105-0915OC. [DOI] [PubMed] [Google Scholar]
- 25.Johansen JS, Bojesen SE, Tybjaerg-Hansen A, Mylin AK, Price PA, Nordestgaard BG. Plasma YKL-40 and total and disease-specific mortality in the general population. Clin Chem. 2010;56(10):1580–91. doi: 10.1373/clinchem.2010.146530. [DOI] [PubMed] [Google Scholar]
- 26.Johansen JS, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK, Bruunsgaard H. High serum YKL-40 level in a cohort of octogenarians is associated with increased risk of all-cause mortality. Clin Exp Immunol. 2008;151(2):260–6. doi: 10.1111/j.1365-2249.2007.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143(1):35–42. doi: 10.1016/j.ijcard.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–85. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 29.Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139(1):165–73. doi: 10.1378/chest.10-1252. [DOI] [PubMed] [Google Scholar]
- 30.Johansen JS, Lottenburger T, Nielsen HJ, Jensen JE, Svendsen MN, Kollerup G, et al. Diurnal, weekly, and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2603–8. doi: 10.1158/1055-9965.EPI-07-2766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.