Abstract
Objectives
Patients with advanced pancreatic neuroendocrine tumors (PNET) have limited therapeutic options. RAD001, an inhibitor of the mammalian target of rapamycin (mTOR) pathway, has been shown to increase progression-free survival, but not overall survival, indicating a need to identify additional therapeutic targets. Inhibition of mTORC1 by RAD001 may induce upstream AKT upregulation. We hypothesized that dual inhibition of AKT along with mTOR will overcome the limited activity of RAD001 alone.
Methods
The BON cell line has been used as a model to study PNET cell biology. Western blots and cell growth assays were performed with mTOR inhibitor RAD001 (50 nM), MEK inhibitor PD0325901 (50 nM), PI3K inhibitor LY294002 (25 μM) or vehicle control. Nude mice were treated daily for 6 weeks with RAD001 (oral gavage), LY29400 (SQ) one week after intrasplenic injection of BON cells.
Results
Cellular proliferation was most attenuated with the combination therapy LY29400 and RAD001. Similarly, the volume of liver metastasis was lowest in the group treated with both LY29400 (100 mg/kg/week, SQ) and RAD001 (2.5 mg/kg/d) compared to vehicle (p=0.04).
Conclusion
The combination LY29400 and RAD001 decreased the cell growth in vitro and progression of liver metastasis in vivo compared vehicle or to single drug.
Keywords: pancreatic neuroendocrine tumor, liver metastasis, RAD001, BON cell
INTRODUCTION
Pancreatic neuroendocrine tumors (PNET) account for approximately 1% of newly diagnosed pancreatic neoplasms, with an annual incidence of 3.2 cases per 100,000 populations.1 Although the incidence has more than doubled over the past two decades,2 the median survival for patients with advanced disease (metastatic) remains ~24 months, with 5-year survival rates of 30 to 40%.3 Unfortunately, 65% of patients present with unresectable or metastatic disease,1 and treatment options for this subgroup of patients are limited.
Recently, everolimus (RAD001) has emerged as a treatment option for treatment of patients with advanced PNET. RAD001, a rapamycin-derivative [40-O-(2-hydroxyethyl)-rapamycin, Novartis] is an orally bioavailable mTOR inhibitor that has been shown in a phase-3 clinical trial for patients with advanced PNETs, to prolong disease-free progression,4 but not overall survival. Furthermore, in 73% of patients on everolimus, the tumor burden remained static with 14% of patients developing progressive disease. These results indicate a need to identify additional therapeutic targets.
The human BON cell, a well-known experimental model for PNET, was derived from a metastatic lymph node of a patient with PNET.5 These cells have been shown to secrete insulin-like growth factor-1 (IGF-1) and activate insulin-like growth factor-1 receptor (IGF-1R) in an autocrine fashion.6 IGF-1R is a receptor tyrosine kinase, which in turn activates insulin receptor substrate (IRS) proteins and phosphatidylinositol 3-kinases (PI3K) mobilization. PI3K bound to IRS converts phosphotidylinositol-4,5-biphosphate into the lipid second-messenger phosphatidylinositol-3,4,5-triphosphate, which recruits protein kinase B(PKB/AKT) to the plasma membrane. There, AKT is activated by phosphatidylinositol-3-dependent kinase 1 (PDK1). Phosphorylated AKT activates the serine/threonine protein kinase, mTOR, which then regulate two downstream substrates known to regulate transcription and translation of growth genes: eukaryotic translation initiation factor 4E (eIF4E)-binding protein (4EBP1) and p70 ribosomal S6 kinase (p70S6K).6
However, inhibition of mTOR Complex 1 (mTORC1) by RAD001 may induce upstream AKT upregulation.7 RAD001 treatment releases the negative-feedback exerted by activated p70S6K on IRS, leading to activation of the PI3K/AKT pathway.8 The IGF-1/IGF1-R signaling axis also induces activation of the mitogen-activated protein kinase (MAPK)/extracellular-regulated kinase (ERK) pathway.6 Thus, upregulation of the ERK pathway and/or the PI3K/AKT pathway (mediated by RAD001) may be responsible for the modest therapeutic effects of RAD001.
We hypothesized that the addition of a MAPK/ERK inhibitor and/or PI3K inhibitor to the standard RAD001 treatment will further limit tumor progression in a preclinical model of PNET using BON cells. RAD001 combined with either PD98059 or PD0325901, MAPK inhibitors, did reduce BON cell growth; however, when RAD001 was combined with LY29400, a PI3K inhibitor, BON cell growth was more markedly attenuated. Similarly, the volume of liver metastasis was lowest in the group treated with both LY29400 and RAD001.
MATERIALS AND METHODS
Reagents
RAD001, PD0325901, and LY294002 were purchased from Selleck Chemicals. PD98059 was purchased from Invitrogen. Everolimus (oral RAD001) was provided by Novartis Pharma (Basel, Switzerland) and used only for in vivo studies. Antibodies against pp70S6 kinase (#9234), pERK (#9102), and pAKT (#9271) were purchased from Cell Signaling (Beverly, MA). The antibody against β-actin (#926-42212), the secondary goat anti-rabbit IR Dye (#926-32211), and goat anti-mouse IRDye (#926-68020) were purchased from LI-COR Biosciences (Lincoln, NE).
Cell culture
BON cells are a PNET cell line derived from a human peripancreatic lymph node5 and has been used as a model for both in vitro and in vivo PNET disease. BON cells are maintained in a 1:1 mixture of DMEM:F12 supplemented with 10% FBS in 5% CO2 at 37°C.
Western blot analysis
Cells (2 × 106) were plated and were incubated in media with 10% FBS over a time course, and treated with RAD001 (50 nM), PD0325901 (50 nM) or PD98059 (20 μM), LY294002 (25 μM) or DMSO (vehicle). Cell were lysed in 50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 1 mM NaF, 1 mM PMSF, and 1 Protease Inhibitor Cocktail Tablet/50 ml (Roche Applied Science, Indianapolis, IN). Protein concentrations (30 μg/lane) were determined using BioRad Dye Reagent (BioRad Laboratories, Inc., Hercules, CA), resolved on 4–12% polyacrylamide gels (Invitrogen, Carlsbad, CA), transferred to a PVDF membrane (Millipore Corporation, Bedford, MA), and blocked with Odyssey blocking buffer (LICOR Biosciences). Primary antibodies (pERK, pAKT, pP70S6K, and β-actin) were used at dilutions of 1:1000. Secondary antibodies were used at a 1:10,000 dilution. Specific immunoreactive bands were detected by the Odyssey® Infrared Imaging System (LI-COR Biosciences). Densitometry of the appropriately sized bands comparing the relative expression of pAKT, pp70S6K, and pERK, were normalized by β actin signal for each lysate, and all samples were compared to a control lysate (vehicle). Quantification was performed using the Odyssey® Molecular Imaging software (LI-COR Biosciences). All protein isolations and Western blots were repeated a minimum of 3 times.
Cell proliferation
Cells (2 × 105) were plated in duplicate in 6-well plates. The following day, the media was changed to 1% FBS with either DMSO, MEK (PD0325901 or PD98095), AKT (LY29400), and/or mTOR (RAD001) inhibitors. Cell growth was measured over time; cells were quantified using a Coulter Counter (Beckman Coulter, Inc.). This experiment was repeated three times.
In vivo liver metastasis assay
For in vivo studies, female nu/nu mice (6 weeks old) were injected intrasplenically (liver metastasis model9 with 3×106 (100 μl) BON cells. One week later, the mice were randomized and treated daily for 6 weeks with RAD001 (2.5 mg/kg/d, oral gavage), LY294002 (100 mg/kg/week, SQ), both RAD001 and LY294002, or placebo controls. The experiment was repeated three times. This study protocol was approved by our Institutional Committee on (IACUC) and conducted according to guidelines for animal care and protection.
Statistical Analysis
Statistical analysis was performed using Fisher’s Exact Test and unpaired t-test (GraphPad InStat 5.0). Significance was set at p<0.05.
RESULTS
Both the AKT/mTOR and MEK/ERK pathways are activated in BON cells
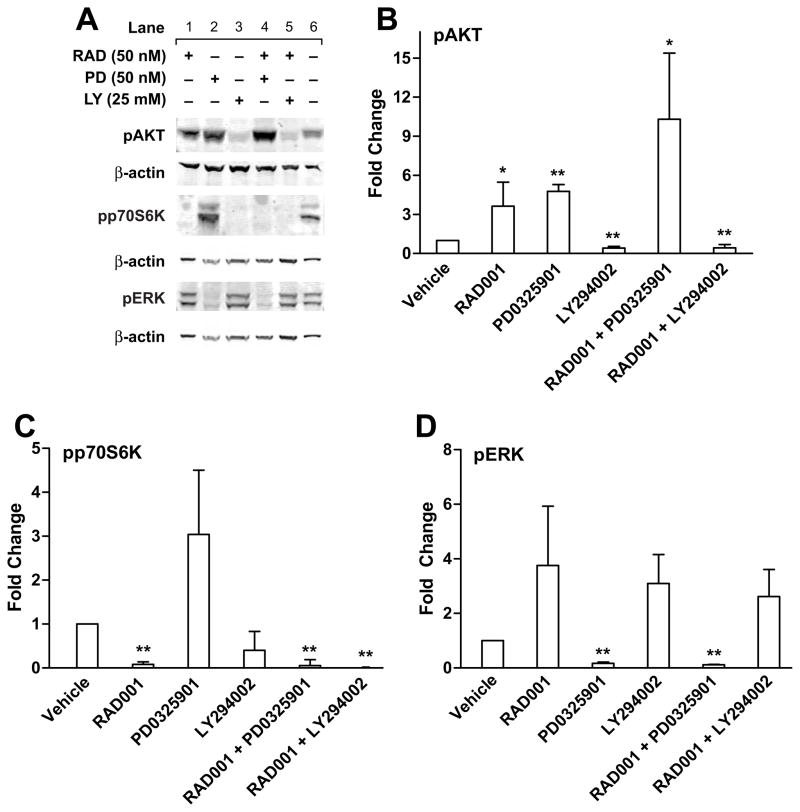
Shah et al10 performed immunohistochemistry on surgically resected neuroendocrine tumors, both primary and metastatic, from 98 patients and demonstrated overexpression of pAKT and pERK in 76% and 96% of the samples. Since RAD001 inhibits the mTOR pathway, we reasoned that upstream inhibition of either the AKT or MAPK pathway, in combination with mTOR inhibition, would further decrease BON cell growth than RAD001 treatment alone. First, we verified that when BON cells are grown in standard media (Fig. 1A: vehicle, lane 6), there is steady state activation of AKT, p70S6K, and ERK1/2 due to the autocrine IGF/IGF1-R signaling pathway, as expected. To downregulate these phospho-proteins, we used LY294002 (a reversible, ATP-competitive PI3K inhibitor), RAD001 (an mTOR inhibitor), and PD0325901 (a MEK inhibitor). BON cells were treated with LY294002 (25 μM), RAD001 (50 nM) and/or PD0325901 (50 nM) for 30 minutes (Figure 1A) and 3 hours (data not shown). BON cells showed a 57% inhibition (Fig. 1A, lane 3 and Fig. 1B) in pAKT protein expression, 92% decrease for pp70S6K (Fig. 1A, lane 1 and Fig. 1C), and a 98% decrease in pERK levels (Fig. 1A, lane 2 and Fig. 1D) when treated with LY294002, RAD001 and PD0325901, respectively. RAD001 increased pAKT levels 3.6-fold at 30 minutes (Fig. 1A, lane 1 and Fig. 1B), and 8.5-fold at 3 hours (p<0.05, data not shown), as previously reported.11 Compared with LY294002 alone (Fig. 1A, lane 3), the addition of with RAD001 with LY294002, no further suppression of AKT or p70S6K activation (Fig. 1A, lane 5 and Fig. 1B, C) was detected. Interestingly, inhibition of MEK increased pAKT 4.7-fold (Fig. 1A, lane 2; Fig. 1B) (p=0.0001); combined inhibition with PD0325901 and RAD001 enhanced pAKT expression 10.31-fold (Fig. 1A, lane 4, Fig. 1B) (p=0.02). These data suggest that the MAPK/MEK pathway suppresses the AKT survival pathway. These experiments have been repeated with the BON cells exposed to an alternate MEK inhibitor PD98095 (10 μM), with similar results (data not shown).
Figure 1.
Effect of mTOR inhibitor RAD001 (RAD), MEK inhibitor PD0325901 (PD) and PI3K inhibitor LY294002 (LY), as single agents and in dual combination, on MAPK and AKT signal transduction. (A) BON cells were treated with the indicated inhibitors, for 30 minutes. Cell lysates were subjected to electrophoresis, followed by Western blotting using the indicated phosphor-specific antibodies. Blots were then stripped and reprobed with βactin to confirm equal protein loading. Representative blots are shown. (B, C, D). Graphical representation of protein expression levels (pAKT, pp70S6K, and pERK1,2, respectively) relative to vehicle control treated BON cells from 3 independent experiments. T-test was used to compare vehicle vs. treatment (*p<0.05, **p<0.01).
Dual inhibition with RAD001 and LY294002 suppressed BON cell growth in vitro
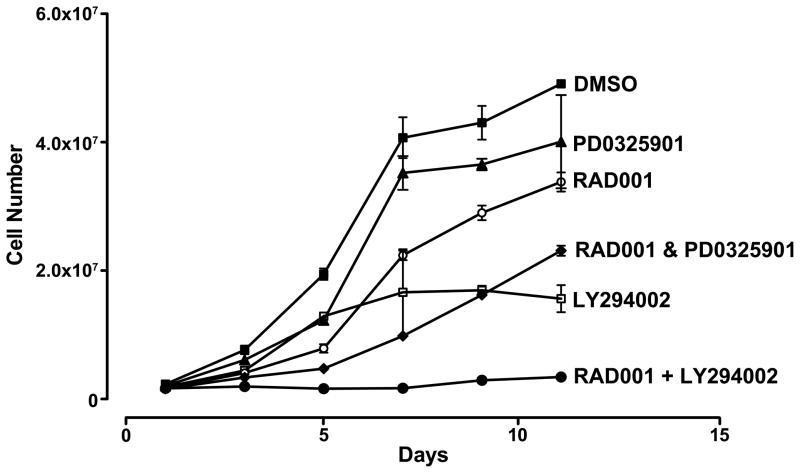
Although we confirmed that RAD001 treatment upregulates pAKT activity in BON cells, we demonstrated that the addition of LY294002 with RAD001 checked pAKT activation (Fig. 1A, lane 5). To test whether BON cell growth can be inhibited with combination therapy, cell numbers were measured over 11 days in duplicate using a Coulter Counter. Cellular growth was only moderately suppressed in the presence of MEK inhibitor PD0325901 (50 nM) or RAD001 (50 nM) alone. The reduction in cell growth over time using the combination of both RAD001 with PD0325901 was similar to the addition of LY294002 (25 μM) alone. Maximal inhibition of cell growth was achieved using both RAD001 and LY294002 (Fig. 2). Treatment with all three inhibitors showed the same decrease in cell growth as with the two inhibitors RAD001 and LY294002 (data not shown).
Figure 2.
Effect of mTOR inhibitor RAD001, MEK inhibitor PD0325901, and PI3K inhibitor LY294002, as single agents and in dual combination, compared to vehicle treatment on BON cell growth in vitro over time.
Dual inhibition with RAD001 and LY294002 suppressed BON cell growth in vivo
Approximately 92% of the patients enrolled into the phase-3 clinical trial testing RAD001 versus best supportive care had pre-existing liver metastases.4 To test whether dual therapy with RAD001 and LY294002 improves outcome by limiting the progression of liver metastases in PNET, we next tested this combination of drugs in vivo. Using an experimental model of liver metastases,9 nu/nu mice were intrasplenically injected (method of portal vein access) with BON cells. After one week, mice were randomized and treated over 6 weeks with vehicle (daily oral gavage of placebo solution and weekly DMSO SQ injection) (Group A), RAD001 via daily oral gavage and weekly DMSO SQ (Group B), daily vehicle gavage and weekly LY294002 SQ (Group C), and both RAD001 and LY294002 (Group D). Table 1 summarizes the combined results of three experiments. Mice were deemed to have bulky liver metastasis if the gross appearance of the liver showed >50% of the liver replaced with tumor (Fig. 3, a single representative experiment). Treatment with placebo and vehicle resulted in 68% of the mice developing significant bulky liver metastases. Single-agent treatment with either RAD001 or LY294002 showed that more than half of the mice at sacrifice had significant liver tumor burden (p=N.S.). The volume of gross liver metastases appeared lowest in the group treated with both LY29400 and RAD001, with only 28% of mice demonstrating >50% of the liver replaced with tumor (p=0.04, comparing placebo/vehicle treatment-Group A with those receiving both therapies-Group D). Comparison of the control group with either single agent therapy did not reach significance.
Table 1.
Summary Results of Three Combined In Vivo Experiments.
| Treatment Group | Number of mice with >50% tumor burden in the liver | P-value |
|---|---|---|
| A. Vehicle | 13/19 (68%) | - |
| B. RAD001 | 6/11 (54%) | N.S. |
| C. LY294002 | 10/18 (56%) | N.S. |
| D. RAD001 & LY294002 | 4/14 (28%) | 0.04 |
Fisher’s Exact Test, compared to Group A.
N.S. = not significant.
Figure 3.
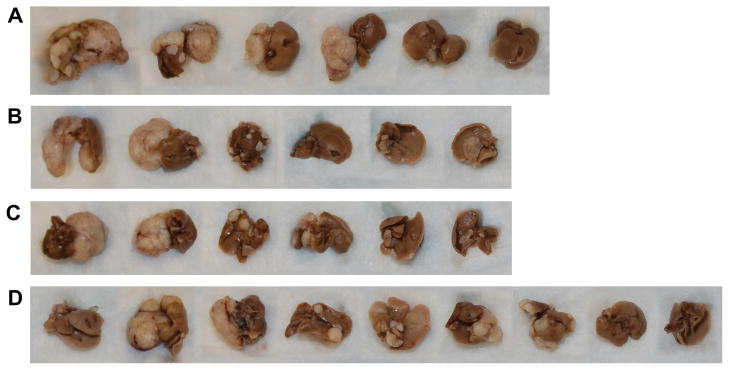
Effect of vehicle treatment (A), mTOR inhibitor RAD001 (B), PI3K inhibitor LY294002 (C) as single agents, and in dual combination (D), in an in vivo model of hepatic metastases from BON cells (representative experiment shown). For example, bulky tumor (>50% of the liver parencyma by gross inspection) was seen in 4/6 livers. See Table 1 for Summary of 3 combined experiments.
DISCUSSION
Patients with advanced/unresectable pancreatic neuroendocrine tumors have limited therapeutic options. Historically, patients with untreated liver metastasis have a 5-year survival of 20–30%.12 Patients treated with RAD001 have an increase in median progression-free survival of 11.4 months compared to those treated with placebo (5.4 months).4 RAD001, targeting the mTOR pathway, only offers modest clinical benefit; therefore, improved therapeutic strategies are needed.
Similar to previous work,7, 11, 13 we show that suppression of mTOR enhances AKT phosphorylation, indicating a well-recognized mechanism of RAD001 resistance. To overcome RAD001-mediated AKT activation, we demonstrated that the addition of LY294002 inhibits the overexpression of pAKT, further decreases cellular growth of BON cells in vitro, and decreases the progression of hepatic tumor burden in vivo. LY294002 has been shown to decrease cell proliferation in BON cells by inducing G1 cell cycle arrest.14 Preclinical studies using the BON cell line have shown that RAD001 also decreases cellular proliferation by inducing G1 cell cycle arrest.11
Additionally, we show that PD0325901-mediated repression of MEK enhanced pAKT protein expression (Fig. 1A, lanes 2, 4). PD0325901, alone or in conjunction with RAD001, only partially decreased cell growth in vitro (Fig. 2). Together, these data support the hypothesis that the MAPK/MEK pathway suppresses the AKT survival pathway in BON cells.
Clinically, the effects of RAD001 are tumoristatic, with 73% of the patients in the phase III trial showing stable tumor size and slowed progression of disease by radiographic criteria, with an overall tumor response rate of only 5%.4 Disease stabilization translates into prolonged progression free survival, but not a significant difference in overall survival, which is consistent with most therapies for unresectable or metastatic solid tumors. Our in vivo data demonstrates that inhibition of both pAKT and mTOR decreases the progression of bulky liver metastases compared to placebo, whereas treatment with a single inhibitor was not significant compared to vehicle. Based on findings from our in vitro studies, the addition of an MEK inhibitor in vitro increased the activation of AKT and furthermore, triple drug therapy did not further decrease in cell proliferation. Therefore, we did not pursue this line of investigation in our mouse model of liver metastasis.15
In summary, we demonstrate the efficacy of dual inhibition of PI3K and mTOR in a preclinical model of PNET. Combination therapy is more effective in vitro and in vivo than either inhibitor alone, suggesting that dual PI3K/mTOR inhibitors such as NVP-BEZ23516, 17 may be useful in patients with advanced PNET. Currently, a phase II clinical trial (NCT01628913, registered at www.clinicaltrials.gov) is accruing patients with advanced PNET to be randomized to NVP-BEZ235 or everolimus. Primary outcome measures include drug safety, efficacy and progression-free survival compared to everolimus. In preclinical studies, Zitzmann and colleagues18 showed that NVP-BEZ235 decreased pAKT expression at two hours in BON cells, whereas RAD001 at the same time point induced upregulation of pAKT; however, after 24 hours of treatment with NVP-BEZ235, expression of pAKT increased to the level of RAD001-treated BON cells. They also used an IGF-IR inhibitor, NVP-AEW541 to downregulate AKT in vitro, but did not show its effects in combination with RAD001. Future lines of investigation are needed to determine whether better clinical outcomes can be achieved combining RAD001 with targeted inhibitors that block at the cell surface receptors on the tumor cell such as NVP-AEW54, whether the inhibitors should be delivered sequentially or concomitantly.
Acknowledgments
We thank Novartis (Basel, Switzerland) for the gift of everolimus (RAD001) and the placebo control. We thank Eileen Figueroa and Steve Schuenke for their assistance with manuscript preparation.
Footnotes
Authors declare no conflicts of interest.
This study was presented as a poster at the Joint APA, IAP 2012 meeting, Miami Beach, Florida on November 1, 2012.
Conflicts of Interest and Source of Funding: This study was supported by grants from the National Institutes of Health (T32-DK007639 to MRH and K08-CA125209 to CC).
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Enewold L, Stojadinovic A, et al. Incidence rates of exocrine and endocrine pancreatic cancers in the United States. Cancer Causes Control. 2010;21:853–861. doi: 10.1007/s10552-010-9512-y. [DOI] [PubMed] [Google Scholar]
- 3.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evers BM, Ishizuka J, Townsend CM, Jr, et al. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci. 1994;733:393–406. doi: 10.1111/j.1749-6632.1994.tb17289.x. [DOI] [PubMed] [Google Scholar]
- 6.von Wichert G, Jehle PM, Hoeflich A, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–4581. [PubMed] [Google Scholar]
- 7.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavrommati I, Maffucci T. mTOR inhibitors: facing new challenges ahead. Curr Med Chem. 2011;18:2743–2762. doi: 10.2174/092986711796011247. [DOI] [PubMed] [Google Scholar]
- 9.Jackson LN, Chen LA, Larson SD, et al. Development and characterization of a novel in vivo model of carcinoid syndrome. Clin Cancer Res. 2009;15:2747–2755. doi: 10.1158/1078-0432.CCR-08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah T, Hochhauser D, Frow R, et al. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J Neuroendocrinol. 2006;18:355–360. doi: 10.1111/j.1365-2826.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 11.Zitzmann K, De Toni EN, Brand S, et al. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology. 2007;85:54–60. doi: 10.1159/000100057. [DOI] [PubMed] [Google Scholar]
- 12.Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–785. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 14.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16:2936–2942. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts PJ, Usary JE, Darr DB, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res. 2012;18:5290–5303. doi: 10.1158/1078-0432.CCR-12-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 17.Baumann P, Mandl-Weber S, Oduncu F, et al. The novel orally bioavailable inhibitor of phosphoinositol-3-kinase and mammalian target of rapamycin, NVP-BEZ235, inhibits growth and proliferation in multiple myeloma. Exp Cell Res. 2009;315:485–497. doi: 10.1016/j.yexcr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Zitzmann K, von Ruden J, Brand S, et al. Compensatory activation of Akt in response to mTOR and Raf inhibitors - A rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Lett. 2010;295:100–109. doi: 10.1016/j.canlet.2010.02.018. [DOI] [PubMed] [Google Scholar]