Abstract
Past studies have suggested that progesterone-derived ovarian hormones contribute to the discriminative stimulus effects of ethanol, particularly via progesterone metabolites that have activity at γ-aminobutyric acid type A (GABAA) receptors. It is unknown whether loss of ovarian hormones in women, for example, after menopause, may be associated with altered receptor mediation of the effects of ethanol. The current study measured the substitution of allopregnanolone, pregnanolone, pentobarbital, midazolam, dizocilpine, TFMPP, and RU 24969 in female sham and ovariectomized (OVX) rats trained to discriminate 1.0 g/kg ethanol from water. The groups did not differ in the substitution of GABAA-positive modulators (barbiturates, benzodiazepines, neuroactive steroids) or the N-methyl-D-aspartate (NMDA) receptor antagonist dizocilpine. Similarly, blood-ethanol concentration (BEC) did not differ between the groups, and plasma adrenocorticotropic hormone (ACTH), progesterone, pregnenolone, and deoxycorticosterone (DOC) were unchanged 30 min after administration of 1.0 g/kg ethanol or water. However, substitution of neuroactive steroids and RU 24969, a 5-HT1A/1B receptor agonist, was lower than observed in previous studies of male rats, and TFMPP substitution was decreased in OVX rats. Ovarian hormones appear to contribute to 5-HT receptor mediation of the discriminative stimulus effects of ethanol in rats.
Keywords: discriminative stimulus effects, ethanol, ethanol discrimination, females, neuroactive steroids, ovariectomy, rat
Introduction
Numerous studies investigating the neuropharmacological effects of ethanol have reported that discriminative stimulus effects occur primarily through three receptor mechanisms: γ-aminobutyric acid type A (GABAA) receptors (Grant et al., 2000), N-methyl-D-aspartate (NMDA)-type glutamate receptors (Vivian et al., 2002), and 5-hydroxytryptamine (5-HT)1A/C and 5-HT2A receptors (Grant et al., 1997b). Cyclical fluctuation in reproductive hormones is associated with variability in the receptor mechanisms mediating the discriminative stimulus effects of ethanol. For example, discriminative stimulus effects of ethanol and of progesterone-derived neuroactive steroids that positively modulate GABAA receptors (e.g., allopregnanolone; Park-Chung et al., 1999) occur more potently in female cynomolgus monkeys during the luteal compared with the follicular phase of their menstrual cycle (1.0 g/kg ethanol training dose; Grant et al., 1997a). As peak progesterone production by the corpus luteum occurs during the primate luteal phase, these data suggest that neuroactive steroids increase sensitivity to the discriminative stimulus effects of ethanol. Furthermore, the absence of differences in sensitivity to the ethanol-like effects of the benzodiazepine midazolam across menstrual cycle phases in cynomolgus monkeys (Green et al., 1999) implies the existence of a receptor mechanism that is independent of the GABAA benzodiazepine site, by which progesteronederived neuroactive steroids modulate sensitivity to the discriminative stimulus effects of ethanol (e.g. Lovick, 2006).
In contrast to the studies referenced above, most studies of the receptor mechanisms mediating the discriminative stimulus effects of ethanol used male rats. Few studies have used female rats (Schechter, 1973; York, 1978). Like female primates, the reproductive function of female rats varies, but according to a 4–5-day estrous cycle. Rising estradiol in female rats precedes a surge of luteinizing hormone that coincides with the onset of proestrus and induces ovulation. In rats, circulating levels of progesterone rise throughout proestrus. Approximately 12 h after the luteinizing hormone surge, ovulation coincides with peak progesterone, which then declines throughout estrus and is low during diestrus (Freeman, 1994). In contrast to primates, for whom progesterone mediates development of the uterine epithelium before menses, progesterone in rats mediates cellular proliferation of the vaginal epithelium. Progesterone-derived steroids are produced by the ovaries of rats (Holzbauer, 1975) and the adrenal glands (Corpéchot et al., 1993). The rapid fluctuation of neuroactive steroids during the rat estrus cycle is associated with altered GABAA receptor function (Finn and Gee, 1993) and subunit composition (Lovick, 2006), which could contribute to sex differences in the receptor mechanisms mediating the discriminative stimulus effects of ethanol in rodents and differences across the estrous cycle in female rats.
In both primates and rodents, aging is associated with cessation of reproductive function, with female rats experiencing estropause after 10–12 months of cycling (Goldman et al., 2007). Postmenopausal women are reported to have greater sensitivity to the subjective (Kerr et al., 2006) and memory-impairing (Jones and Jones, 1980) effects of ethanol. In addition, aged women suffer from the negative health consequences of heavy ethanol consumption after lower lifetime doses relative to men (Mancinelli et al., 2009). The presence of reproductive hormones may therefore be protective by increasing sensitivity to ethanol intoxication and therefore decreasing consumption. The decline in levels of ovarian hormones in humans with age can be modeled by ovariectomy.
In a recent study in our laboratory of the effects of aging in cynomolgus macaques that previously acquired ethanol discrimination, a female monkey that had been trained to discriminate 1.0 g/kg ethanol over a decade earlier demonstrated a decrease in the substitution potency of ethanol with age, which further decreased after bilateral ovariectomy (Helms and Grant, 2011). This trend suggests that the absence of ovarian-derived hormones alters the mechanisms mediating the discrimination of ethanol. The current study investigated the contribution of ovarian-derived neurosteroids to the receptor pharmacology mediating the discriminative stimulus effects of ethanol using ovariectomized and intact female rats. In addition, the effect of an acute intragastric administration of ethanol or water on the reproductive hormone (progesterone), stress hormones (adrenocorticotropic hormone, ACTH and deoxycorticosterone, DOC), and their metabolic precursor, pregnenolone, was compared across groups. Morrow et al. (2006) suggested that many of the acute effects of ethanol could be related to ethanol-induced steroidogenesis by stimulation of hypothalamic–pituitary–adrenal axis and subsequent release of ACTH, which then promotes the synthesis of the precursor hormone pregnenolone in the adrenal cortex (Lavoie and King, 2009). Pregnenolone can then be metabolized into numerous neuroactive steroid based upon the presence of specific steroidal enzymes (Helms et al., 2012). Differential hormonal response to the training dose of ethanol could be related to differences in substitution patterns of test drugs.
Methods
Subjects
Female Long–Evans sham (n=13) and ovariectomized (n=13) rats (Harlan Laboratories, Livermore, California, USA) were obtained at 57–69 days of age. Sham surgeries and ovariectomies were performed by Harlan Laboratories (dorsal midline) 6 days before the rats arrived. Vaginal lavages revealed an estrous cycle in one ovariectomized rat, which was subsequently reclassified as a sham rat, thus resulting in a total of 14 sham and 12 ovariectomized rats. Upon arrival, the rats were individually housed and habituated for 5 days to a temperature (22°C)-controlled vivarium on a 12-h light–dark cycle (lights on at 06:00 h). After habituation, the rats were weighed and handled daily. Food restriction began 2 weeks later, when the rats were ~90 days of age. Nutritionally complete rat chow was provided to maintain the rats at their 90-day weights. Water was freely available except during experimental sessions. Experimental sessions began 3 weeks after the rats’ arrival. A daily food ration was provided at the end of each experimental session. These studies were conducted according to the guidelines of the Oregon Health & Science University Animal Care and Use Committee and the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (National Research Council, 1996).
Apparatus
Experimental sessions took place 5–7 days/week, between 11:00 and 17:00 h, in ventilated and sound-attenuating chambers (Med Associates Inc., St Albans, Vermont, USA). Set into one panel of the chamber were two retractable levers with three LED lights (amber, green, and red) above each lever and a central white light. The opposite panel contained a food tray for reinforcer delivery (45 mg sugar pellets; Bio-Serv, Frenchtown, New Jersey, USA). The pellets were delivered through vinyl tubing connected to a feeder located outside of the chamber. Event scheduling and data acquisition were controlled by a PC-compatible or Macintosh-compatible computer connected to an interface (Med Associates Inc.) programmed with LabView software (National Instruments, Austin, Texas, USA).
Procedure
Discrimination training
Rats were shaped to respond on a single lever, using successive approximation with a fixed ratio (FR)-1 schedule. Once a rat completed 20 responses in 15 min or less for two consecutive sessions, the response requirement increased to FR-2. The FR incremented by 1 after each session in which a rat received 20 reinforcers in 30 min or less. After FR-5, the response requirement incremented to FR-10, followed by FR-15. Beginning with the first FR-15 session, a 30-min time-out before insertion of the levers was introduced over six consecutive sessions in 5-min increments (i.e. first session, 5-min time-out; second session, 10-min time-out, etc.). All lights were switched off during the time-out, after which a lever was extended and the lights above the lever illuminated.
Response training continued until response rates were stable (SD<0.05) for five consecutive sessions, with at least one of the sessions reaching a 30-min time-out, or after 25 sessions of the FR-15 schedule, whichever occurred first. After completing response training, the rats were gavaged with tap water (6.7 ml/kg) before five consecutive sessions and reinforced for responding on the water-appropriate lever, the only lever that extended after the 30-min pretreatment. Before the next five sessions, the rats were gavaged with 1 g/kg ethanol (15% w/v in tap water, 6.7 ml/kg) and reinforced for responding on the ethanol-appropriate lever, which was the only lever extended after a 30-min pretreatment.
Discrimination training began after these 10 forcedchoice gavage sessions. Rats were administered either water or a training dose of ethanol and were immediately placed into a chamber. After 30 min, both levers extended into the chamber and all lights were illuminated. A pellet was delivered once the rat completed the FR requirement (FR-15) on the appropriate lever. Responding on the inappropriate lever reset the FR count on the appropriate lever to zero. Ethanol–water discrimination was trained using a double-alternating schedule (i.e. water, water, ethanol, ethanol), except that the treatment condition was switched after a session in which 90% of total responses, and 70% of responses in the first FR, were on the appropriate lever. Once the above criteria were maintained for five consecutive sessions, the discrimination training was considered complete.
During testing, completing a FR requirement on either lever resulted in delivery of a pellet. Water was always administered for the first test, followed by 1.0 g/kg ethanol, then intermediate and increasing doses of ethanol to obtain an ED50. The other test drugs were administered intraperitoneally generally in the following order: allopregnanolone, pregnanolone (neuroactive steroids), pentobarbital (barbiturate), midazolam (benzodiazepine), dizocilpine (NMDA receptor antagonist), TFMPP (nonselective 5-HT receptor agonist), and RU 24969 (5-HT1A/1B receptor agonist; Doods et al., 1985). However, tests of individual drugs were widely distributed over time resulting in great overlap. Rats were immediately placed in the operant chamber for the 30- min pretreatment interval after drug administration. After 30min, all lights were illuminated and both levers extended into the chamber. Each session ended after 20 reinforcers were delivered or after 30 min, whichever occurred first. Drugs were tested after water and/or ethanol training sessions if 90% of responses in the entire session and 70% of responses in the first FR were to the appropriate lever for two consecutive sessions, or three consecutive sessions following a session in which discrimination performance did not match these criteria. For the current study, 603/782 (77.1%) of doses tested were administered after both a water and an ethanol training session (double determination), with 65 tests (8.3%) occurring after a water training session only and 103 tests (13.2%) occurring after an ethanol training session only (single determination). All results contributing to calculation of the ED50 were double determined. Single determinations most often reflect that the dose tested resulted in zero responses during the session and occasionally that the rat failed to consume an entire meal the following day such that the dose was not retested. Because of experimenter error, 1.4% of tests were conducted twice with both tests occurring after ethanol or water training sessions.
Vaginal lavage
Between 10:00 and 13:00 h, cells were sampled from the vaginal epithelium by briefly restraining the rat and inserting a glass pipette with a rubber bulb 2–3mm into the vagina. A small amount (<50 μl) of sterile water was introduced into the vagina and then retrieved by the pipette two to three times to obtain the cells. The sample was placed on a glass slide and dried before cytological assessment to determine estrous cycle phase (Everett, 1989). During acquisition and testing, estrous cycle phase was determined using cytological examination of vaginal smears. A majority of sessions occurred during diestrus (60.9%) with 13.6% of sessions occurring in estrus and 20.7% of sessions occurring during proestrus. Because of absent or insufficient samples, 4.9% of sessions were not characterized for estrous cycle phase. Intact estrous cycles were observed in all sham rats throughout the (mean±SD) 324±24 days of the experiment on which vaginal cytology was examined.
Blood-ethanol concentration
All rats were administered 1.0 g/kg ethanol and 30 min later, 20 μl blood was collected from the medial saphenous vein using a 25G needle and microcapillary tube. The samples were immediately diluted into 500 μl of a matrix of 4mmol/l n-propanol in deionized water. The blood sample in matrix was then capped and thoroughly vortexed in a 2.0 ml crimp top vial. Analysis was performed using ambient headspace sampling gas chromatography (Agilent 6890N GC, using a DB-ALC1 column; Agilent, Wilmington, Delaware, USA) on a 30 μl aliquot, as previously described (Finn et al., 2007). Six sets of ethanol standards (0.1–3.0 mg/ml), including n-propanol (internal standard), were run in parallel with the samples.
Drugs
Anhydrous ethanol (1.0 g/kg; Pharmco-Aaper, Shelbyville, Kentucky, USA) was diluted to 15% (w/v) with tap water and administered (<7 ml/kg, intragastrically) 30 min before each training session. Allopregnanolone and pregnanolone were suspended in 45% β-cyclodextrin (Sigma, St Louis, Missouri, USA) the night before the test session, and stirred overnight in a walk-in refrigerator (4°C). Allopregnanolone and pregnanolone were administered in volumes less than 3ml (intraperitoneally). Steroids in solution were used up to 4 days after they were initially prepared, then discarded. Pentobarbital (1–17mg/kg), midazolam (0.30–5.6mg/kg; Oregon Health & Science University Pharmacy, Portland, Oregon, USA), TFMPP (trifluoromethylpiperazine; 0.1–1.7 mg/kg) and RU24969 [5-methoxy-3-(1,2,3,6-tetrahydropyridin-4-yl) 1H-indole, 0.1–1.0 mg/kg; Sigma] were prepared fresh immediately before each test (intraperitoneally). The dose range was selected based on previous tests in male rats.
Hormone assays
Half of the rats from each group were administered water and half were administered 1.0 g/kg ethanol with these subgroups matched for ethanol substitution ED50. Thirty minutes later, trunk blood (~5 ml) was obtained following decapitation without anesthesia because previous reports indicated that ACTH levels from trunk blood obtained by decapitation under anesthesia (CO2 or pentobarbital) were 2–13-fold greater than when anesthesia was absent (Vahl et al., 2005), which could confound an effect of ethanol. The whole blood was stored on ice (<60 min) until centrifuged (15 min, 4°C). The plasma was stored at − 80°C until assayed for ACTH, pregnenolone, DOC and progesterone by radioimmunoassay (RIA) and enzyme immunoassay (EIA) at the Endocrine Technology and Support Core Lab at the Oregon National Primate Research Center/Oregon Health & Science University. Briefly, plasma samples (20 μl) were extracted with 6ml diethyl ether, dried under an air stream and redissolved in assay buffer (0.1% gel PBS) for a specific RIA. Hormonal values were corrected for extraction losses, determined by radioactive trace recovery at the same time with sample extraction, which ranged between 80 and 90% recovery rate. The sensitivity was less than 5 pg/tube for DOC. The intraassay variation was 8.61%. Plasma progesterone was determined using a RIA kit (Diagnostics Systems Lab, Webster, Texas, USA). This assay had a sensitivity of 0.34 ng/ml and a range up to 60 ng/ml. The kit came with two controls. The intra-assay variation was 5.15%. Rat ACTH was determined using a multispecies direct ACTH EIA using 200 μl per sample (DRG International Inc., Mountainside, New Jersey, USA). The assay had a sensitivity and range of 7.8–584.0 pg/ml with high and low controls provided in the kit. The intra-assay variation was 1.51%. For pregnenolone, 100 μl of each plasma sample was extracted using 5ml of ether before they were assayed by a specific EIA kit (Alpco, Salem, New Hampshire, USA). The assay had a sensitivity and range of 0.1–25.6 ng/ml with a control included in the assay kit. The intra-assay variation was 10.29%.
Data analysis
Percentage responding on the ethanol-appropriate lever (total responses on the ethanol-appropriate lever/total responses) and percentage of baseline response rate were the primary-dependent variables. Ethanol-appropriate responding was averaged from all determinations when multiple determinations of the same test dose were conducted. Complete and partial substitutions were defined, respectively, as at least 80% and at least 20–79% of responses on the ethanol-appropriate lever. Baseline response rate was calculated by averaging response rates (total responses/session time) for each ethanol and water training session immediately preceding a test. The dependent variables were analyzed separately for each drug, using linear mixed models with rat, group, and dose as classification variables and dose as a repeated variable. Separate models were run exclusively for sham rats that included estrous cycle phase and drug dose only. The results are from models using optimal covariance structures determined by Schwarz’s Bayesian Information Criteria. Bonferroni’s corrected pair-wise comparisons were used to evaluate main effects and interactions. The dose at which response rate was decreased by 50% from baseline (ED50) was computed using linear interpolation between the two doses that encompassed the 50% effect (Excel). When a drug completely substituted for ethanol, the ED50 was computed similarly. The two groups were compared using independent-samples t-tests for which the Satterthwaite approximation (as indicated by degrees of freedom) was used when variance significantly differed between the groups according to a folded F-test. For all tests, α was 0.05. Analyses were conducted using SPSS 14.0 (SPSS Inc., Chicago, Illinois, USA) and SAS 9.2. (SAS Inc., Cary, North Carolina, USA)
Results
The groups did not differ significantly in the total number of sessions necessary to acquire responding up to FR-10 (mean±SD: sham, 26.6±8.3; OVX, 34.3±13.9 sessions) [t(24)=1.74, P=0.09]. Sham and OVX rats’ responding on the FR-15 schedule stabilized after a similar number of sessions (sham, 12.5±7.3; OVX, 11.3±7.2 sessions) [t(24)= − 0.44] with three sham rats and two OVX rats being trained for the maximum of 25 sessions. Response rate at the final reinforcement schedule did not differ between sham (0.32±0.18 responses/s) and OVX (0.32±0.17 responses/s) rats. The number of sessions required for complete discrimination training, excluding the 10 forced-choice sessions, did not differ significantly for OVX (65.8±42.6 sessions) compared with sham (49.6±20.1 sessions) rats [t(15.1)=1.2, P=0.25]. The groups also did not differ in blood-ethanol concentration (BEC) 30 min after administration of 1.0 g/kg ethanol (intragastrically), t(17.3)= − 1.6, P=0.13 (OVX, 76.3±5.7mg/dl; sham, 82.2±12.2 mg/dl).
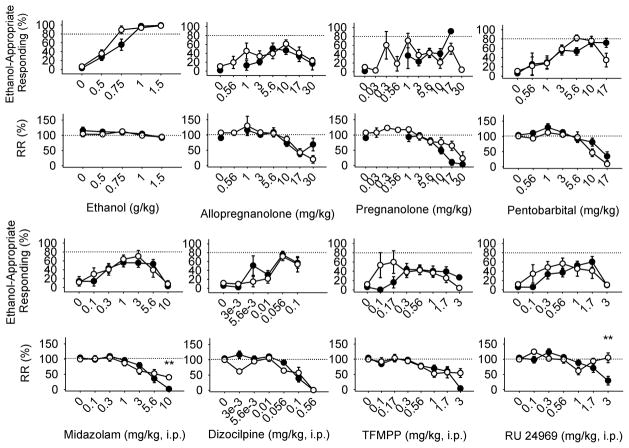
Percent ethanol-appropriate responding increased dose dependently after administration of every test drug [Fig. 1; ethanol, F(4, 217)=148.0, P<0.001; allopregnanolone, F(8, 209)=9.5, P<0.001; pregnanolone, F(9, 167)=4.5, P<0.001; pentobarbital, F(7, 191)=15.3, P<0.001; midazolam, F(10, 190)=6.9, P<0.001; dizocilpine, F(6, 160)=24.7, P<0.001; TFMPP, F(8, 182)=3.8, P=0.001; RU 24969, F(8, 178)=1090.6, P<0.001]. There were no significant interactions between dose and group. The absence of significant interactions between dose and estrus cycle phase for a majority of drugs indicated that percentage ethanol-appropriate responding did not vary across estrus cycle phase [ethanol: F(10, 110)=1.9, P=0.056; allopregnanolone, F(11, 95)=1.3; midazolam, F(10, 79)=0.5; pentobarbital, F(8, 78)=0.8; dizocilpine, F(7, 61)=0.4; RU24969, F(10, 55)=1.4]. However, a significant interaction for TFMPP [F(11, 69)=2.0, P<0.05] and follow-up post-hoc tests, indicated that 0.56 mg/kg TFMPP resulted in greater ethanol-appropriate responding during estrus (mean±SD, 69±46%, n=4) compared with diestrus (26±43%, n=12) but not proestrus (61±49%, n=2), but this probably reflected intersubject variability because different rats were represented across the different phases.
Fig. 1.
Mean (±SEM) ethanol-appropriate responding and percentage of baseline response rate (RR) by test drug dose in female sham (closed circles) and ovariectomized (open circles) rats trained to discriminate 1.0 g/kg ethanol from water. The dashed lines show the criteria for complete substitution and response rate equivalent to baseline. On average, each rat was tested with a (mean±SD) minimum of 5.5±1.5 doses and a maximum of 12.1±1.8 doses of each drug; **P<0.01. i.p. intraperitoneally.
Response rate was not suppressed by ethanol [F(4, 217)=2.0, P=0.1]. In contrast, the percentage of baseline response rate decreased dose dependently after administration of allopregnanolone [F(8, 220)=9.5, P<0.001], dizocilpine [F(7, 169)=12.0, P<0.001], and TFMPP [F(8, 190)=9.1, P<0.001], and did not vary between the groups. Administration of pregnanolone resulted in a dose-dependent suppression of response rate [F(9, 191)=5.6, P<0.001]. There was no significant interaction between group and dose, but there was a significant main effect of group [F(1, 191)=4.3, P<0.05] reflecting a smaller overall suppression of response rate in OVX (mean±SD, 90±46%) compared with sham (76±46%) rats administered pregnanolone. Midazolam likewise suppressed response rate as indicated by a significant main effect of dose [F(10, 196)=20.2, P<0.001]. A significant interaction between group and dose [F(8, 196)=2.7, P=0.007] reflected less suppression after 10.0mg/kg midazolam in OVX (40±15%, n=4) compared with sham (2±1%, n=2) rats (Fig. 1). Pentobarbital suppressed response rate [F(8, 203)=16.3, P<0.001] and a significant main effect of group [F(1, 203)=6.1, P=0.01] was due to a overall slightly lower response rate suppression in sham (96±47%) compared with OVX (90±48%) rats. Response rate was decreased by RU24969 [F(8, 187)=3.6, P=0.001], and there was a significant interaction between dose and group [F(6, 187)=3.3, P=0.005] reflecting a greater suppression of responding in sham (30±38%, n=7) compared with OVX (105±28%, n=3) rats in tests with 3.0mg/kg. The absence of significant interactions between dose and estrus cycle phase indicated that percentage of baseline response rate did not vary across estrus cycle phase [ethanol: F(10, 110)=1.9, P=0.058; allopregnanolone, F(11, 101)=1.6, NS; midazolam, F(11, 80)=0.8; pentobarbital, F(8, 83)=0.5; dizocilpine, F(8, 65)=0.9; TFMPP, F(11, 75)=1.4; RU24969, F(10, 60)=1.3]. There was a significant interaction between estrus cycle phase and pregnanolone dose [F(9, 83)=2.7, P<0.01], and post-hoc tests indicated that response rate was suppressed by 5.6 mg/kg pregnanolone during diestrus (mean± SEM, 65±12%, n=19) but not proestrus (126±19%, n=6; estrus, 100±13%, n=3). Greater response rate suppression was also observed during diestrus (35± 14%, n=10; proestrus, 32±30%, n=3) compared with estrus (96±20%, n=6) after administration of 10 mg/kg pregnanolone.
As expected, all rats showed complete substitution in tests with ethanol. Two-sample tests of proportion revealed no significant difference between the percentage of OVX and sham rats for which the following drugs completely substituted for ethanol: allopregnanolone (OVX, 58%; sham, 50%: Z= − 0.4), pregnanolone (OVX, 67%; sham, 36%: Z= − 1.6, P=0.12), pentobarbital (OVX, 75%; sham, 86%: Z=0.7), midazolam (OVX, 58%; sham, 78%: Z=1.1), dizocilpine (OVX, 83%; sham, 64%: Z= − 1.1), or RU 24969 (OVX, 50%; sham, 64%: Z=0.7). In contrast, TFMPP completely substituted in a greater percentage of sham (64%) compared with OVX (25%; Z=2.0, P=0.045; Fig. 2). The substitution patterns of individual rats for all test drugs showed that complete substitution of every test drug was observed only in two rats (OVX, 6 and 11), indicating robust production of ethanol-like stimulus effects via multiple, as well as individual, receptor subtypes. However, in the majority of sham (64%) and a proportion of the OVX rats (25%), several test drugs completely substituted and one or two test drugs produced partial substitution. In many OVX (50%) and some sham rats (36%), a majority of the test drugs produced partial or no substitution.
Fig. 2.
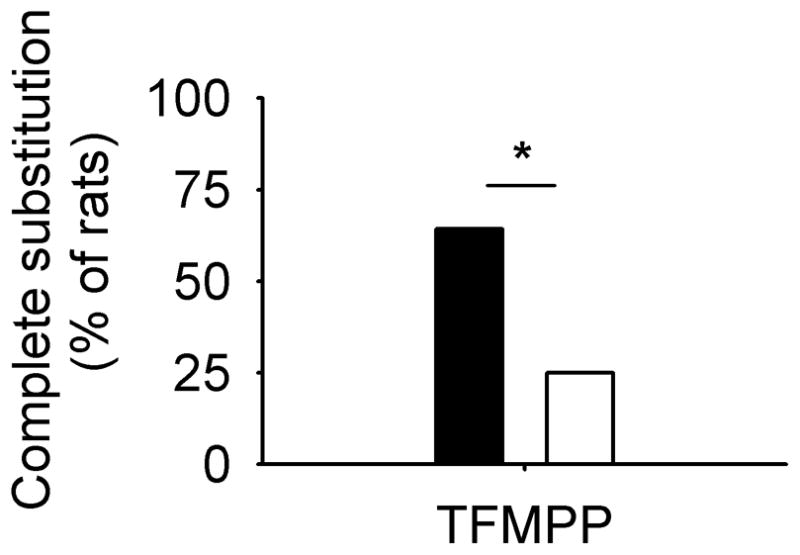
Percentage of sham and ovariectomized (OVX) female rats showing complete substitution of TFMPP for 1.0 g/kg ethanol; *P<0.05.
Among rats for which ED50 was calculable, the ED50 for substitution did not differ significantly between the groups for any of the drugs tested according to independent-samples t-tests [ethanol, t(24)= − 0.7; allo-pregnanolone, t(12)= − 0.5; pregnanolone, t(11)=0.2; pentobarbital, t(19)= − 1.8; midazolam, t(16)=0.3; dizocilpine, t(17)=0.9; TFMPP, t(10)= − 1.0]. Among rats for which RU 24969 completely substituted for 1.0 g/kg ethanol, however, the mean ED50 for OVX rats tended to be lower than for intact rats [t(13)= − 2.1, P=0.052; Table 1]. Among rats for which the test drugs decreased response rate to 50% or less of baseline, the potency of response rate suppression did not differ between the groups for a majority of drugs [allopregnanolone, t(10.8)= − 0.6; pregnanolone, t(7.1)=1.5; pentobarbital, t(13)= − 1.2; midazolam, t(11)= − 0.7; dizocilpine, t(12)=0.2; TFMPP, t(12)= − 0.7]. However, RU 24969 decreased response rate more potently in OVX compared with sham rats [t(10)= − 2.4, P<0.05].
Table 1.
Mean (± SD) ED50 for substitution of test drugs and suppression of response rate amongst sham and ovariectomized (OVX) female rats.
| Group | Substitution
|
Response rate
|
||
|---|---|---|---|---|
| Sham | OVX | Sham | OVX | |
| Ethanol | 640 ± 141 | 592 ± 199 | - | - |
| Allopregnanolone | 6.8 ± 3.1 | 5.8 ± 3.9 | 13.9 ± 1.2 | 13.2 ± 3.3 |
| Pregnanolone | 6.3 ± 4.2 | 638 ± 5.6 | 8.5 ± 4.0 | 7.1 ± 3.7 |
| Pentobarbital | 5.0 ± 3.2 | 2.9 ± 1.7 | 10.2 ± 4.8 | 6.5 ± 3.7 |
| Midazolam | 1.0 ± 1.0 | 0.6 ± 0.5 | 3.5 ± 1.9 | 1.9 ± 1.4 |
| Dizocilpine | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.08 ± 0.01 | 0.06 ± 0.03 |
| TFMPP | 0.59 ± 0.36 | 0.34 ± 0.39 | 0.8 ± 0.6 | 0.6 ± 0.2 |
| RU 24969 | 0.79 ± 0.38 | 0.38 ± 0.33 | 1.3 ± 0.4 | 0.8 ± 0.3* |
OVX, ovariectomized.
p < 0.05.
One-way analysis of variance indicated no significant main effects or interactions involving group or treatment on plasma hormones. Among sham rats, mean (±SD) ACTH was 3.2±4.4 and 2.3±5.7 pg/ml after treatment with water and ethanol, respectively, and 12.0±13 and 3.7±4.5 pg/ml among OVX rats after the same treatments. Sham rats’ pregnenolone concentrations after water and ethanol treatment were 0.8±0.2 and 1.0±0.3 ng/ml, and OVX rats had 0.9±0.3 and 0.8±0.3 ng/ml after these treatments, respectively. Pro-gesterone concentrations were 7.1±5.0 and 10.6±5.4 ng/ml after water and ethanol treatment among sham rats, respectively, with the same treatments resulting in 5.7±3.2 and 5.6±4.0 ng/ml progesterone in OVX rats. Lastly, DOC concentrations after water and ethanol treatment were, respectively, 13.2±5.8 and 8.7±2.5 ng/ml in sham rats, and 10.7±4.1 and 10.4±4.8 ng/ml in OVX rats.
Discussion
The present study indicates that, in rodents, the receptors known to mediate the discriminative stimulus effects of ethanol in males are also major mediators in females. Past studies of males in this lab, using the same training dose and pretreatment interval as the current study, showed complete substitution of allopregnanolone and pregnanolone for ethanol (80–100% substitution, Long–Evans; Bowen et al., 1999). In the current study, substitution of neuroactive steroids was lower (67%) in females, suggesting that neuroactive steroids produced weaker ethanol-like discriminative stimulus effects compared with male rodents in previous studies. This sex difference is not observed in cynomolgus macaques, for which both allopregnanolone and pregnanolone completely substituted in both sexes (Grant et al., 2008), possibly because of species differences in cycle duration particularly with respect to the prolonged luteal phase in primates. Similarly, we observed lower efficacy of RU 24969 in female compared with male rats previously studied under the same training conditions (100% of male rats showing complete substitution; Grant et al., 1997b), indicating that 5-HT1A/1B receptors have a weaker role in the discriminative stimulus effects of ethanol in female rodents. In contrast, substitution of the nonselective 5-HT receptor agonist TFMPP in the current study was similar to the 60% substitution observed in a study of male rats (Grant and Colombo, 1993b). Barbiturate, benzodiazepine and NMDA receptor antagonist substitution for the discriminative stimulus effects of ethanol was similar between females in the current study and males in past studies. Previously, pentobarbital substituted for 1.0 g/kg ethanol (30-min pretreatment; Grant and Colombo, 1993a) in 80–100% of male Long–Evans rats tested, within range, but not overlapping, with the percentage substitution observed in the current study. The substitution of midazolam in our study was similar to the 75% substitution observed in male rats (Grant, 1999) and the partial substitution of dizocilpine in female rats in the current study is consistent with partial substitution observed by Grant and Colombo (1993b).
The data suggest that female rodents and non-human primates differ greatly in the receptor mediation of the discriminative stimulus effects of ethanol, as the discriminative stimulus effects of ethanol in non-human primates are produced by redundant receptor mechanisms (Helms et al., 2008). In contrast, the current study indicates that ethanol effects on multiple receptor subtypes are necessary for female rats to discriminate ethanol from water. Individual substitution patterns indicated that for most of the female rats in the current study tests involving any single receptor type produced only one component of the ethanol stimulus, resulting in partial substitution. The present study tested a majority of the receptor types known to mediate the discriminative stimulus effects of ethanol to survey the role of gonadal hormones. However, additional receptor subtypes not tested include sigma receptors and metabotropic glutamate receptors (Besheer et al., 2006). For example, previous studies reported bidirectional substitution of ethanol and pregnanolone in some rats (Bowen et al., 1999; Gerak et al., 2008), and a sigma receptor agonist fully substituted for pregnanolone (Engel et al., 2001), and for ethanol (Hundt et al., 1998). Variation in endogenous hormones could therefore affect the contribution of these receptor subtypes not included in the current study, particularly sigma receptors.
Removal of ovarian hormones by ovariectomy primarily affected 5-HT receptor mediation of the discriminative stimulus effects of ethanol. The potency of RU 24969 substitution was on average lower among intact females (0.79 mg/kg) compared with OVX rats (0.38mg/kg) in the current study and male rats in previous studies (0.3 mg/kg; Grant et al., 1997b), suggesting that progesteronederived ovarian hormones affect the signaling of 5-HT receptors in the presence of ethanol, although the current study cannot exclude a role for estrogen. Significantly fewer OVX rats showed complete substitution of TFMPP compared with sham rats. The suppression of response rates by RU 24969 at the highest dose tested was greater in sham rats, suggesting that ovarian hormones increased the sedative effects of 5-HT receptor agonists. Previous studies reported that 5-HT neuron firing in the dorsal raphe nucleus is significantly greater in male rats compared with free-cycling or OVX females, and 5-HT neuronal firing increased during pregnancy in concordance with increasing levels of circulating progesterone (Klink et al., 2002). A similar trend is present in nonhuman primates. Long-term ovariectomy in Japanese macaques was associated with decreased 5-HT cell number and density of the 5-HT1A autoreceptor, Fev transcription factor differentiating and maintaining serotonergic neurons, serotonin reuptake transporter, and TPH2 (gene coding for the rate-limiting enzyme in 5-HT synthesis; Bethea et al., 2011). Thus, ovarian hormones modulate serotonergic neurotransmission in rodents and primates. Low concentrations of progesterone are associated with decreased 5-HT transmission, consistent with lower efficacy of TFMPP to produce ethanol-like discriminative stimulus effects of ethanol in OVX compared with sham rats as indicated by the percentage of rats showing complete substitution. Down-regulation of 5-HT1A/1B receptors to compensate for decreased serotonergic neurotransmission (Cahir et al., 2007) may be related to the trend toward greater potency of RU 24969 to produce ethanol-like discriminative stimulus effects in OVX compared with sham rats. Future studies could investigate whether ovariectomy alters the role of other 5-HT receptor subtypes in the discriminative stimulus effects of ethanol, as 5-HT3 (Hodge et al., 2006) but not 5-HT2 (Szeliga and Grant, 1998) receptors have been implicated in ethanol discrimination. Additional studies focusing the interaction between ovarian hormones and 5-HT receptor mediation of the discriminative stimulus effects of ethanol could test drugs with selectivity for single receptor subtypes that have been previously studied in males, for example 5-HT1B (CGS 12066B; Grant et al., 1997b) and 5-HT1A (8-OH-DPAT, buspirone (Grant et al., 1997b; Signs and Schechter, 1988).
The similar BEC after administration of 1.0 g/kg ethanol between OVX and sham rats in the current study suggests that elimination of ovarian hormones did not affect ethanol pharmacokinetics and cannot account for group differences in test drug substitution. We did not observe a relationship between BEC and estrous cycle phase. Previous studies in female rats demonstrated that BEC following an acute high dose of ethanol (4 g/kg, intraperitoneally) was significantly reduced during proestrus compared with the first day of diestrus (Stojanović et al., 1996), indicating an effect of reproductive hormone fluctuation on metabolism of sedative doses of ethanol to which discriminative stimulus effects are not relevant because of the absence of behavior during sedation. Additional studies are needed in female rats trained to discriminate 2.0 g/kg ethanol, which could uncover greater differences between sham and OVX rats, particularly in NMDA receptor mechanisms (Grant and Colombo, 1992). Female rats tend to have higher BEC than male rats (Rivier, 1993). However, another study reported similar ethanol concentration within the nucleus accumbens, a brain area mediating the discriminative stimulus effects of ethanol (Hodge et al., 2001), between males and females, and across estrous cycle phases (Crippens et al., 1999). Sex differences in receptor mediation of the discriminative stimulus effects of ethanol, as suggested by comparison of the current study in females with previous studies of males, are unlikely to be related to ethanol distribution and metabolism.
Individual differences in hormone concentrations precluded any significant differences between the groups. Sham rats had on average slightly greater progesterone compared with OVX rats, which would be expected because of removal of the ovaries. The difference in progesterone between the groups in the current study was less than previously reported (e.g. sham, 17.4 ng/ml; OVX, 2.7 ng/ml; Ford et al., 2004) using a different surgical method. Other research indicated that the presence of plasma progesterone in OVX rats is expected because peripheral reproductive hormones are also produced by the adrenal glands (Corpéchot et al., 1993). A study by Flores et al. (2008) revealed that the adrenals are the main source of progesterone during the rat estrous cycle, as bilateral ovariectomy did not decrease serum progesterone, whereas bilateral adrenalectomy resulted in a significant decrease in progesterone. In another study, ovariectomy resulted in a greater than three-fold decrease in serum concentration of the progesterone metabolite allopregnanolone, which was only slightly decreased in frontal cortex (Pluchino et al., 2008). Minimal differences between sham and OVX rats in the receptor mechanisms mediating the discriminative stimulus effects of ethanol could therefore be related to brain steroidogenesis.
The precursors pregnenolone and DOC (precursor to THDOC) did not increase after acute ethanol. The absence of differences in plasma hormone levels between the groups after either gavage treatment is consistent with the absence of differences in test drug substitution. Under the conditions of the current study in rats, ethanol did not significantly influence plasma hormone concentrations and, therefore, ethanol-induced release of peripheral hormones is unlikely to have contributed to the discriminative stimulus effects of ethanol. In contrast, in male rats, acute ethanol increased ACTH (1.0 g/kg, Rivier, 1993; 1.5 g/kg, Boyd et al., 2010b), allopregnanolone (1.5 g/kg; Porcu et al. 2010), DOC (2 g/kg; Khisti et al., 2005), and the precursors progesterone and pregnenolone (1.5 g/kg; Boyd et al., 2010b). The absence of ethanol effects in the current study could be related to sex, dose or experimental history, for example chronic ethanol results in tolerance to ethanol-induced ACTH, progesterone and pregnenolone (Boyd et al., 2010a).
Overall, these studies suggest that, unlike primates, for which redundant receptor mechanisms mediate the discriminative stimulus effects of ethanol, female rodents use a combination of receptor mechanisms, with fewer female rats using neuroactive steroid-sensitive and 5-HT receptors compared with males. The lower sensitivity of females to the ethanol-like discriminative stimulus effects of neuroactive steroids in the current study resembles the lower sensitivity to allopregnanolone of ethanol drinking in females compared with males (mice; Finn et al., 2010). Additional studies could determine whether sex differences in the ethanol-like discriminative stimulus effects of neuroactive steroids could contribute to the biological bases underlying sex differences in ethanol self-administration.
Acknowledgments
The authors thank Chris Snelling for operation of the gas chromatograph, Hilary Gray and Marrie Getman-Pierce for assistance with vaginal cytology, and Matthew Ford for helpful comments. This work was supported by NIH/ NIAAA AA017040 (C.M.H.), AA007468 and AA020741 (T.M.M.), OD011092 and an Oregon National Primate Research Undergraduate Summer Fellowship (Silver Family Foundation, S.L.H.). This work was presented at the meeting of Experimental Biology – American Society for Experimental Therapeutics, Anaheim, California, 2010 (C116.767.7).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Smith AW, Centeno ML, Reddy AP. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2011;192:675–688. doi: 10.1016/j.neuroscience.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effects of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O’Buckley TK, Morrow AL. Chronic ethanol exposure produces tolerance to elevations in neuroactive steroids: mechanisms and reversal by exogenous ACTH. J Neurochem. 2010a;115:142–152. doi: 10.1111/j.1471-4159.2010.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O’Buckley TK, Porcu P, Morrow AL. Ethanol induction of steroidogenesis in rat adrenal and brain is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. J Neurochem. 2010b;112:784–796. doi: 10.1111/j.1471-4159.2009.06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT2A receptor binding in the rat. Psychopharmacology (Berl) 2007;190:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, et al. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Crippens D, White ML, George MA, Jaworski JN, Brunner LJ, Lancaster FE, Gonzales RA. Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcohol Clin Exp Res. 1999;23:414–420. [PubMed] [Google Scholar]
- Doods HN, Kalkman HA, De Jonge A, Thoolen MJMC, Wilffert B, Timmermans PBMWM, Van Zwieten PA. Differential selectivities of RU 24969 and 8-OH-DPAT for the purported 5-HT1A and 5-HT1B binding sites. Correlation between 5-HT1A affinity and hypotensive activity. Eur J Pharmacol. 1985;112:363– 370. doi: 10.1016/0014-2999(85)90782-4. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;267:489–495. [PubMed] [Google Scholar]
- Everett JW. Neurobiology of reproduction in the female rat. A fifty-year perspective. Monogr Endocrinol. 1989;32:1–133. [PubMed] [Google Scholar]
- Finn DA, Gee KW. The influence of estrus cycle on neurosteroid potency at the gamma-aminobutyric acid A receptor complex. J Pharmacol Exp Ther. 1993;265:1374–1379. [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: sex differences in the effects of alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Gallegos AI, Valasco J, Mendoza FD, Montiel C, Everardo PM, et al. The acute effects of bilateral ovariectomy or adrenalectomy on progesterone, testosterone and estradiol serum levels depend on the surgical approach and the day of the estrous cycle when they are performed. Reprod Biol Endocrinol. 2008;6:48. doi: 10.1186/1477-7827-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. New York: Raven Press, Ltd; 1994. pp. 613–658. [Google Scholar]
- Gerak LR, Moerschbaecher JM, Winsauer PJ. Overlapping, but not identical, discriminative stimulus effects of the neuroactive steroid pregnanolone and ethanol. Pharmacol Biochem Behav. 2008;89:473–479. doi: 10.1016/j.pbb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Def Res. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1992;264:1241–1247. [PubMed] [Google Scholar]
- Grant KA, Colombo G. Pharmacological analysis of the mixed discriminative stimulus effects of ethanol. Alcohol Alcohol. 1993a;2:445–449. [PubMed] [Google Scholar]
- Grant KA, Colombo G. Substitution of the 5-HT1 agonist trifluoromethylphenylpiperazine (TFMPP) for the discriminative stimulus effects of ethanol: effect of training dose. Psychopharmacology (Berl) 1993b;113:26–30. doi: 10.1007/BF02244329. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology (Berl) 1997a;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Colombo G, Gatto GJ. Characterization of the ethanol-like discriminative stimulus effects of 5-HT receptor agonists as a function of ethanol training dose. Psychopharmacology (Berl) 1997b;133:133–141. doi: 10.1007/s002130050383. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABAA receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CL, Rogers LS, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008;326:354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Azarov AV, Szeliga KT, Purdy RH, Grant KA. The influence of menstrual cycle phase on sensitivity to ethanol-like discriminative stimulus effects of GABAA-positive modulators. Pharmacol Biochem Behav. 1999;64:379–383. doi: 10.1016/s0091-3057(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Helms CM, Grant KA. The effect of age on the discriminative stimulus effects of ethanol and its GABAA receptor mediation in cynomolgus monkeys. Psychopharmacology (Berl) 2011;216:333–343. doi: 10.1007/s00213-011-2219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rogers LSM, Waters CA, Grant KA. Zolpidem generalization and antagonism in male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol. Alcohol Clin Exp Res. 2008;32:1197–1206. doi: 10.1111/j.1530-0277.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rossi DJ, Grant KA. Neurosteroid influences on sensitivity to ethanol. Front Endocrinol. 2012;3:1–19. doi: 10.3389/fendo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25:1441–1447. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Grant KA, Becker HC, Besheer J, Crissman AM, Platt DM, et al. Understanding how the brain perceives alcohol: neurobiological basis of ethanol discrimination. Alcohol Clin Exp Res. 2006;30:203–213. doi: 10.1111/j.1530-0277.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- Holzbauer M. Physiological variations in the ovarian production of 5alphapregnane derivatives with sedative properties in the rat. J Steroid Biochem. 1975;6:1307–1310. doi: 10.1016/0022-4731(75)90357-x. [DOI] [PubMed] [Google Scholar]
- Hundt W, Danysz W, Hölter SM, Spanagel R. Ethanol and N-methyl-Daspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998;135:44–51. doi: 10.1007/s002130050484. [DOI] [PubMed] [Google Scholar]
- Jones MK, Jones BM. The relationship of age and drinking habits to the effects of alcohol on memory in women. J Stud Alcohol. 1980;41:179–186. doi: 10.15288/jsa.1980.41.179. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Midanik LT. How many drinks does it take you to feel drunk? Trends and predictors for subjective drunkenness. Addiction. 2006;101:1428–1437. doi: 10.1111/j.1360-0443.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Boyd KN, Kumar S, Morrow AL. Systemic ethanol administration elevates deoxycorticosterone levels and chronic ethanol exposure attenuates this response. Brain Res. 2005;1049:104–111. doi: 10.1016/j.brainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Neuropharmacology. 2002;43:1129–1138. doi: 10.1016/s0028-3908(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes STARD1, CYP11A1 and HSD3B. Exp Biol Med. 2009;234:880–907. doi: 10.3181/0903-MR-97. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrus cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol. 2006;91:655–660. doi: 10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- Mancinelli R, Vitali M, Ceccanti M. Women, alcohol and the environment: an update and perspectives in neuroscience. Funct Neurol. 2009;24:77–81. [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic–pituitary–adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. p. 125. [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acid A receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Lenzi E, Casarosa E, Cela V, Begliuomini S, Ninni F, et al. Dydrogesterone increases allopregnanolone in selected brain areas and in serum of female rats. Fertil Steril. 2008;89:1384–1389. doi: 10.1016/j.fertnstert.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/ 3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Ethanol as a discriminative cue: reduction following depletion of brain serotonin. Eur J Pharmacol. 1973;24:278–281. doi: 10.1016/0014-2999(73)90085-x. [DOI] [PubMed] [Google Scholar]
- Signs SA, Schechter MD. The role of dopamine and serotonin receptors in the mediation of the ethanol interoceptive cue. Pharmacol Biochem Behav. 1988;30:55–64. doi: 10.1016/0091-3057(88)90424-8. [DOI] [PubMed] [Google Scholar]
- Stojanović N, Budec M, Jovcić G, Bugarski D, Todorović V. Effect of a single dose of ethanol on granulopoiesis in female rats: relationship to phase of estrous cycle. J Stud Alcohol. 1996;57:344–348. doi: 10.15288/jsa.1996.57.344. [DOI] [PubMed] [Google Scholar]
- Szeliga KT, Grant KA. Analysis of the 5-HT2 receptor ligands dimethoxy- 4-indophenyl-2-aminopropane and ketanserin in ethanol discriminations. Alcohol Clin Exp Res. 1998;22:646–651. doi: 10.1111/j.1530-0277.1998.tb04306.x. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl-D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology (Berl) 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- York JL. A comparison of the discriminative stimulus effects of ethanol, barbital, and phenobarbital in rats. Psychopharmacology (Berl) 1978;60:19–23. doi: 10.1007/BF00429173. [DOI] [PubMed] [Google Scholar]