Abstract
The amygdala has been described as a structure affected by mesial temporal lobe epilepsy (MTLE). Indeed, it is suggested that amygdala abnormalities are related to the co-morbid depression and anxiety reported in MTLE. In this context, we investigated the relation between functional connectivity (FC) emerging from this structure in fMRI and depression and anxiety levels reported in MTLE patients. We focused on resting-state BOLD activity and evaluated whether FC differences emerge from each of three amygdala subdivisions (laterobasal, centromedial and superficial) in left and right MTLE groups, compared with healthy controls. Results revealed significant differences between patient groups and controls. Specifically, the left MTLE group showed abnormal FC for the left-sided seeds only. Furthermore, regardless of the seed, we observed more reliable differences between the right MTLE group and controls. Further analysis of these results revealed correlations between these impaired connectivities and psychiatric symptoms in both MTLE groups. Opposite relations, however, were highlighted: the more depressed or anxious the right MTLE patients, the closer their FC values approached controls; whereas the less anxious the left MTLE patients, the closer their FC values were normative. These results highlight how MTLE alter FC emerging from the limbic system. Overall, our data demonstrate that right TLE has a more maladaptive impact on emotion-related networks, in ways specific to the amygdala region, and the emotion symptom involved, than left TLE.
Keywords: resting state functional connectivity, amygdala, depression, anxiety, epilepsy
1 Introduction
Mesial temporal lobe epilepsy (MTLE) is the most frequent form of refractory epilepsy, and is commonly associated with mesial temporal sclerosis (MTS). There is a growing body of evidence that brain abnormalities in MTLE are not limited to the hippocampus and epileptogenic temporal lobe, but extend into several widespread areas in both hemispheres (review of Gross, 2011), as a direct result of the pathological processes inherent to seizure generalization and spread. The amygdala appears to be a structure particularly influenced by these effects. In temporal lobe epilepsy the amygdala is considered to be part of the epileptogenic network, and it is commonly removed during a standard en bloc resection of the anterior temporal lobe (ATL).
Some studies describe the amygdala ipsilateral to the epileptogenic hippocampus as atrophic, with the contralateral amygdala remaining normal in unilateral MTLE (Alhusaini et al., 2012; Bernasconi et al., 2003; Bernasconi et al., 2005). Other studies (Tebartz Van Elst et al., 2002) describe an enlargement of both amygdala in these patients. The functional consequences of these potential amygdala effects appear to be in the realm emotion processing and psychiatric symptoms, a consequence that appears relevant for the case of TLE, as depression and anxiety represent the most commonly reported comorbid symptoms in TLE (Tracy et al., 2007a; Tracy et al., 2007b). Some neuropsychological studies have strongly supported the notion that depression in TLE is caused by pathological epileptic activity (Reuber et al., 2004). Given the major role of the amygdala in the processing of fear and related emotions (LeDoux, 2000; Phelps and LeDoux, 2005), several studies have suggested and described the role of this structure to the expression of such co-morbid emotional conditions (see review of Kondziella et al., 2007). Indeed, a positive correlation has been described between the left amygdala volume and depression severity in left TLE patients (Tebartz van Elst et al., 2000; Tebartz van Elst et al., 1999), with the authors suggesting the amygdala is hyperactive in anxiety and mood disorders (Tebartz van Elst et al., 1999). This hypothesis is also coherent with physiological studies that have demonstrated a positive correlation between the density of neuropeptide Y positive neurons in the amygdala and depression scores in MTLE patients (Frisch et al., 2009). Also, psychiatric studies have revealed a direct relation between atypical amygdala responsiveness, and either anxiety (Killgore and Yurgelun-Todd, 2005; Thomas et al., 2001) or depression level (Abercrombie et al., 1998; Roberson-Nay et al., 2006).
Using neuroimaging techniques, independent of the level of depression, there is a growing body of evidence that functional abnormalities in MTLE are not limited to the epileptogenic region, and involve amygdala among other regions. For instance, task-driven functional MRI imaging provided evidence of amygdala abnormalities in MTLE. Bonelli et al. (2009) showed that left MTLE patients had significantly reduced activation in left and right amygdala compared to controls and right MTLE patients during the viewing of fearful and neutral faces. In addition, these authors demonstrated that in right but not the left MTLE patients, bilateral amygdala activation was significantly related to the level of anxiety and depression reported. More recently, Broicher et al. (2012) examined unilateral MTLE patients and described unilateral amygdala activation lateralized to the contralateral side in response to an animated fearful face task, in contrast to the bilateral activation observed in healthy controls. Resting-state functional magnetic resonance imaging (fMRI) studies have started to provide evidence of connectivity abnormalities with the amygdala contralateral to the epileptogenic source in unilateral MTLE patients. Indeed, Bettus et al. (2010) described increased functional connectivity preferentially involving the amygdala and hippocampus in the non-epileptogenic hemisphere, relative to controls. Zhang et al. (2011) also showed an increase of FC emerging from the amygdala with the rest of the brain in patients suffering from idiopathic generalized epilepsy. Chen et al. (2012) described the opposite pattern with a reduced activity in the left amygdala in TLE patients suffering from depressive disorders. Overall, results from the neuroimaging studies involving amygdala activity in MTLE are still inconsistent, particularly with regard to the strength of the relationship to specific emotions and the hemispheric bias that might be at work.
One potential explanation to these inconsistencies is that the majority of these studies considered the amygdala as one homogeneous structure. However, more and more evidence suggests this region should not be considered a single structure, but rather as a complex of structurally and functionally heterogeneous nuclei. Indeed, both structural (Sheline et al., 1998) and functional (Morris et al., 2001; Whalen et al., 2004) neuroimaging studies have demonstrated such distinctions in the human amygdala. Accordingly, three major subdivisions have been described recently by Amunts et al. (2005). The laterobasal (LB) subregion includes the lateral, basolateral, basomedial, and basoventral nuclei, with its functional role described as facilitating associative learning processes (LeDoux, 2003; Phelps and LeDoux, 2005). The centromedial (CM) subregion, composed of the central and medial nuclei, considered to play a role in generating behavioral responses (Davis, 1997; LeDoux, 2003). Finally, the superficial (SF) subdivision of the amygdala laying adjacent to the laterobasal region, appearing to be the structure most closely associated with affective processes (Gonzalez-Lima and Scheich, 1986). Using this partition, Roy et al. (2009) demonstrated distinct functional connectivity patterns throughout the brain, emerging from each amygdala subregion in a sample of healthy participants. More specifically, spontaneous activity in the LB subregion showed specific activity in temporal and frontal regions, while activity in the CM nuclei was associated with activity primarily in striatum. Activity in the SF subdivision was positively correlated with activity throughout the limbic system. In mesial temporal lobe epilepsy, to the best of our knowledge, these distinct structural/functional relationships have not been investigated.
In the current project, we sought to investigate functional connectivity (FC) changes within the network emerging from each amygdala's subdivision in right and left unilateral MTLE patients, in comparison to healthy participants. Accordingly, we utilized fMRI resting-state data from groups of right MTLE (RMTLE), left MTLE (LMTLE) patients, and healthy controls. We postulated that the left and right MTLE patients would show quite different patterns of FC abnormalities, relative to controls. We also explored the possibility that these potential FC abnormalities in patients may be related to their level of depression or anxiety, evaluated through a well-standardized instrument: the Personality Assessment Inventory (PAI, Morey, 1991). We hypothesized that a FC-based approach will clarify the contribution of each amygdala region to anxiety and depression symptomatology in unilateral MTLE.
2 Material and Methods
2.1. Participants
2.1.1. MTLE Patients
A total of 22 patients with refractory mesial temporal lobe epilepsy, all with unilateral temporal sclerosis (11 left and 11 right), were recruited from the Thomas Jefferson University Comprehensive Epilepsy Center. A combination of EEG, MRI, PET, and neuropsychological testing was used to lateralize the side of seizure focus (Sperling et al., 1992). All patient participants met the following inclusion criteria: unilateral temporal lobe seizure onset through surface video/EEG recordings (i.e., a single unilateral mesial temporal lobe focus); MRI evidence of mesial temporal lobe pathology in the epileptogenic temporal lobe; concordant PET finding of hypometabolism in the temporal lobe (available for most patients, and no patient had a non-concordant PET); Full-Scale IQ of at least 75. MTLE patients were excluded from the study for any of the following: medical illness with central nervous system impact other than epilepsy; head trauma; prior or current alcohol or illicit drug abuse; extratemporal or multifocal epilepsy; contraindications to MRI; psychiatric diagnosis other than an Axis-I Depression or Anxiety Disorder; or hospitalization for any Axis I disorder listed in the Diagnostic and Statistical Manual of Mental Disorders, IV. Participants provided written informed consent. The study was approved by the Institutional Review Board for Research with Human Subjects at Thomas Jefferson University. Table 1 outlines the demographic, clinical and neuropsychological characteristics of the subjects. The Edinburgh handedness scale was used as a measure of handedness (Oldfield, 1971).
Table 1.
Clinical information and characteristics of MTLE patients and controls.
| RMTLE | LMTLE | Controls | |
|---|---|---|---|
| N (female) | 11 (6) | 11 (8) | 19 (10) |
| age (m ± std, years) | 49 ± 12 | 42 ± 9 | 42 ± 13 |
| Right-Handers (N) | 10 | 10 | 16 |
| Duration of Epilepsy (m ± std, yrs.) | 27 ± 16 | 25 ± 15 | - |
| Seizure Type | CPS: 8 | CPS: 6 | - |
| CPS*: 1 | CPS*: 2 | - | |
| CPS**: 0 | CPS**: 2 | - | |
| CPS/SPS: 1 | CPS/SPS: 1 | - | |
| SPS: 1 | SPS: 0 | - | |
| Full Scale IQ (m ± std) | 94 ± 14 | 94 ± 15 | - |
| Anti-Depressive Medication % (N) | Lexapro: 18% (2) | Lexapro: 9% (1) | Lexapro: 0 |
| Zyprexa: 9% (1)¥ | Zyprexa: 0 | Zyprexa: 0 | |
| Ativan: 9% (1)¥ | Ativan: 0 | Ativan: 0 | |
| Zoloft: 9% (1) | Zoloft: 0 | Zoloft: 0 | |
| Celexa: 9% (1) | Celexa: 9% (1) | Celexa: 0 | |
| Depressive and Anxiety level (m ± std) | |||
| PAI-ARD | 52 ± 12 | 52 ± 11μ | 50 ± 10π |
| PAI-ANX | 56 ± 8 | 55 ± 13μ | 50 ± 10π |
| PAI-DEP | 55 ± 13 | 55 ± 12μ | 50 ± 10π |
¥: Same patient; π: Normative results obtained on 1000 healthy participants (Morey, 1991); μ : Average done on 9 LMTLE patients; μ : Average done on 9 LMTLE patients
CPS as primary type with rare secondary generalized seizures
CPS as primary type with rare generalized tonic-clonic seizures.
Abbreviations: PAI: Personality Assessment Inventory, ANX: Anxiety subscale, ARD: Anxiety-Related Disorders subscale, DEP: Depression subscale; CPS: Complex Partial Seizures; SPS: Secondary Partial Seizures.
2.1.2. Normal Controls
A total of 19 healthy normal controls (NCs) were also recruited from the Thomas Jefferson University community, in order to match the patient participants in age and gender (Table 1). All controls were free of psychiatric or neurological disorders based on health screening measure. This study was submitted for approval by the Institutional Review Board for Research with Human Subjects at Thomas Jefferson University and all participants have provided a written informed consent.
2.2. EEG Procedures for TLE patients
EEG was obtained using the 10-20 system with anterior temporal electrodes and, at times, sphenoidal electrodes that used a Grass-Telefactor 32 channel acquisition system. At least 96 hours of continuous EEG recording, with selected samples in wakefulness and sleep examined both by registered EEG technologists, board certified electroencephalographers, and through commercial automated spike detection program (SZAC, Grass Telefactor) to determine location and lateralization of interictal spikes and seizures.
2.3. Participant MRI Data Acquisition
All patients and normal controls underwent Magnetic Resonance Imaging on a 3-T X-series Philips Achieva clinical MRI scanner (Amsterdam, the Netherlands) using an 8-channel head coil. A total of 5 minutes of a resting state condition was collected on all subjects. Anatomical and functional acquisitions were similar for all patients. Single shot echoplanar gradient echo imaging sequence acquiring T2* signal was used with the following parameters: 120 volumes, 34 axial slices acquired parallel to the AC-PC line, TR=2.5 sec, TE=35 msec, FOV=256 mm, 128×128 data matrix isotropic voxels, flip angle=90°, bandwidth=1.802(±241.1 kHz). The in-plane resolution was 2*2mm2 and the slice thickness was 4 mm. Prior to collection of the T2* images, T1-weighted images (180 slices) were collected using an MPRage sequence (256×256 isotropic voxels; TR=640 msec, TE=3.2 msec, FOV=256 mm, flip angle=8°) in positions identical to the functional scans to provide an anatomical reference. The in-plane resolution for each T1 slice was 1 mm3 (axial oblique; angle following anterior, posterior commissure line). Survey and field reference inhomogeneity images were collected prior to the start of the study. Each EPI imaging series started with three discarded scans to allow for T1 signal stabilization. Subjects lay in a foam pad to comfortably stabilize the head, were instructed to remain still throughout the scan, not focus on any particular activity or thing, and to keep their eyes closed during the entirety of the scan.
2.4. Imaging Processing
Data from the MTLE patients and normal controls were preprocessed identically using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Slice timing correction was used to adjust for variable acquisition time over slices in a volume, with the middle slice in every volume used as reference. Next, a six-parameter variance cost function rigid body affine registration was used to realign all images within a session to the first volume. Motion regressors were computed and later used as regressors of no interest. To maximize mutual information, coregistration between functional scans and the MNI305 (Montreal Neurological Institute) template was carried out using six iterations and resampled with a 7th-Degree B-Spline interpolation. Functional images were then normalized into standard space (MNI305) to allow for signal averaging across subjects. We utilized the standard normalization method in SPM5, which minimizes the sum-of-squared differences between the subject's image and the template (MNI305), while maximizing the prior probability of the transformation. Segmentation of the data in the grey matter, white matter (WM) and cerebro-spinal fluid (CSF) classes was also realized. All normalized images were smoothed by convolution with a Gaussian kernel, with a full width at half maximum of 8 mm in all directions. For each individual, the time-courses of both WM and CSF were estimated in the relevant brain tissue classes defined at the segmentation step. Sources of spurious variance were then removed from the data through linear regression: six parameters obtained by rigid body correction of head motion, the CSF and WM signals. Finally, fMRI data were temporally filtered using the REST Toolbox (low cutoff frequency = 0.008 Hz – high cutoff frequency 0.1 Hz) (Song et al., 2011).
2.5. Definition of the seed regions
Each unilateral amygdala was subdivided into 3 separate and independent regions, based on the probabilistic amygdala maps of Amunts and colleagues (2005) (www.fzjuelich.de/ime/spm_anatomy_toolbox). These seed regions were created in our MNI standard space, using the Anatomy toolbox available in SPM8 (Figure 1). Consequently, a total of 6 seed regions were then computed (3 per hemisphere), including the amygdala Latero-Basal (LB), CentroMedial (CM) and Superficial (SF) subregions (Roy et al., 2009). Only voxels with a probability of at least 50% of belonging to each subdivision (LB, CM, SF) were included, with each voxel was assigned to only one subdivision, the subdivision for which they had the highest probability of inclusion. The same seed regions were applied to both patients and controls, as all the participants had normalized functional data. Finally, data analyses within each patient included calculation of the mean signal time course in each seed region.
Figure 1.
Amygdala subdivisions used as seeds. Red: Centromedial (CM) part; Green: Laterobasal (LB) part; Blue: Superficial (SF) part. Neurological orientation.
2.6. Measures of Depression and Anxiety in MTLE patients
Depression and Anxiety levels in patient participants were assessed using the Personality Assessment Inventory (PAI). The PAI is a well-normed measure of psychopathology and provides results along 22 non-overlapping clinical scales based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) (Morey, 1991). The scales capturing the level of ‘Anxiety’ (ANX), ‘Anxiety-Related Disorders’ (ARD) and ‘Depression’ (DEP) Scales were used in the present study. In detail, the ANX scale focuses on phenomenology and observable signs of anxiety with an emphasis on assessment across different response modalities; the ARD scale focuses on symptoms and behaviors related to specific anxiety disorders (such phobias, traumatic stress, and obsessive-compulsive symptoms); and the DEP scale represents concerns about symptoms and phenomenology of depressive disorders (unipolar). These scores are reported in Table 1. Because two LMTLE patients did not complete neuropsychological/psychological testing at our center, our analyses involving these psychiatric measures were conducted on 11 RMTLE and 9 LMTLE patients.
2.7. Statistical analyses
2.7.1. First-level analyses
At the individual level, a correlation map was produced by extracting the mean BOLD time course from each amygdala subregion (LB, CM and SF, right and left hemispheres, separately) seed and then computing the correlation between that time course and the time course from all other brain voxels. Next, this matrix of correlation coefficients was submitted to a Fisher r-to-z transformation (Z(r)), yielding an approximate normal distribution for the sampled data. All second or group level statistical analyses were conducted on these transformed data.
2.7.2. Second-level analyses
Individual Z(r) values maps were entered into a second-level random-effects analyses to determine if differences in functional connectivity existed between the experimental groups. First, we performed two-sample t-tests to detect differences in controls versus left MTLE patients, controls versus right MTLE patients, or left MTLE versus right MTLE patients, using each amygdala subdivision, run separately for each hemisphere. A statistical whole-brain map was generated, where the height threshold was fixed at p< 0.0001 (uncorrected, corresponding to a T > 4.27) and the cluster-level threshold was set at a corrected alpha level of p < 0.05.
2.7.3. Correlation between the psychiatric symptoms and the abnormal functional connectivity (Z(r)) values in MTLE patients
If two-sample t-test results associated with a seed region showed significant clusters, these clusters were extracted as regions of interest (ROIs). The mean time series of these ROIs were computed. The resulting individual Z(r) computed between each ROI and the seed region were then correlated to patients’ depression and anxiety levels (each of the 3 scales: PAI-DEP, PAI-ANX and PAI-ARD, separately), using a non-parametric Spearman's correlation analysis. All correlations reported are at a significance (alpha) level of at least p ≤ 0.005.
3 Results
3.1. Participants
The RMTLE and LMTLE groups did not differ in their scores on any of the three PAI scales as well as control values (Table 1). Indeed, based on the PAI manual, the mean normative t-values are 50 ± 10 (Morey, 1991). No correlation between PAI scores and seizure duration was observed (p>0.2). Also, the proportion of patients on anti-depressant medications in the right versus left TLE group did not differ (Chi-squared test, p>0.3). For the RMTLE, no difference on the PAI scales was observed when comparing the patients on versus not on psychotropic drugs (anti-anxiety, anti-depression) (t-tests, p>0.2). It was not possible to test this effect on the LMTLE given the small sample size of patients on psychotropic drugs (2 vs 9).
The 3 experimental groups did not differ in age (p>0.2) and gender (p>0.1). The PAI scores listed for the normal controls in Table 1 is based on the normative t-values provided in the manual for a healthy sample (Morey, 1991).
3.2. Analysis of Amygdala Subdivisions
Analyses did not reveal significant FC differences emerging for any of the seeds between the LMTLE and the RMTLE groups. In contrast, for both patient groups, several differences were present compared to the healthy controls for several of the seeds. These differences are enumerated below (Tables 2-4; Figure 2).
Table 2.
Whole brain functional connectivity differences between the patient and control groups with either the left or right CM amygdala seed.
| Contrasts | Region | Ke | p (FWE corrected) | T | x | y | z | Z(r) grp 1 (STD) | Z(r) grp 2 (STD) |
|---|---|---|---|---|---|---|---|---|---|
| Left CM Seed | |||||||||
| CTL - RMTLE | L Cereb. | 453 | <0.001 | 8.65 | −20 | −44 | −36 | 0.36 (0.18) | −0.1 (0.2) |
| L Cereb. | 5.43 | −28 | −62 | −34 | |||||
| L Cereb. | 5.4 | −32 | −72 | −34 | |||||
| L Mid Tp | 226 | <0.001 | 6.24 | −34 | 10 | −42 | 0.41 (0.25) | −0.14 (0.18) | |
| L Inf Tp | 6.19 | −4 2 | −4 | −40 | |||||
| RMTLE - CTL | R Inf Frt | 3456 | <0.001 | 8.37 | 42 | 18 | 8 | 0.25 (0.12) | −0.41 (0.23) |
| R PostCG. | 8.05 | 64 | −12 | 30 | |||||
| R PostCG. | 7.87 | 56 | −10 | 22 | |||||
| CTL - LMTLE | L Sup Tp | 81 | 0.02 | 5.3 | −48 | 14 | −10 | 0.33 (0.22) | −0.11 (0.24) |
| LMTLE - CTL | R Inf Frt | 115 | 0.006 | 6.04 | 36 | 24 | 14 | 0.11 (0.19) | −0.35 (0.23) |
| R Insula | 4.82 | 36 | 14 | 12 | |||||
| R Inf Frt | 4.65 | 40 | 20 | 22 | |||||
| R Mid Frt | 92 | 0.013 | 5.13 | 24 | 6 | 46 | 0.14 (0.21) | −0.31 (0.22) | |
| R Mid Frt | 4.35 | 40 | 6 | 40 | |||||
| Right CM | |||||||||
| CTL - RMTLE | Null | ||||||||
| RMTLE - CTL | Null | ||||||||
| CTL - LMTLE | Null | ||||||||
| LMTLE - CTL | Null | ||||||||
Abbreviations: Cereb: Cerebellum, Frt: Frontal cortex, Tp: Temporal cortex, PostCG: Postcentral gyrus, Inf: Inferior, Mid: Middle, Sup: Superior, R: Right hemisphere, L: Left hemisphere. Z(r) grp 1 (Z(r) grp 2): corresponds to the averaged FC between the region of interest and the seed in the group 1 (group 2, respectively) (for the contrast: group 1 – group 2). (STD) indicates the standard deviation of Z(r) among the participants of the group 1. For instance, for the difference between the CTL and the RMTLE groups in the cerebellum, the CTL group has an average Z(r) of 0.36 (with a standard deviation of 0.18), whereas the RMTLE group has an average Z(r) of −0.1 (with a standard deviation of 0.2). Patient Z(r) values are bolded to facilitate viewing the direction of the group difference.
Table 4.
Whole brain functional connectivity differences between the patient and control groups with either the left or right SF amygdala seed.
| Contrasts | Region | Ke | p (FWE corrected) | T | x | y | z | Z(r) grp 1 | Z(r) grp 2 |
|---|---|---|---|---|---|---|---|---|---|
| Left SF Seed | |||||||||
| CTL - RMTLE | L Cereb | 63 | 0.03 | 4.92 | −28 | −80 | −28 | 0.24 (0.2) | −0.13 (0.17) |
| RMTLE - CTL | R Mid Frt | 61 | 0.033 | 6.93 | 46 | −2 | 60 | 0.05 (0.1) | −0.3 (0.17) |
| R PostCG | 400 | <0.001 | 6.9 | 64 | −12 | 30 | 0.17 (0.16) | −0.27 (0.17) | |
| R PostCG | 5.76 | 66 | −10 | 20 | |||||
| R PostCG | 5.74 | 54 | −8 | 20 | |||||
| R Sup Tp | 55 | 0.044 | 5.18 | 54 | −16 | −6 | 0.29 (0.2) | −0.15 (0.28) | |
| R Sup Tp | 4.58 | 58 | −8 | −2 | |||||
| CTL-LMTLE | Null | ||||||||
| LMTLE - CTL | R Inf Frt | 77 | 0.017 | 5.84 | 54 | 24 | 24 | 0.01 (0.11) | −0.31 (0.15) |
| R Inf Frt | 5.41 | 44 | 18 | 18 | |||||
| Right SF Seed | |||||||||
| CTL - RMTLE | R Sup Par | 66 | 0.032 | 5.59 | 20 | −52 | 62 | 0 (0.15) | −0.32 (0.13) |
| R Sup Tp | 59 | 0.044 | 5.08 | 54 | −30 | 18 | 0.3 (0.2) | −0.03 (0.05) | |
| R Sup Tp | 4.38 | 62 | −38 | 16 | |||||
| RMTLE – CTL | Null | ||||||||
| CTL - LMTLE | Null | ||||||||
| LMTLE - CTL | Null | ||||||||
Abbreviations: Cereb: Cerebellum, Frt: Frontal cortex, Par: Parietal cortex, Tp: Temporal cortex, PostCG: Postcentral gyrus, Inf: Inferior, Sup: Superior, R: Right hemisphere, L: Left hemisphere. . Z(r) grp 1 (Z(r) grp 2): corresponds to the averaged FC between the region of interest and the seed in the group 1 (group 2, respectively) (for the contrast: group 1 – group 2). (STD) indicates the standard deviation of Z(r) among the participants of the group 1. Patient Z(r) values are bolded to facilitate viewing the direction of the group difference.
Figure 2.
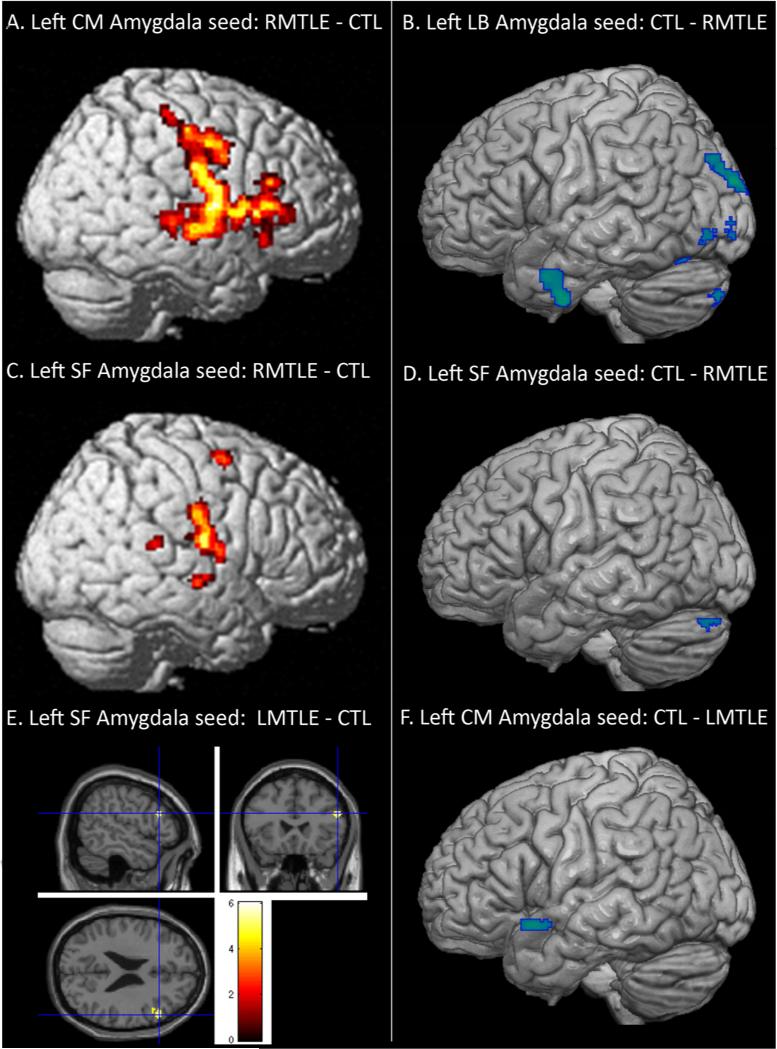
Regions showing functional connectivity differences between the patient and control groups, emerging from some of the seeds. A: Left centromedial amygdala seed with increased FC for the RMTLE group, B: Left laterobasal amygdala seed with reduced FC for the RMTLE group, C: Left superficial amygdala seed with increased FC for the RMTLE group, D: Left superficial amygdala seed with reduced FC for the RMTLE group, E: Left superficial amygdala seed with increased FC for the LMTLE group, F: Left centromedial amygdala seed with reduced FC for the RMTLE group. Yellow-red and blue colors represent increased and decreased FC, respectively, in the patient groups relative to controls.
3.2.1. Centromedial Seed
3.2.1.1. RMTLE versus Controls
Within the functional network associated with the right CM amygdala, the two-sample t test did not reveal significant differences between the controls and the RMTLE patients.
In contrast, for the left CM seed, the RMTLE patients showed several major FC differences with controls (Table 2). The largest cluster showing higher connectivity in the RMTLE group with the left CM amygdala was in the right contralateral inferior frontal cortex, extending to the postcentral cortex along the central sulcus (Ke=3,456; T=8.4; Figure 2A), relative to the controls. Also, significantly lower FC values were observed in the patient group between the left CM seed and 2 ipsilateral clusters: the largest regional difference was detected between the seed and the left cerebellum (Ke=453; T=8.7) (Figure 3A, left panel), and to a lesser degree, these RMTLE patients also showed reduced FC in a cluster located in the left inferior temporal cortex (Ke=226; T=6.2).
Figure 3.
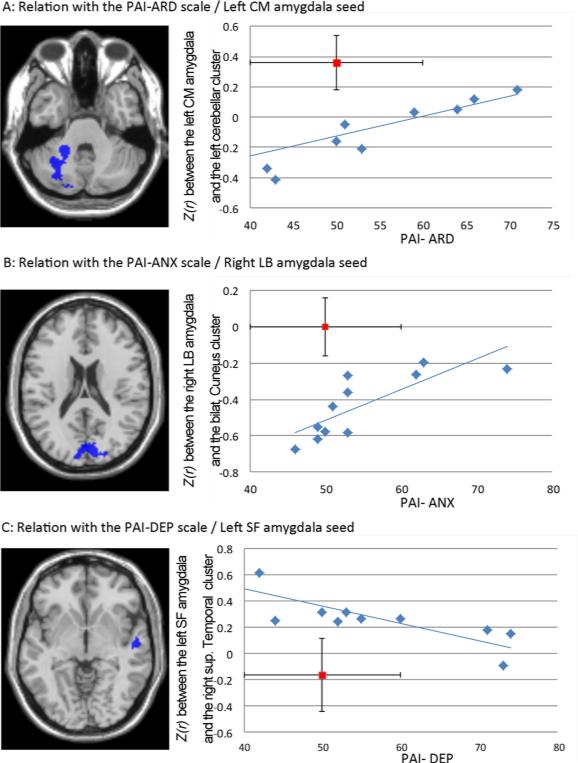
Significant relationships between abnormal reduced FC in the RMTLE group. A: with the anxiety-related disorder level scale (PAI-ARD). B: with the anxiety level scale (PAI-ANX). C: with the Depression level scale (PAI-DEP). The mean FC value for normal controls (with SD as vertical line) and the PAI-manual referenced value for normals (with SD as horizontal line) is displayed in red.
3.2.1.2. LMTLE versus Controls
As for the RMTLE group, no significant differences were detected between the LMTLE and control group for the right (non-pathologic) CM seed.
For the left (pathologic) CM seed, the two-sample t test revealed differences (Table 2). The LMTLE group showed higher FC in 2 clusters located in the right contralateral inferior frontal cortex / insula (Ke=115; T=6.0) and the right middle frontal cortex (Ke=92; T=5.1); relative to controls. In contrast, the normal group showed higher FC between the seed and the left superior temporal cortex (Ke=81; T=5.3) (Figure 2F), relative to the LMTLE group.
3.2.2. Laterobasal Seed
3.2.2.1. RMTLE versus Controls
We only revealed reduced FC with the right (pathologic) LB seed in the RMTLE group, relative to the control group (Table 3). The main bilateral cluster was located in the cuneus (Ke= 734; T=6.3) (Figure 3B, left panel). To a lesser degree, two other clusters located in the right parietal (Ke= 313; T=6.3) and the inferior temporal cortex (Ke= 90; T=6.2) were observed, respectively.
Table 3.
Whole brain functional connectivity differences between the patient and control groups with either the left or right LB amygdala seed.
| Contrasts | Region | Ke | p (FWE corrected) | T | x | y | z | Z(r) grp 1 | Z(r) grp 2 |
|---|---|---|---|---|---|---|---|---|---|
| Left LB seed | |||||||||
| CTL - RMTLE | L Sup Occ | 303 | <0.001 | 6.69 | −14 | −92 | 22 | 0.16 (0.17) | −0.28 (0.21) |
| L Sup Occ | 5.3 | −22 | −86 | 28 | |||||
| L Mid Occ | 5.28 | −28 | −82 | 32 | |||||
| L Inf Tp | 197 | <0.001 | 5.77 | −46 | −4 | −40 | 0.63 (0.24) | 0.09 (0.24) | |
| L Mid Tp | 5.47 | −56 | 6 | −28 | |||||
| L Mid Tp | 4.81 | −50 | 0 | −30 | |||||
| L Cereb | 232 | <0.001 | 5.45 | −28 | −68 | −16 | 0.37 (0.23) | −0.14 (0.23) | |
| L Cereb | 5.24 | −16 | −68 | −12 | |||||
| L Cereb | 5 | −26 | −56 | −16 | |||||
| L Mid Occ | 124 | 0.004 | 5.45 | −26 | −88 | 0 | 0.34 (0.29) | −0.15 (0.24) | |
| L Inf Occ | 5.17 | −36 | −76 | −8 | |||||
| L Inf Occ | 4.54 | −34 | −84 | −4 | |||||
| RMTLE - CTL | R putamen | 1096 | <0.001 | 5.88 | 26 | 4 | 16 | 0.22 (0.22) | −0.41 (0.2) |
| R caudate | 5.72 | 16 | −14 | 22 | |||||
| R Rolandic | 151 | 0.002 | 5.78 | 54 | −12 | 12 | 0.15 (0.15) | −0.26 (0.23) | |
| CTL - LMTLE | L Inf Tp | 80 | 0.22 | 5.31 | −44 | 2 | −38 | 0.63 (0.23) | 0.17 (0.22) |
| LMTLE - CTL | Null | ||||||||
| Right LB seed | |||||||||
| CTL - RMTLE | L Cuneus | 734 | <0.001 | 6.32 | −6 | −88 | 24 | 0 (0.17) | −0.43 (0.18) |
| R Cuneus | 5.78 | 12 | −84 | 24 | |||||
| R Sup Occ | 5.75 | 14 | −88 | 36 | |||||
| R Sup Par | 313 | <0.001 | 6.27 | 18 | −58 | 62 | 0.07 (0.16) | −0.38 (0.2) | |
| R Sup Par | 6 | 28 | −56 | 62 | |||||
| R Sup Par | 5.47 | 16 | −52 | 68 | |||||
| R Inf Tp | 90 | 0.014 | 6.2 | 58 | −68 | −2 | 0.27 (0.18) | −0.17 (0.22) | |
| R Inf Tp | 4.77 | 52 | −56 | −4 | |||||
| RMTLE - CTL | Null | ||||||||
| CTL – LMTLE | Null | ||||||||
| LMTLE – CTL | Null | ||||||||
Abbreviations: Cereb: Cerebellum, Occ: Occipital cortex, Par: Parietal cortex, Tp: Temporal cortex, PostCG: Postcentral gyrus, Inf: Inferior, Mid: Middle, Sup: Superior, R: Right hemisphere, L: Left hemisphere. . Z(r) grp 1 (Z(r) grp 2): corresponds to the averaged FC between the region of interest and the seed in the group 1 (group 2, respectively) (for the contrast: group 1 – group 2). (STD) indicates the standard deviation of Z(r) among the participants of the group 1. Patient Z(r) values are bolded to facilitate viewing the direction of the group difference.
For the left (non-pathologic) LB seed, compared to controls, the RMTLE group displayed higher FC between the seed and right subcortical areas, including a part of the putamen and the caudate nuclei (Ke= 1,096; T=5.9) and the right rolandic operculum (Ke= 151; T=5.8). Also, the patient group showed reduced connectivity between the seed and clusters located in the left lateral occipital cortex (superior/middle: Ke=303; T=6.7; inferior/middle: Ke=124; T=5.45); in the left cerebellum (Ke= 232; T=5.5) and in the left inferior/middle temporal cortex (Ke= 197; T=5.8) (Figure 2B).
3.2.2.2. LMTLE versus Controls
No significant differences were detected between the LMTLE and control group for the right (nonpathologic) LB amygdala seed.
For the left (pathologic) LB seed, compared to controls, the LMTLE group only displayed lower FC between the seed and left inferior temporal cortex (Ke= 80; T=5.3) (Table 3; Figure 4, left panel).
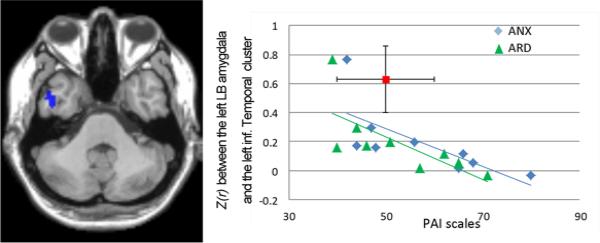
Figure 4.
Negative relationship between abnormal increased FC between the left LB amygdala and the left inferior temporal cluster and both PAI-ARD and PAI-ANX scales in the LMTLE group. The mean FC value for normal controls (with SD as vertical line) and the PAI-manual referenced value for normals (with SD as horizontal line) is displayed in red.
3.2.3. Superficial Seed
3.2.3.1. RMTLE versus Controls
We detected that the right ipsilateral superior parietal (Ke= 66; T=5.6) and superior temporal (Ke= 59; T=5.1) cortex had reduced FC values with the right (pathologic) SF seed in the RMTLE group than the controls.
For the left (non-pathologic) SF seed, the RMTLE group showed increased FC with clusters located in the right contralateral hemisphere, relative to controls (Figure 2C). They are located in the postcentral gyrus (Ke=400; T=6.9), the middle frontal cortex (Ke= 61; T=6.9) and the superior temporal cortex (Ke=55; T=5.2; Figure 3C, left panel). To a lesser degree, the RMTLE also demonstrated a reduced FC with a cluster located in the left cerebellum (Ke=63; T=4.9) (Figure 2D), compared with controls.
3.2.3.2. LMTLE versus Controls
As for the two other right-sided seeds, no significant differences were detected between the LMTLE and control group for the right (non-pathologic) SF amygdala seed.
The LMTLE group had increased connectivity between the left (pathologic) SF seed and a small cluster located in the right inferior frontal cortex (Ke=77; T=5.8) (Figure 2E).
3.2.4. Summary
Overall, the LMTLE group consistently displayed impaired FC with seeds located in the left pathologic/ictal hemisphere, functional impairments not present with the right-sided (non pathologic/ictal) seeds. However, regardless of the seed region, the LMTLE group showed much less functional impairments with any amygdala subdivisions than the RMTLE group, relative to controls. Indeed, the RMTLE had demonstrated an abnormal network 17 times more extended than the LMTLE (445 voxels versus 7676 voxels). Finally, when specifically considering the results associated with the RMTLE, the present data revealed that this group showed more abnormal higher than reduced FC, relative to the control group (‘RMTLE – Controls’: 5,219 voxels versus ‘Controls – RMTLE’: 2,457 voxels).
3.3. Correlation between PAI Scales and FC Changes
Using the regions displaying a significant increase or reduction in FC in either patient group relative to the controls, we sought to determine whether these observed FC differences were correlated with the level of anxiety and depression scores from the PAI scales reported by the patients.
We found 3 significant relations between the PAI scores and abnormal FCs in the RMTLE patients (Figure 3). Two positive correlations involving the FC between the left non-pathologic CM amygdala seed and the left cerebellum cluster, and the PAI-ARD (r=0.78, p=0.004) (Figure 3A), and the FC between the right pathologic LB amygdala and the bilateral cuneus cluster and the PAI-ANX (r=0.88, p<0.001) were present (Figure 3B). This correlation indicated that higher levels of PAI scores in RMTLE patients were associated with higher FC values between these regions, moving the FC values closer to the control group. We also revealed one negative correlation involving the FC between the non-pathologic left SF amygdala seed and the right superior temporal cluster, and the PAI-DEP (r=−0.80, p=0.003) (Figure 3C). This correlation indicates that patients with higher levels of PAI-DEP displayed lower FC values between these regions, moving their FC values closer to the controls.
Regarding the LMTLE group, we found that the impaired reduced FC between the left pathologic LB amygdala and the left inferior temporal cluster was negatively correlated with both the PAI-ANX and PAI-ARD scales (ANX: r=−0.87, p=0.002; ARD: r=−0.83, p=0.005; Figure 4). To a lesser degree, this FC was also negatively correlated with the third PAI-DEP scale (r=−0.78, p=0.01). This relation indicates that patients with higher levels of anxiety disorders displayed lower FC values between these regions, moving their FC values even further from the controls. We should note that the ANX and ARD scales are correlated within the LMTLE group (r=.9, p<.001), and likely reflect similar underlying emotion-related constructs. Thus, it is not surprising that they bear similar relationships with our FC measures. Of note, as shown on Figure 4, one patient was an outlier. We re-did these analyses without this patient, and found that the correlation remained significant for the 3 scales (r<−0.76; p<0.05).
4 Discussion
The present work highlights a clear difference between the RMTLE and LMTLE groups in terms of the resting-state FC emerging from the amygdala, with the LMTLE group only showing small differences involving the pathologic-sided seeds, compared with controls. In contrast, the RMTLE group displayed several FC differences with a consistent pattern involving reduced FC in the hemisphere ipsilateral to the seed, while showing increased FC with regions contralateral regions to the seed, relative to controls. Our data also showed that the nature of the FC values varies by amygdala region, suggesting regionally distinct networks exist and, in the setting of MTLE, important hemispheric differences are present, with right temporal lobe seizures perturbing amygdala networks more significantly than left-temporal seizures compared to controls. In addition to these network disruptions, several FC abnormalities emerging from distinct amygdala subdivisions were related to the level of depression and anxiety in the patients. These results suggest that the present method using amygdala subdivision and the PAI scales may be an efficient way to distinguish patients with either right or left-sided seizures.
Quantitative comparisons between the 3 experimental groups, for each seed, revealed that the major differences were between the right MTLE and control groups. More specifically, we found that the FC between the amygdala (regardless of the subdivision) and several brain regions were abnormal in the RMTLE, relative to the controls, including the postcentral gyrus, cerebellum and occipital cortex. Regarding the two latter regions, we demonstrated that their FC with the seed was highly negative in the patients, but positive in the controls. Previous studies have already demonstrated that both these regions are anatomically connected to the amygdala and highly involved in emotion processing in healthy participants (Amaral et al., 1992; Chao et al., 2012; Sang et al., 2012). Thus, our data are consistent with these studies and suggest that the emotional impairments reported in TLE (Helmstaedter et al., 2004) may be related to these functional impairments. In contrast, we also found that the RMTLE patients showed increased FC between the amygdala and the contralateral primary motor cortex, compared to controls. Indeed, in primates, the amygdala has been shown to have direct projections to the motor cortex (Morecraft et al., 2007). More specifically, we found that FC between the amygdala and contralateral postcentral cortex is negative in controls in contrast to the RMTLE patients. Altogether, these results may be another sign of the inhibitory neurotransmission disruptions in TLE described by our lab and others (Ge et al., 2011; Tracy et al., 2012), which in this case may represent maladaptive modulations of the normative anti-correlated activity. Ultimately, with the present data, we cannot conclude whether these abnormal functional connectivities are the sign of a gain or loss of inhibition. Previous physiologic studies, however, have shown that human TLE is associated with a loss of GABA-mediated inhibition leading to an excitatory/inhibitory imbalance (review of Hines et al., 2012), an imbalance that in the context of the present study would be consistent with either a significantly higher or lower level of FC in patients than in controls. On this basis, we suggest that our result reflects a loss of balance and proper modulation of inhibitory control in emotion-related networks, which might be a key feature of right unilateral TLE patients.
Regarding the LMTLE patients, we only revealed abnormal FC emerging from the left-sided (pathologic/ictal) amygdala. This result is consistent with functional studies that described reduced FC emerging from the left but not the right amygdala in left MTLE, relative to a normal control group (Pittau et al., 2012). We suggest that this result is directly related to the impact of epileptic compared to the non-epileptic hemisphere on the amygdala and related mesial temporal lobe structures, decreasing both afferent and efferent communications. Several studies have described the epileptogenic amygdala as significantly more atrophic compared to controls, while the contralateral amygdala shows much less atrophy (Bonilha et al., 2010; Cendes et al., 1993).
A striking, but more perplexing, finding is that in both patient groups we consistently demonstrated that the patients had increased connectivity with contralateral regions and reduced connectivity with regions ipsilateral to the seed, relative to controls. In contrast, the controls only showed positive FC with regions located in the same hemisphere of the seed, but negative FC with contralateral regions. The only region that did not follow this unilateral pattern was the bilateral cluster located in the cuneus, for the RMTLE. One explanation for this is that the occipital lobe is composed of homologous regions that are highly functionally and structurally connected (Cordes et al., 2001; van den Heuvel et al., 2009), causing them to be less sensitive to or less prone to lateralization effects. Thus, our data show that MTLE patients suffer from FC abnormalities at rest, in a manner suggesting there is a hemispheric bias to these effects. The key question then becomes understanding inversion of the normal balance between the hemispheres in TLE.
Based on the present results, we believe that right- and left- lateralized intractable temporal lobe seizures are associated with distinct patterns of functional reorganization emerging from the limbic system. Importantly, our data show that this pathology-based reorganization is associated with the level of psychiatric symptomatology. It is well-known that both the amygdala – and more generally the limbic system – of the right hemisphere is more highly involved in emotional processing (see reviews of Davidson, 2003; Gainotti, 2012), and displays higher levels of panic and other emotional disorders than the left hemisphere (Sazgar et al., 2003). Indeed, Sazgar et al. (2003) described that patients suffering from a lesion located in the right hemisphere, especially in the temporal lobe, suffer from panic disorders more systematically compared with patients with left-lateralized lesions. Accordingly, the distinct patterns we observed in RMTLE and LMTLE may be caused by the fact that the right hemisphere plays a greater role in emotional dysregulation. Our data suggests this is the case even though the right and left MTLE groups did not differ in the level of anxiety and depression reported on the PAI. Indeed, an intriguing possibility is that the functional connectivity differences we revealed between the patients and the controls may reflect emotional perturbations that have not been yet observed at a behavioral level, or at a level of awareness that can get expressed through self-report psychiatric symptom inventories. To our knowledge, there is no current comparable finding in the literature. Further work is certainly needed to investigate the same amygdala partitions in MTLE patients, comparing patients that do or do not suffer from severe psychiatric symptoms.
It has been previously proposed by our lab and others that the presence of left-hemisphere dominance for language is associated with distinct asymmetric patterns of FC within the MTL (Pereira et al., 2010) as well as outside the temporal lobe (Doucet et al., 2012). Indeed, Pereira et al. (2010) suggested that resting-state functional connectivity differs between right and left hippocampi, and, in turn, between the RMTLE and LMTLE patients because of left-hemisphere dominance for language processing. In our previous study (Doucet et al., 2012), we extended these results by showing right and left MTLE patients differ in their whole-brain functional connectivity associated with a memory-related network, suggesting a cognitive reorganization of the memory-related system in these patients. In the same vein, but applied to emotional processing, with the current results we further extend those prior findings by highlighting that the (right) emotion-dominant hemisphere (Gainotti, 2012) plays a role in compelling a functional reorganization of networks. In essence then, the functional connectivity differences in our TLE groups may reflect the impact of hemisphere dominance for emotion processing. The important implication of our data is that right temporal lobe seizures may preferentially burden affect-specific systems of the right hemisphere in ways comparable to the impact of left-hemisphere seizures on dominant hemisphere language networks.
An important aspect of our current findings is that unilateral seizures perturb different networks in association with distinct subregions of the amygdala, with these distinct networks bearing different relationships with psychiatric symptomatology. Indeed, we demonstrated that each of the three PAI scales may be associated with a distinct amygdala subpart. To the best of our knowledge, this is the first time that such results have been described in MTLE, bringing new clues and potential markers to investigate the neuropsychiatric impairments associated with this pathology. For instance, our data show that, in RMTLE, the left (non-pathologic) CM nuclei may be associated with the level of stress or phobia (i.e., the ARD, anxiety-related disorders, scale), the left SF nuclei may be related to the level of depression, and the right (pathologic) LB nuclei may be rather related to the level of anxiety. Altogether, these findings are concordant with the idea that each of these subdivisions is associated with a different affective state and emphasize the importance of not considering the amygdala as a single functional unit (LeDoux, 2003; Roy et al., 2009). Regarding the specific directions of these effects, our results appear counter-intuitive as amygdala FC values closer to controls were related to higher levels of anxiety and depression in the RMTLE patients. Nevertheless, because this specific relation is present in patients suffering from seizure activity emerging from their right hemisphere, we suggest it represents a maladaptive change in spontaneous activity driven by seizures, hurting the limbic system, and perturbing the substrate for emotion processing. In contrast, for the left TLE, we described a more expected negative relation involving the left (pathologic) LB amygdala and the PAI scales, showing that the less the patients are anxious and stress responsive, the closer their FC values are to normal. Consistent with the above interpretation, we suggest that left-sided epileptic seizures emerging from the hemisphere not dominant for emotion processing, disturb the limbic and emotion system to a lesser degree, as evidenced by more normal levels of FC coinciding with more normative psychiatric symptom levels. Overall, we suggest that our data reflects differences in the ability of the right and left hemispheres FC networks to regulate affective states in response to either internal or external demands.
Such an interpretation helps us understand why the right and the left MTLE patients demonstrated opposite relationships with the psychiatric symptoms, relative to controls. Namely, right- but not left-sided epileptic seizures initiate maladaptive emotion-related networks, leading to abnormal and unexpected relationships between psychiatric symptoms and the neural network communications emerging from the limbic system. Indeed, our data are consistent with the possibility that RMTLE patients may be both more prone to maladaptive functional connectivity changes within the limbic system, and less capable of recruiting other regions to take over functionality and reorganize skills such as emotional processing.
Regarding the other regions that showed impaired FC with the amygdala subdivisions in the MTLE groups, not bearing direct relations with the anxiety or depression scores, one possibility is that these impairments reflect subtle extra-temporal structural abnormalities, which have been describe in unilateral TLE, abnormalities not picked up by MRI and standard pre-surgical assessment algorithms. Indeed, recent studies have demonstrated that MTLE patients have extensive white-matter abnormalities in limbic pathways (Concha et al., 2009), in addition to structural atrophy in regions such as the lateral parietal lobe, subcortical, and cerebellum (Bonilha et al., 2007; Mueller et al., 2010).
Overall, we confirm an association between the amygdala and negative emotions, but provide greater regional specificity for MTLE, providing hints that different emotion symptoms may involve different sub-divisions of the amygdala. In this sense, our result provides strong evidence that the amygdala is not composed of one homogeneous region. By showing that the amygdala sub-divisions have distinct functional connectivity patterns, our data implies they may have different roles and relations to specific aspect of emotion processing. Furthermore, and importantly, our finding points to the possibility that each amygdala subdivision and its connectivity integrity may serve as a marker of a specific type of mood disorder.
In terms of the limitations of our findings, a significant proportion of the sample (45%) were on anti-depressant medication, perhaps explaining why self-reported depression (PAI-DEP), anxiety (PAI-ANX), and anxiety-related disorders (PAI-ARD) levels were below thresholds typically used to identify clinically significant symptoms. Yet, within the RMTLE, the group showing most of the effects discussed in this paper, there was no difference in ANX, DEP, and ARD levels amongst patients taking versus not taking psychotropic medications. In fact, one could argue that observing such significant relationship between FC and psychiatric symptoms in the presence of a medication, medication that is presumably reducing psychiatric symptoms, makes any observed relationships all the stronger. Similarly, all the patients were on anticonvulsant medication, reducing the level of current seizure burden. However, observing perturbed amygdala networks (compared to controls), and then finding a relationship with psychiatric symptoms suggests that the association may be even stronger in the presence of stronger seizure activity. Several of our comparisons, however, involved low statistical power due to the small sample sizes, and we cannot preclude some role for psychotropic medications (or the absence of such) in the effects we report.
Regarding the control group used in the present study, one concern is that we were unable to obtain PAI scores for the controls. However, each normal control completed a health screening questionnaire, and was interviewed by a trained neuropsychologist to confirm the absence of factors that would influence resting-state brain activity such as psychiatric, medical, or neurologic problems, or current or past use of a central nervous system medication. Note, however, the main purpose of the project was to quantify and measure FC impairments relative to normal, and determine if any observed impairments were related to levels of psychiatric symptomatology in patients.
Finally, another limitation is the small sample size in our patient groups. This may explain why we failed to find significant differences between RMTLE and LMTLE. However, our results are concordant with our previous study (Doucet et al., 2012), which used the MTL as a seed, where we did not find significant differences between RMTLE and LMTLE groups, but again observed differences between the patient and control groups.
5 Conclusion
In summary, we showed that FC emerging from subdivisions of the amygdala are distinct and vary with the side of the epileptic pathology, with right MTLE patients showing more functional network impairments involving the amygdala compared to controls than the left MTLE patients. In terms of emotional/psychiatric symptoms, there was no difference between the RMTLE, LMTLE patients and controls in the present study. Despite this observation, the functional data indicate that lateralized epileptic pathology may disturb specific emotion processes and psychiatric symptoms, with indications that different symptoms are subserved by different functional connectivity networks. Thus, epileptic pathology in the emotion-dominant right hemisphere appears to negatively impact the expression of emotion-related networks, in ways specific to the amygdala region and the emotion or symptom involved. Also, our data highlight opposite functional connectivity relations between anxiety, depression, and brain functioning in right and left MTLE. Therefore, our data argue against a simple or highly general conceptualization of reorganization in unilateral TLE. There may be several types of reorganization, with right hemisphere pathology playing a crucial role in the reorganization of emotion functions. More specifically, we suggest that brain networks in MTLE are perturbed according to the side of the seizures, the hemisphere involved, and the function, cognitive or emotional in nature, implemented by these networks. When incorporating the current result with the past findings, one potential emerging hypothesis is that hemispheric function and dominance may have a large impact on the nature of seizure-related reorganization, with left MTLE patients showing more hippocampal-based disruptions than right in association with a verbal memory task (Doucet et al., 2012), and right MTLE patients showing more amygdala-based disruptions in association with emotional states and emotion processing. To our knowledge, this is the first description of resting-state FC alterations in MTLE involving a specific limbic structure with results that have relevance to the emotional presentation and problems of these patients.
TLE patients have impaired functional connectivity emerging from the amygdala at rest
Results differ in function of the amygdala subdivisions
Impaired functional connectivity correlates with anxiety and depression scores in TLE
Right TLE has a more maladaptive impact on emotion-related networks
Acknowledgments
The authors thank Dr. Karol Osipowicz for his participation in the data acquisition.
This work was supported, in part, by the National Institute for Neurological Disorders and Stroke (NINDS) [grant number R21 NS056071-01A1] to Dr. Joseph I. Tracy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure: None of the authors have any conflict of interest to disclose.
References
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9(14):3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Alhusaini S, Doherty CP, Scanlon C, Ronan L, Maguire S, Borgulya G, Brennan P, Delanty N, Fitzsimons M, Cavalleri GL. A cross-sectional MRI study of brain regional atrophy and clinical characteristics of temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2012;99(1-2):156–166. doi: 10.1016/j.eplepsyres.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: A. W., editor. The amygdala neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 1–66. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210(5-6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126(Pt 2):462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65(2):223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2010;81(10):1147–1154. doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Powell R, Yogarajah M, Thompson PJ, Symms MR, Koepp MJ, Duncan JS. Preoperative amygdala fMRI in temporal lobe epilepsy. Epilepsia. 2009;50(2):217–227. doi: 10.1111/j.1528-1167.2008.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Edwards JC, Kinsman SL, Morgan PS, Fridriksson J, Rorden C, Rumboldt Z, Roberts DR, Eckert MA, Halford JJ. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia. 2010;51(4):519–528. doi: 10.1111/j.1528-1167.2009.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Halford JJ, Eckert M, Appenzeller S, Cendes F, Li LM. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(3):286–294. doi: 10.1136/jnnp.2006.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broicher SD, Frings L, Huppertz HJ, Grunwald T, Kurthen M, Kramer G, Jokeit H. Alterations in functional connectivity of the amygdala in unilateral mesial temporal lobe epilepsy. J Neurol. 2012;50(1):118–128. doi: 10.1007/s00415-012-6533-3. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, Melanson D, Olivier A, Peters T, Lopes-Cendes I, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43(4):719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- Chao LL, Lenoci M, Neylan T. Effects of post-traumatic stress disorder on occipital lobe function and structure. Neuroreport. 2012;23:412–419. doi: 10.1097/WNR.0b013e328352025e. [DOI] [PubMed] [Google Scholar]
- Chen S, Wu X, Lui S, Wu Q, Yao Z, Li Q, Liang D, An D, Zhang X, Fang J, Huang X, Zhou D, Gong QY. Resting-state fMRI study of treatment-naive temporal lobe epilepsy patients with depressive symptoms. Neuroimage. 2012;60(1):299–304. doi: 10.1016/j.neuroimage.2011.11.092. [DOI] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(3):312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Darwin and the neural bases of emotion and affective style. Ann N Y Acad Sci. 2003;1000:316–336. doi: 10.1196/annals.1280.014. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9(3):382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22059. doi: 10.1002/hbm.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch C, Hanke J, Kleineruschkamp S, Roske S, Kaaden S, Elger CE, Schramm J, Yilmazer-Hanke DM, Helmstaedter C. Positive correlation between the density of neuropeptide y positive neurons in the amygdala and parameters of self-reported anxiety and depression in mesiotemporal lobe epilepsy patients. Biol Psychiatry. 2009;66(5):433–440. doi: 10.1016/j.biopsych.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia. 2012;50(2):205–218. doi: 10.1016/j.neuropsychologia.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Ge YX, Liu Y, Tang HY, Liu XG, Wang X. ClC-2 contributes to tonic inhibition mediated by alpha5 subunit-containing GABA(A) receptor in experimental temporal lobe epilepsy. Neuroscience. 2011;186:120–127. doi: 10.1016/j.neuroscience.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. Classical conditioning of tone-signaled bradycardia modifies 2-deoxyglucose uptake patterns in cortex, thalamus, habenula, caudate-putamen and hippocampal formation. Brain Res. 1986;363(2):239–256. doi: 10.1016/0006-8993(86)91009-7. [DOI] [PubMed] [Google Scholar]
- Gross DW. Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52 Suppl. 2011;4:32–34. doi: 10.1111/j.1528-1167.2011.03149.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Sonntag-Dillender M, Hoppe C, Elger CE. Depressed mood and memory impairment in temporal lobe epilepsy as a function of focus lateralization and localization. Epilepsy Behav. 2004;5(5):696–701. doi: 10.1016/j.yebeh.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABA(A) receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22(3):552–558. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Alvestad S, Vaaler A, Sonnewald U. Which clinical and experimental data link temporal lobe epilepsy with depression? J Neurochem. 2007;103(6):2136–2152. doi: 10.1111/j.1471-4159.2007.04926.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4-5):727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007;500(1):134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morey LC. The Personality Assessment Inventory professional manual. Odessa, FL: 1991. [Google Scholar]
- Morris JS, Buchel C, Dolan RJ. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13(6 Pt 1):1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, Cardenas-Nicolson V, Weiner MW. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia. 2010;51(8):1436–1445. doi: 10.1111/j.1528-1167.2009.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia. 2012;53(6):1013–1023. doi: 10.1111/j.1528-1167.2012.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber M, Andersen B, Elger CE, Helmstaedter C. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129–135. doi: 10.1016/s1059-1311(03)00073-6. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60(9):966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, Yu C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage. 2012;61:1213–1225. doi: 10.1016/j.neuroimage.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Sazgar M, Carlen PL, Wennberg R. Panic attack semiology in right temporal lobe epilepsy. Epileptic Disord. 2003;5(2):93–100. [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9(9):2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: A Toolkit for Resting-State Functional Magnetic Resonance Imaging Data Processing. PLoS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, O'Connor MJ, Saykin AJ, Phillips CA, Morrell MJ, Bridgman PA, French JA, Gonatas N. A noninvasive protocol for anterior temporal lobectomy. Neurology. 1992;42(2):416–422. doi: 10.1212/wnl.42.2.416. [DOI] [PubMed] [Google Scholar]
- Tebartz Van Elst L, Baeumer D, Lemieux L, Woermann FG, Koepp M, Krishnamoorthy S, Thompson PJ, Ebert D, Trimble MR. Amygdala pathology in psychosis of epilepsy: A magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002;125(Pt 1):140–149. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Woermann F, Lemieux L, Trimble MR. Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neurosci Lett. 2000;281(2-3):103–106. doi: 10.1016/s0304-3940(00)00815-6. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Woermann FG, Lemieux L, Trimble MR. Amygdala enlargement in dysthymia--a volumetric study of patients with temporal lobe epilepsy. Biol Psychiatry. 1999;46(12):1614–1623. doi: 10.1016/s0006-3223(99)00212-7. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Dechant V, Sperling MR, Cho R, Glosser D. The association of mood with quality of life ratings in epilepsy. Neurology. 2007a;68(14):1101–1107. doi: 10.1212/01.wnl.0000242582.83632.73. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Lippincott C, Mahmood T, Waldron B, Kanauss K, Glosser D, Sperling MR. Are depression and cognitive performance related in temporal lobe epilepsy? Epilepsia. 2007b;48(12):2327–2335. doi: 10.1111/j.1528-1167.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Osipowicz K, Spechler P, Sharan A, Skidmore C, Doucet G, Sperling MR. Functional Connectivity Evidence of Cortico-Cortico Inhibition in Temporal Lobe Epilepsy. Human Brain Mapping. 2012 doi: 10.1002/hbm.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tokoglu F, Negishi M, Arora J, Winstanley S, Spencer DD, Constable RT. Social network theory applied to resting-state fMRI connectivity data in the identification of epilepsy networks with iterative feature selection. J Neurosci Methods. 2011;199(1):129–139. doi: 10.1016/j.jneumeth.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]