Abstract
Objectives
Published data have reported that components of the peripheral blood are significant prognostic factors in hematologic and solid malignancies. Thus, we sought to investigate if the pre-operative absolute lymphocyte count (ALC) and absolute monocyte count (AMC) affects disease progression and survival after complete surgical resection of advanced malignant melanoma.
Methods
We retrospectively reviewed records of 227 patients with resected advanced malignant melanoma (153 stage III and 74 stage IV) that were treated at the Mayo Clinic from 2000 to 2010. Survival analysis was performed using the Kaplan-Meier method, log-rank tests, and the Cox proportional hazards model for the univariate and multivariate analysis.
Results
Surgically resected stage III melanoma patients with a pre-operative AMC < 0.6 × 109/L experienced a longer overall survival (OS) versus AMC ≥ 0.6 × 109/L (median: 63.9 vs. 34.8 months, respectively, P < 0.008). Multivariate analysis showed AMC to be an independent predictor for OS in stage III patients. Stage IV resected melanoma patients with an ALC ≥ 1.9 × 109/L experienced a superior median relapse-free survival (RFS) compared to patients with an ALC < 1.9 × 109/L (median: 11.4 months vs. 5.4 months, respectively, P < 0.006). Multivariate analysis showed ALC to be an independent predictor for RFS in stage IV patients.
Conclusions
These data showed, that in surgically resected stage III melanoma, pre-operative AMC is an independent prognostic factor OS. In contrast, a higher pre-operative ALC is an independent prognostic for longer RFS in surgically resected stage IV melanoma.
Keywords: malignant melanoma, advanced stage, absolute lymphocyte count, absolute monocyte count, survival
Introduction
Advanced malignant melanoma remains a major source of mortality despite recent advances in treatment. In the United States, approximately 9,400 individuals will die from malignant melanoma in 2013 (1.6% of cancer-related deaths) 1. Current prognostic factors are based on the American Joint Committee on Cancer (AJCC) 7th edition TNM staging system, which incorporates information about the primary tumor thickness, presence of ulceration, number of lymph nodes affected, and distant sites of metastases 2. Melanoma progression and subsequent distant spread are believed to be at least in part regulated by host immunity (tumor micro-environment 3–6, the sentinel lymph node 7, and systemically 8). Interestingly, despite the recognition of the relevance of the immune system in melanoma biology, there is currently no routine use of biomarkers to reflect a host’s immune response to cancer.
An inexpensive and clinically utilized estimate of systemic immunity in humans is the absolute concentration of peripheral blood lymphocytes. The absolute lymphocyte count (ALC) at the time of diagnosis has been identified as an independent prognostic factor for survival in multiple hematologic malignancies 9–12, and some solid tumors 13. Similarly, the absolute monocyte count (AMC), another peripheral blood biomarker of immune competence, has also been reported as a negative prognostic factor in several cancers 11,12,14,15. In melanoma, both ALC and AMC appear to impact clinical outcomes in patients with unresectable disseminated metastatic melanoma who have been treated with immunotherapy 14,16. In these studies, patients with normal or increased lymphocyte count and decreased monocyte count in the peripheral blood appear to have better clinical outcomes relative to those that do not. In this same regard, patients that are able to undergo surgical resection of all metastatic disease can also experience excellent outcomes, despite no additional therapy. However, the prognostic significance of pre-operative ALC or AMC in resectable melanoma has not been studied. An inexpensive biomarker of immune competence may improve patient selection for metastectomy and adjuvant therapy. Thus, we postulate that immune competence may play an important role in the clinical outcomes of patients undergoing complete surgical resection of advanced melanoma. Therefore we conducted a retrospective study to assess the prognostic significance of pre-operative ALC and AMC, in patients with resected advanced melanoma.
Materials and Methods
Study population
Patients with complete resected stage III or stage IV melanoma who were followed at Mayo Clinic, Rochester, Minnesota from 2000 through 2010 were considered for study participation. All study subjects had a pathology report available for review, with confirmation of melanoma. Staging was assigned based on the American Joint Committee on Cancer (AJCC) 7th edition TNM staging system 2. The stage III cohort included patients with an initial diagnosis of stage III, as well as patients that had for the first time a loco-regional recurrence. Of the 246 eligible patients for the study, 19 patients were excluded for the following reasons: 4 had a history of organ transplant and were taking several immunosuppressive therapies, 3 had a diagnosis of pancytopenia, 2 were in chronic immunosuppressive treatment for an autoimmune disease, and 10 patients had a concomitant malignant diagnosis, such as lymphoma or pancreatic cancer. Thus, our study sample included 227 evaluable patients (153 stage III and 74 stage IV) who had undergone complete resection of all clinically or radiologically evident disease. For patients with multiple resections, only the first date of resection was used. Demographic, clinical and pathological data were collected and manage using REDCap electronic data capture tools hosted at the Mayo Clinic17. All patients had previously given consent for their medical record to be reviewed. Study approval was obtained from the Mayo Clinic Institutional Review Board.
End points
The primary endpoint for this study was to determine the prognostic significance of the pre-operative ALC and AMC values in patients undergoing complete surgical resection of high-risk melanoma with respect to relapse-free survival (RFS) and overall survival (OS). The ALC and AMC were obtained from a standardized complete blood count (CBC) prior to surgical resection. The secondary endpoint was to determine whether the ALC and AMC before complete resection of stage III/IV disease was an independent prognostic factor for RFS and OS in melanoma patients. RFS was measured from the date of surgery to the date of relapse, death or last follow-up. OS was measured from date of surgery to the date of death or last follow-up.
Statistical Analysis
Continuous variables were summarized with means, standard deviations, and ranges; while categorical variables were summarized with frequency counts and percentages. A separate analysis was performed for the stage III and stage IV cohorts, due to heterogeneity in the extent of disease. RFS and OS were estimated using the Kaplan-Meier method. Differences in survival curves were tested for statistical significance using the two-tailed log rank test. The ALC and AMC were analyzed as both continuous and dichotomized variables. The optimal cut-off point for each cohort (stage III and stage IV) was based on the χ2 from the long-rank statistics that yielded the greatest difference in survival of RFS and OS, when analyzing different cut-off points from the interquartile range (25th–75th) for the pre-surgical ALC and AMC. The Cox proportional hazards model was used for the univariate and multivariate analysis to evaluate the ALC and AMC as prognostic factors for RFS and OS. All prognostic factors with a P-value < 0.2 in the univariate analysis were included in the multivariate analysis. P-values were not adjusted for multiple comparisons. All tests were two-sided and P-values < 0.05 were considered statistically significant.
Results
Patients characteristics
Two-hundred twenty-seven patients who had undergone complete resection of their metastatic melanoma (153 stage III and 74 stage IV) qualified for the study (Table 1). The median age at the time of resection for patients with stage III melanoma was 59 years (range: 18–88 years). Of these, 63% of patients were men. The median follow-up for this group was 30 months (range: 2–126 months). The median age at time of resection for the 74 patients with stage IV metastatic melanoma was 56 years (range: 26–91 years) and 69% were male. The median follow-up for this cohort was 24 months (range: 2–117 months). In terms of previous treatment, 31% had received granulocyte-macrophage colony stimulating factor (GM-CSF), 12% had received prior interferon-α (IFN), 4% had participated in a vaccine trial, and 4% had received prior chemotherapy. The majority of patients with stage IV disease had only one distant metastatic lesion (85%), with only 14% having two organs involved and only one patient (1%) had three organ involvement. Additional patient characteristics are summarized in Table 1.
Table 1.
Summary of features for patients 153 with stage III melanoma and 74 patients with stage IV melanoma
| Stage III Melanoma | Stage IV Melanoma | ||
|---|---|---|---|
| Feature | Mean ± SD (Median; Range) | Feature | Mean ± SD (Median; Range) |
| Age at resection (years) | 58.4 ± 15.8 (59; 18 – 88) | Age at resection (years) | 55.5 ± 13.8 (56; 26 – 91) |
| Breslow thickness (mm) | 3.8 ± 3.2 (2.8; 0.5 – 20.0) | Breslow thickness (mm) | 3.0 ± 3.4 (2.0; 0.5 – 20.0) |
| Number of positive lymph nodes | 3 ± 3 (1; 0 – 20) | Disease-free interval (months)a | 25.0 ± 41.2 (12.8; 0 – 211.4) |
| Sex | N (%) | Sex | N (%) |
| Female | 56 (37) | Female | 23 (31) |
| Male | 97 (63) | Male | 51 (69) |
| Primary site | Primary site | ||
| Head/Neck | 45 (29) | Head/Neck | 21 (28) |
| Trunk | 36 (24) | Trunk | 21 (28) |
| Lower extremity | 36 (24) | Upper extremity | 12 (16) |
| Upper extremity | 9 (6) | Lower extremity | 5 (7) |
| Unknown | 27 (18) | Subungual | 2 (3) |
| Unknown | 13 (18) | ||
| Sub-stage at resection | Site of resectionb | ||
| IIIA | 39 (25) | Lung | 31 (42) |
| IIIB | 56 (37) | Distant subcutaneous tissue | 15 (20) |
| IIIC | 58 (38) | Distant lymph nodes | 13 (18) |
| Distant skin metastases | 4 (5) | ||
| Liver | 4 (5) | ||
| Small intestine | 3 (4) | ||
| Brain | 3 (4) | ||
| Bone | 4 (5) | ||
| Other | 9 (12) | ||
| Number of positive lymph nodes | Number of sites of resection | ||
| <4 | 124 (81) | 1 | 63 (85) |
| ≥4 | 29 (19) | 2 | 10 (14) |
| 3 | 1 (1) | ||
| In transit metastases | 20 (13) | Intervening regional diseasec | |
| Yes | 28 (38) | ||
| No | 46 (62) | ||
| Prior systemic treatment | Prior systemic treatment | ||
| None | 152 (99) | None | 38 (51) |
| GM-CSF | 1 (1) | GM-CSF | 23 (31) |
| IFN | 9 (12) | ||
| Vaccine | 3 (4) | ||
| Chemotherapy | 3 (4) | ||
| Adjuvant systemic treatment | Adjuvant systemic treatment | ||
| None | 91 (59) | None | 29 (39) |
| GM-CSF | 53 (35) | GM-CSF | 40 (54) |
| IFN | 7 (5) | IFN | 2 (3) |
| Vaccine | 2 (1) | Chemotherapy | 2 (3) |
| Other | 1 (1) | ||
| Adjuvant radiation | 37 (24) | Adjuvant radiation | 6 (8) |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon.
From date resected free of disease to date of diagnosis with stage IV disease
Some patients had more than one site resected
Defined as appearance of regional lymph nodes or in-transit metastases after diagnosis of primary melanoma, but before diagnosis of stage IV disease.
Relapse-free survival and overall survival for stage III resected melanoma
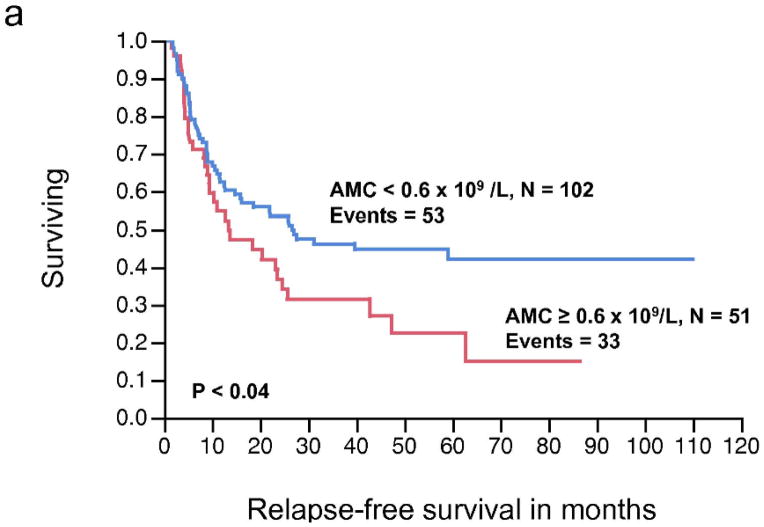
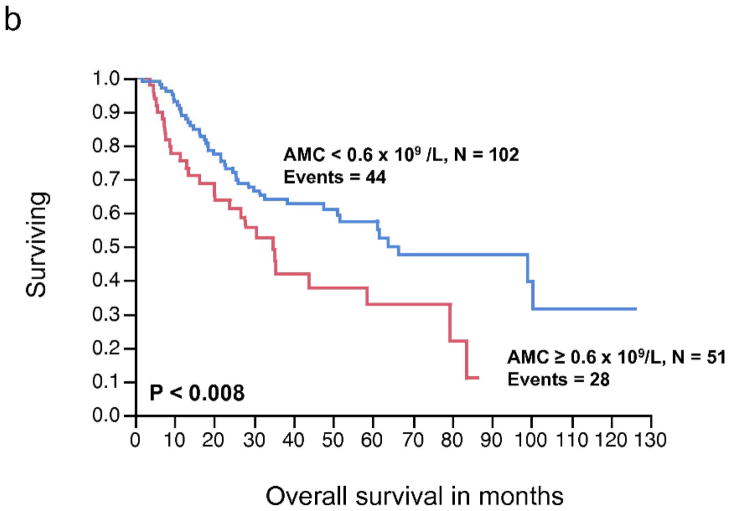
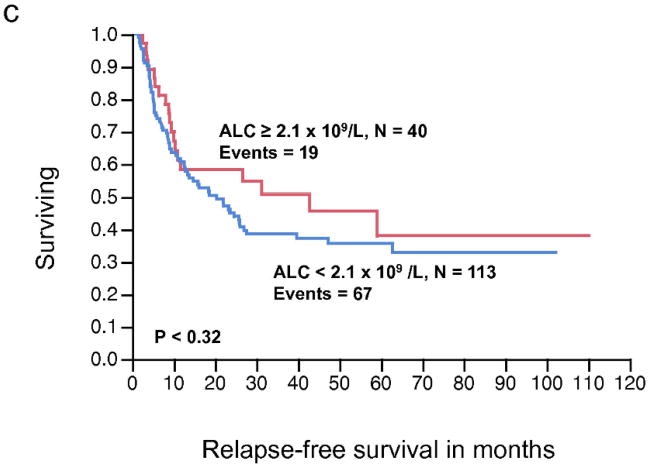
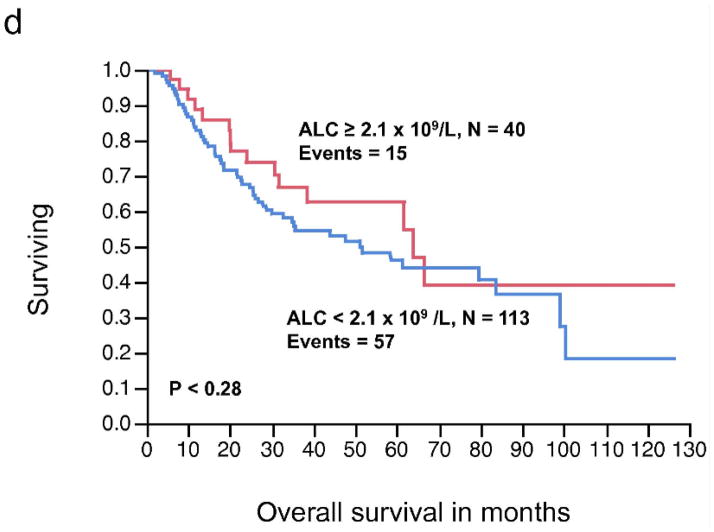
There was no significant relationship between the pre-operative ALC, as a continuous or dichotomous variable, in resected stage III melanoma with RFS and OS (Table 2). The AMC was not significantly associated with RFS; however, increasing AMC was significantly associated with a worse OS (P < 0.04). The AMC was also analyzed as a dichotomous variable. The choice of cut-off for the AMC ≥ 0.6 × 109/L was supported by the data based on the χ2 value (χ2 = 6.37, P < 0.01), using the log-rank test analysis for different cut-off points between the 25% and 75% quartile values (0.62 – 0.43 × 109/L). A pre-operative AMC ≥ 0.6 × 109/L was associated with inferior OS on univariate analysis (HR = 1.89, P < 0.01) (Table 2). We next examined the RFS and OS curves stratified by the AMC for visual representation (Fig. 1a, 1b, respectively). Although the RFS curve for stage III melanoma patients with an AMC < 0.6 × 109/L vs. AMC ≥ 0.6 × 109/L was statistically significant (Fig. 1a), it failed to show prognostic significance as a continuous variable, in the univariate analysis. In the OS curve (Fig. 1b), a pre-operative AMC ≥ 0.6 × 109/L (n= 51) was associated with an inferior survival when compared to patients with an AMC < 0.6 × 109/L (n=102) (median OS, 34.8 months vs. 63.9 months, respectively, P < 0.008). The difference in survival curves when stratified by ALC was not statistically significant (Fig. 1c, 1d).
Table 2.
Univariate and multivariate analysis for relapse-free survival (RFS) and overall survival (OS) for patients with stage III melanoma
| Univariate Analysis-Stage III | ||||||
|---|---|---|---|---|---|---|
| Covariate | RFS | OS | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | 1.01 | 1.00–1.03 | < 0.03 | 1.02 | 1.00–1.03 | < 0.02 |
| Sex | 1.79 | 1.13–2.93 | < 0.01 | 1.91 | 1.14–3.31 | < 0.01 |
| Breslow thickness | 1.12 | 1.04–1.19 | < 0.004 | 1.09 | 1.01–1.18 | < 0.03 |
| ALC (continuous) | 0.78 | 0.51–1.17 | 0.23 | 0.71 | 0.45–1.11 | 0.14 |
| ALC < 2.1 × 109/L | 1.29 | 0.79–2.21 | 0.31 | 1.36 | 0.79–2.50 | 0.27 |
| AMC (continuous) | 1.74 | 0.67–4.19 | 0.25 | 2.82 | 1.07–6.77 | < 0.04 |
| AMC ≥ 0.6 × 109/L | 1.57 | 1.00–2.41 | < 0.05 | 1.89 | 1.15–3.04 | < 0.01 |
| LN ≥ 4 | 2.32 | 1.4–3.70 | < 0.001 | 2.56 | 1.49–4.23 | < 0.001 |
| Adjuvant therapy | 1.07 | 0.69–1.63 | 0.76 | 0.93 | 0.58–1.49 | 0.78 |
| Multivariate Analysis-Stage III | ||||||
| Covariate | RFS | OS | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | 1.02 | 0.99–1.03 | 0.07 | 1.02 | 0.99–1.03 | 0.09 |
| Sex | 2.22 | 1.19–4.44 | < 0.01 | 1.74 | 0.87–3.79 | 0.12 |
| Breslow thickness | 1.11 | 1.02–1.19 | < 0.02 | 1.09 | 0.99–1.18 | 0.07 |
| AMC ≥ 0.6 × 109/L | 1.50 | 0.87–2.54 | 0.14 | 1.79 | 1.00–3.15 | < 0.05 |
| ALC < 2.1 × 109/L | 1.10 | 0.63–2.07 | 0.74 | 0.99 | 0.53–2.01 | 0.99 |
| LN ≥ 4 | 1.93 | 1.03–3.45 | < 0.04 | 2.27 | 1.15–4.24 | < 0.02 |
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count; LN, lymph nodes.
Figure 1.
(a) Relapse-free survival (RFS) curves for stage III malignant melanoma stratified by pre-operative absolute monocyte count (AMC). (b) Overall survival (OS) curves for stage III malignant melanoma stratified by pre-operative AMC. (c) RFS curves for stage III malignant melanoma stratified by pre-operative absolute lymphocyte count (ALC) (d) OS curves for stage III melanoma stratified by pre-operative ALC.
Additional prognostic factors were examined in univariate analysis as indicated in Table 2. Age (continuous variable), sex, Breslow’s thickness (continuous variable), and number of positive lymph nodes (< 4 vs. ≥ 4) were significant predictors of RFS and OS in the univariate analysis. All prognostic factors with a P-value < 0.2 were included in the multivariate analysis with both ALC and AMC as binary variables. As summarized in Table 2, sex, Breslow’s thickness, and positive lymph nodes ≥ 4 were independently significant prognostic factors for RFS. The AMC ≥ 0.6 × 109/L and positive lymph nodes ≥ 4 remained independent predictors of OS on multivariate analysis.
Relapse-free survival and overall survival for stage IV resected melanoma
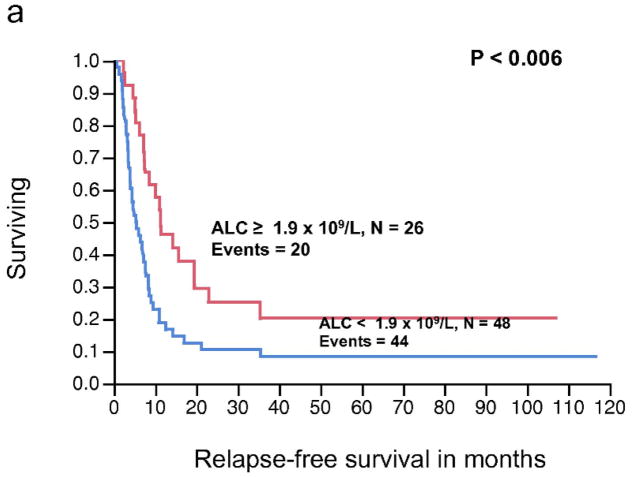
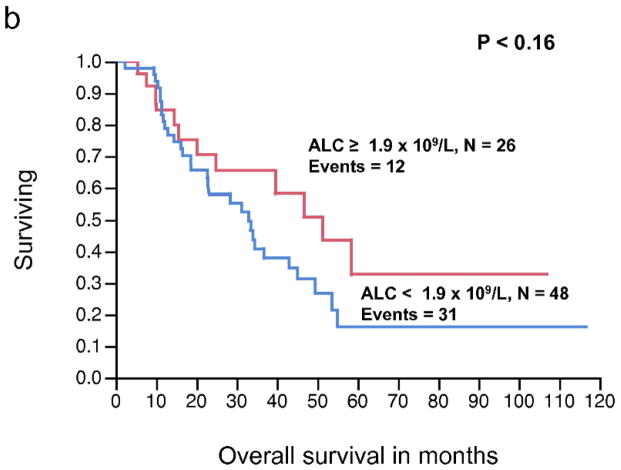
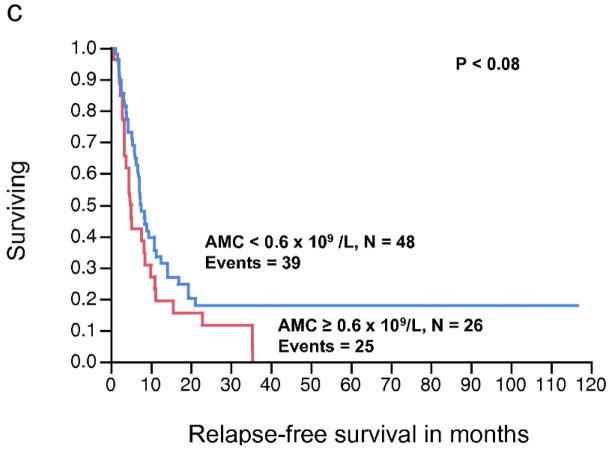
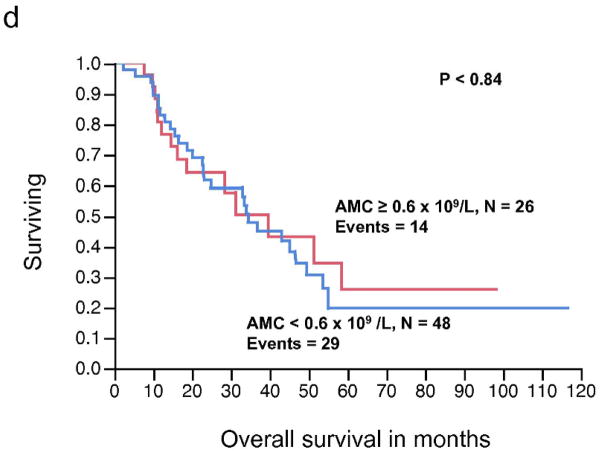
The relationship between pre-operative AMC (continuous variable) and RFS, approached, but did not reach statistical significance (P < 0.08). Similarly, AMC as a continuous variable was not significantly associated with OS (P < 0.84). In contrast, the pre-operative ALC as a continuous variable was significantly associated with RFS (P < 0.008), whereas the relationship between ALC and OS did not reached statistical significance (P < 0.20) (Table 3). The pre-operative ALC was dichotomized into < 1.9 × 109/L and ≥ 1.9 × 109/L. This cut-off point was supported by the data and based on the χ2 value (χ2 = 7.67, P < 0.0056) analyzed at different cut-off points between the 25% and 75% quartiles (1.1 – 1.9 × 109/L), from the log-rank test. Patients with an ALC ≥ 1.9 × 109/L (n = 26) experienced a superior median RFS compared to patients with an ALC < 1.9 × 109/L (n = 48) (median RFS, 11.4 months vs. 5.4 months, respectively, P < 0.006) (Fig. 2a). However, an ALC ≥ 1.9 × 109/L was not associated with superior OS when compared to the group with an ALC < 1.9 × 109/L (median OS, 51.3 months vs. 33 months, respectively, P = 0.16) (Fig. 2b). An ALC ≥ 1.9 × 109/L was associated with an estimated 3-year OS of 65% (95% confidence interval 44%–82%). In contrast, an ALC < 1.9 × 109/L had an estimated 3-year OS of 38% (95% confidence interval 24%–54%). There was no statistically significant difference in survival curves when stratified by AMC (Fig. 2c, 2d).
Table 3.
Univariate and multivariate analysis for relapse-free survival (RFS) and overall survival (OS) for patients with stage IV melanoma
| Univariate Analysis-Stage IV | ||||||
|---|---|---|---|---|---|---|
| Covariate | RFS | OS | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | 1.02 | 1.00–1.04 | < 0.05 | 1.01 | 0.99–1.03 | 0.23 |
| Sex | 1.34 | 0.79–2.35 | 0.27 | 1.75 | 0.91–3.64 | 0.09 |
| Breslow thickness | 1.09 | 0.97–1.20 | 0.15 | 1.09 | 0.98–1.18 | 0.12 |
| ALC (continuous) | 0.54 | 0.34–0.85 | < 0.008 | 0.68 | 0.37–1.23 | 0.20 |
| ALC < 1.9 × 109/L | 2.07 | 1.23–3.60 | < 0.006 | 1.61 | 0.84–3.28 | 0.15 |
| AMC (continuous) | 3.50 | 0.87–12.4 | 0.08 | 0.84 | 0.14–4.19 | 0.84 |
| AMC ≥ 0.6 × 109/L | 1.54 | 0.92–2.54 | 0.09 | 0.93 | 0.48–1.74 | 0.84 |
| Prior systemic therapy | 1.23 | 0.75–2.01 | 0.41 | 1.05 | 0.56–1.93 | 0.87 |
| Disease-free interval | 0.99 | 0.99–1.00 | 0.26 | 1.00 | 0.99–1.01 | 0.97 |
| No. metastatic sites (> 1) | 0.99 | 0.48–1.87 | 0.99 | 0.58 | 0.20–1.34 | 0.22 |
| Intervening regional disease | 1.17 | 0.70–1.94 | 0.53 | 0.99 | 0.53–1.81 | 0.98 |
| Adjuvant therapy | 0.67 | 0.41–1.12 | 0.12 | 0.68 | 0.37–1.24 | 0.20 |
| Multivariate Analysis-Stage IV | ||||||
| Covariate | RFS | OS | ||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | 1.01 | 0.98–1.04 | 0.43 | 1.02 | 0.99–1.06 | 0.09 |
| Breslow thickness | 1.08 | 0.96–1.20 | 0.20 | 1.11 | 0.99–1.21 | 0.08 |
| ALC < 1.9 × 109/L | 2.22 | 1.07–4.88 | < 0.03 | 1.05 | 0.42–2.82 | 0.91 |
| AMC ≥ 0.6 × 109/L | 1.58 | 0.79–3.10 | 0.19 | 0.93 | 0.39–2.09 | 0.86 |
| Adjuvant therapy | 1.09 | 0.57–2.15 | 0.79 | 0.99 | 0.49–2.03 | 0.99 |
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count.
Figure 2.
(a) Relapse-free survival (RFS) curves for stage IV malignant melanoma stratified by pre-operative absolute lymphocyte count (ALC). (b) Overall survival (OS) curves for stage IV malignant melanoma stratified by pre-operative ALC. (c) RFS curves for stage IV malignant melanoma stratified by pre-operative absolute monocyte count (AMC) (d) OS curves for stage IV melanoma stratified by pre-operative AMC.
ALC (binary and continuous) and AMC (binary and continuous), as well as other significant factors such as age (continuous variable), sex, Breslow’s thickness (continuous variable), prior systemic therapy (yes or no), disease-free interval before presentation with distant metastasis (continuous variable), adjuvant therapy (yes or no), number of metastatic sites (1 or >1), and the presence of intervening regional disease (yes or no) were examined in univariate analysis. As demonstrated in Table 3, besides the ALC, age was the other significant factor in univariate analysis for RFS. None of the factors were significant on univariate analysis for OS. The multivariate model accounted for age, Breslow’s thickness, pre-operative AMC ≥ 0.6 × 109/L, adjuvant therapy, as well as pre-operative ALC < 1.9 × 109/L. As summarized in Table 3, only ALC was an independent prognostic factor for RFS, with over a two-fold decrease in RFS for ALC < 1.9 × 109/L (HR = 2.22, 95% confidence interval 1.07–4.88, P < 0.03).
Discussion
Our study is the first to demonstrate the prognostic value for the pre-operative ALC and AMC for survival in completely resected advanced melanoma. The ALC and AMC are inexpensive markers of systemic immunity that can be utilized in clinical practice to select appropriate candidates for resection of metastatic melanoma and to guide adjuvant therapies. Our data suggests that the AMC may have an immune-regulatory function in locally advanced melanoma, as an AMC ≥ 0.6 × 109/L is strongly associated with poor OS in stage III melanoma patients. In contrast, a higher pre-operative ALC is associated with superior RFS in patients with more advanced melanoma (stage IV), both as a continuous and as dichotomous variable. This marker may be a useful tool to stratify patients who are at higher risk of recurrence at time of surgical resection. More importantly, these associations remained significant when controlling for other known clinical and pathological factors.
The role of the host immune status is crucial in the understanding of the natural history and biology of malignant melanoma. Immunotherapy is of great interest for the treatment of advanced melanoma, with reports of improved overall survival in patients treated with ipilimumab, which potentiates an antitumor T-cell response, but at the cost of severe side effects18. Moreover, autoimmunity, as measured by autoantibodies and autoimmune manifestations, has been associated with prolonged survival in patients with melanoma receiving adjuvant immunotherapy as reported by Gogas and colleagues 8. A survival benefit has also been reported in patients with metastatic melanoma receiving immunotherapy that develop vitiligo (an autoimmune disease) 19. This is further evidence of the significance of host immunity and the role in outcomes of melanoma.
To our knowledge, this is the first study describing ALC as a prognostic indicator of relapse after surgical resection of metastatic melanoma. The prognostic significance of the ALC is well established in several hematologic malignancies, as well as solid tumors, either at time of diagnosis or after autologous stem cell transplant 9,10,20–26. Similarly, other authors have reported the prognostic role of the ALC in malignant melanoma; however the patient population in these studies was very different from ours, since they looked at patients receiving immunotherapy. One study reported superior OS in patients with metastatic melanoma who had an ALC ≥ 1,000 cells/μL after two ipilimumab treatments 16. However, this study had several differences from ours, which may explain why we detected a superior RFS in patients with a high ALC, but were not able to identify ALC as predictive marker for OS. First, the patient population of this study consisted on unresectable stage III/IV, which by definition has extensive disease not amenable for surgical resection. This is a key factor since it has been reported that patients with resectable stage IV melanoma have a median OS of approximately 21 months 27 vs. 6.2 months 28 for patients with unresected stage IV melanoma. This illustrates that the extent of disease burden is not equivalent in the study by Ku et al16 when compared to ours, hence the differences in ALC predictive value for OS. Second, the predictive value of the ALC was assessed during treatment with ipilimumab in the Ku study16, whereas we were interested in the predictive value of ALC before surgical resection of metastatic disease. Other studies have investigated peripheral blood lymphocyte subsets in patients with metastatic melanoma, reporting improved survival in patients with an increased CD4+/CD8+ ratio at the beginning of chemoimmunotherapy 29,30. Similarly, a statistically significant correlation has been reported between the occurrence of CD4+ lymphocytes in metastatic lesions and therapeutic benefit of interferon-α in melanoma patients with regional and metastatic disease 6. Further supporting the theory that the host’s immune system is a key player in the anti-tumor response, it has been reported that increased tumor-infiltrating lymphocytes in lymph node melanoma metastases have superior survival 31,32. The pre-operative AMC was an independent factor that influenced OS after surgical resection of tumors in stage III patients, and we hypothesized that this is due to the effect of monocytes on immunosuppression. Previous studies have suggested several mechanisms by which the peripheral blood monocytes might influence cancer progression. Some investigators have shown that the immunosuppressive cytokine IL-10 is produced mainly by monocytes in melanoma patients, and that IL-10 levels are inversely correlated with survival 33. Other investigators have shown that the presence of marker CD163 in serum of patients with stage I/II melanoma is associated with poor overall survival 34. Consistent with our results regarding monocytes and relapse of disease, there is a lack of association between serum CD163 and progression-free survival34. The scavenger receptor CD163 is expressed on circulating monocytes and tissue macrophages, and is associated with anti-inflammatory mediators that promote tumor growth and metastases 35,36.
The different results we report in this study between patients with stage III and stage IV disease may be due to several factors. First, stage III melanoma patients have regional disease, which is still limited to the area of the primary tumor and its lymphatic drainage. The patients in this cohort may have not yet developed any serious shift of the lymphocytes in their peripheral blood. However, stage IV patients have distant disease that has propagated beyond the original tumor, affecting distant organs. This may explain the difference in the predictive value of pre-operative ALC in stage III and stage IV patients. The AMC was a predictive factor for OS in stage III patients, and however it failed to show prognostic significance in stage IV patients. As described before, the localized nature of stage III disease may provide a niche for migration of monocytes, before a full blown systemic effect is noted.
The study has several weaknesses, including the small sample size and limited power to detect statistical differences. However, given the retrospective nature of this study, we can’t change our patient number. Another limitation is that we were not able to include serum LDH level in the statistical model, which is a known prognostic factor, because it was not available for the majority of the patients included in this study. We recognize the importance of serum LDH in the prognosis of stage IV patients and encourage future prospective studies to collect information on this variable.
Our data suggests that advanced melanoma has a different immune response between regional metastatic disease and distant disease, hence the difference in predictive role of the ALC and AMC in stage III and stage IV disease. The study showed better RFS in patients with resected stage IV melanoma who presented with higher ALC before surgical resection of the metastatic lesions, suggesting an important role of the host immunity in this group of patients. However, the pre-operative AMC in stage III patients has a predictive role in overall survival. In regard to all stated limitations of our study, these results have potentially meaningful implications in the management of patient with advanced melanoma for whom surgical resection is considered a standard of care. These inexpensive markers of immunity could be used clinically to identify patients at higher risk of relapse who may benefit from adjuvant treatment. Full understanding of the utility of ALC and AMC in clinical practice will require a confirmatory prospective study.
Acknowledgments
Funding Sources: This project was supported by CTSA Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
No financial disclosures from any author
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliday GM, Patel A, Hunt MJ, et al. Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells. World J Surg. 1995;19:352–8. doi: 10.1007/BF00299157. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RC, Patel A, Panageas KS, et al. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–75. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 5.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Hakansson A, Gustafsson B, Krysander L, et al. Tumour-infiltrating lymphocytes in metastatic malignant melanoma and response to interferon alpha treatment. Br J Cancer. 1996;74:670–6. doi: 10.1038/bjc.1996.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansfield AS, Holtan SG, Grotz TE, et al. Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod Pathol. 2011;24:487–94. doi: 10.1038/modpathol.2010.227. [DOI] [PubMed] [Google Scholar]
- 8.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 9.Ege H, Gertz MA, Markovic SN, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol. 2008;141:792–8. doi: 10.1111/j.1365-2141.2008.07123.x. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui M, Ristow K, Markovic SN, et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol. 2006;134:596–601. doi: 10.1111/j.1365-2141.2006.06232.x. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502–9. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 12.Porrata LF, Ristow K, Colgan J, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin lymphoma. Haematologica. 2011 doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi CH, Kang H, Kim WY, et al. Prognostic value of baseline lymphocyte count in cervical carcinoma treated with concurrent chemoradiation. Int J Radiat Oncol Biol Phys. 2008;71:199–204. doi: 10.1016/j.ijrobp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273–8. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoma MD, Huneke TJ, Decook LJ, et al. Peripheral Blood Lymphocyte and Monocyte Recovery and Survival in Acute Leukemia Postmyeloablative Allogeneic Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658–63. doi: 10.1038/sj.jid.5700545. [DOI] [PubMed] [Google Scholar]
- 20.Joao C, Porrata LF, Inwards DJ, et al. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–71. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 21.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–85. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 22.Porrata LF, Inwards DJ, Ansell SM, et al. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant. 2008;14:807–16. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porrata LF, Inwards DJ, Ansell SM, et al. New-onset lymphopenia assessed during routine follow-up is a risk factor for relapse postautologous peripheral blood hematopoietic stem cell transplantation in patients with diffuse large B-cell lymphoma. Biol Blood Marrow Transplant. 2010;16:376–83. doi: 10.1016/j.bbmt.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Porrata LF, Inwards DJ, Micallef IN, et al. Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin’s disease. Br J Haematol. 2002;117:629–33. doi: 10.1046/j.1365-2141.2002.03478.x. [DOI] [PubMed] [Google Scholar]
- 25.Porrata LF, Litzow MR, Tefferi A, et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–8. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 26.Porrata LF, Ingle JN, Litzow MR, et al. Prolonged survival associated with early lymphocyte recovery after autologous hematopoietic stem cell transplantation for patients with metastatic breast cancer. Bone Marrow Transplant. 2001;28:865–71. doi: 10.1038/sj.bmt.1703236. [DOI] [PubMed] [Google Scholar]
- 27.Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: Results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011 doi: 10.1002/cncr.26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 29.Hernberg MM, Hahka-Kemppinen MH, Pyrhonen SO. The prognostic role of CD4+ and CD8+ lymphocytes during chemoimmunotherapy in metastatic melanoma. Melanoma Res. 2004;14:493–500. doi: 10.1097/00008390-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Hernberg M, Muhonen T, Turunen JP, et al. The CD4+/CD8+ ratio as a prognostic factor in patients with metastatic melanoma receiving chemoimmunotherapy. J Clin Oncol. 1996;14:1690–6. doi: 10.1200/JCO.1996.14.5.1690. [DOI] [PubMed] [Google Scholar]
- 31.Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–34. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–7. [PubMed] [Google Scholar]
- 33.Torisu-Itakura H, Lee JH, Huynh Y, et al. Monocyte-derived IL-10 Expression Predicts Prognosis of Stage IV Melanoma Patients. J Immunother. 2007;30:831–8. doi: 10.1097/CJI.0b013e318158795b. [DOI] [PubMed] [Google Scholar]
- 34.Jensen TO, Schmidt H, Moller HJ, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–7. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 35.Moestrup SK, HJM CD163: A regulated hemoglobin scavenger receptor with a role in the antiinflammatory response. Ann Med. 2004;36:347–54. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen TT, Schwartz EJ, West RB, et al. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol. 2005;29:617–24. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]