Abstract
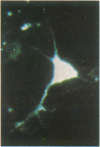
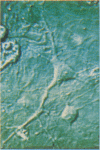
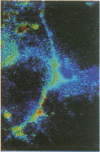
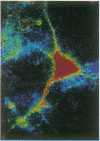
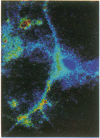
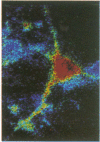
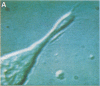
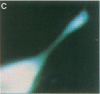
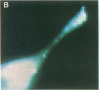
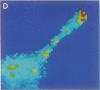
Intracellular free calcium levels have been measured in cultured central nervous system (CNS) cells by using the fluorescent indicator fura-2 and digital imaging techniques. Cells were plated from rat embryo diencephalon (embryonic day 17 or 18), with nearly all of the cells surviving dissociation having undergone final mitosis within the previous 24 hr. The initially spherical cells were observed within the first 24 hr in culture when they were extending processes but had not established a network of fibers that would prevent the identification of the origin of a given fiber. Cells that were rapidly extending showed high Ca2+ levels in the regions of growth. Where processes had just emerged from the soma or where growth was proceeding from more than one pole, Ca2+ levels were uniform and estimated levels of 500 nM were commonly seen. In active growth cones distant from the soma, Ca2+ levels exceeded 200 nM, whereas the soma levels were in the 60-80 nM range. Nonextended and extended cells that had stalled had uniform Ca2+ levels in the range of 30-70 nM. The results show that high Ca2+ levels are at least a correlate of extension in CNS cells and that under some conditions the region of high calcium can be localized to a small part of the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Walker P. S., Fellows R. E. Properties of neurons from dissociated fetal rat brain in serum-free culture. J Neurosci. 1983 Dec;3(12):2448–2462. doi: 10.1523/JNEUROSCI.03-12-02448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Anglister L., Farber I. C., Shahar A., Grinvald A. Localization of voltage-sensitive calcium channels along developing neurites: their possible role in regulating neurite elongation. Dev Biol. 1982 Dec;94(2):351–365. doi: 10.1016/0012-1606(82)90353-0. [DOI] [PubMed] [Google Scholar]
- Freeman J. A., Manis P. B., Snipes G. J., Mayes B. N., Samson P. C., Wikswo J. P., Jr, Freeman D. B. Steady growth cone currents revealed by a novel circularly vibrating probe: a possible mechanism underlying neurite growth. J Neurosci Res. 1985;13(1-2):257–283. doi: 10.1002/jnr.490130118. [DOI] [PubMed] [Google Scholar]
- Graubard K., Ross W. N. Regional distribution of calcium influx into bursting neurons detected with arsenazo III. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5565–5569. doi: 10.1073/pnas.82.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Farber I. C. Optical recording of calcium action potentials from growth cones of cultured neurons with a laser microbeam. Science. 1981 Jun 5;212(4499):1164–1167. doi: 10.1126/science.7233210. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Keith C. H., Ratan R., Maxfield F. R., Bajer A., Shelanski M. L. Local cytoplasmic calcium gradients in living mitotic cells. 1985 Aug 29-Sep 4Nature. 316(6031):848–850. doi: 10.1038/316848a0. [DOI] [PubMed] [Google Scholar]
- Kruskal B. A., Keith C. H., Maxfield F. R. Thyrotropin-releasing hormone-induced changes in intracellular [Ca2+] measured by microspectrofluorometry on individual quin2-loaded cells. J Cell Biol. 1984 Sep;99(3):1167–1172. doi: 10.1083/jcb.99.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]