Abstract
Purpose
Major alterations in body composition, such as with obesity and weight loss, have complex effects on the measurement of bone mineral density (BMD) by dual-energy x-ray absorptiometry (DXA). The effects of altered body fat on quantitative computed tomography (QCT) measurements are unknown.
Methods
We scanned a spine phantom by DXA and QCT before and after surrounding with sequential fat layers (up to 12 kg). In addition, we measured lumbar spine and proximal femur BMD by DXA and trabecular spine BMD by QCT in 13 adult volunteers before and after a simulated 7.5 kg increase in body fat.
Results
With the spine phantom, DXA BMD increased linearly with sequential fat layering at the normal (p<0.01) and osteopenic (p<0.01) levels, but QCT BMD did not change significantly. In humans, fat layering significantly reduced DXA spine BMD values (mean ± SD: −2.2 ± 3.7%, p=0.05) and increased the variability of measurements. In contrast, fat layering increased QCT spine BMD in humans (mean ± SD: 1.5 ± 2.5%, p=0.05). Fat layering did not change mean DXA BMD of the femoral neck or total hip in humans significantly, but measurements became less precise. Associations between baseline and fat-simulation scans were stronger for QCT of the spine (r2 = 0.97) than for DXA of the spine (r2 = 0.87), total hip (r2 = 0.80), or femoral neck (r2 = 0.75). Bland-Altman plots revealed that fat-associated errors were greater for DXA spine and hip BMD than for QCT trabecular spine BMD.
Conclusions
Fat layering introduces error and decreases the reproducibility of DXA BMD spine and hip measurements in human volunteers. Although overlying fat also affects QCT BMD measurements, the error is smaller and more uniform than with DXA BMD. Caution must be used when interpreting BMD changes in humans whose body composition is changing.
Keywords: bone densitometry, bone QCT, body composition
Introduction
The number of women over 60 years old who are overweight or obese is approaching 70% (1). As obesity becomes increasingly prevalent, it becomes important to obtain accurate bone mineral density (BMD) measurements in people at all weights and body mass indices (BMI). Some models of dual-energy x-ray absorptiometry (DXA) scanners can now accommodate patients up to 450 pounds (Hologic Inc., Waltham, MA). DXA, however, is subject to various types of scanning artifacts that may adversely affect the measurement of BMD in the setting of obesity and/or weight change. Although it is known that DXA precision declines with increasing BMI (2), the impact of weight on the accuracy of BMD measurements is still unclear.
The effects of body composition on the precision and accuracy of DXA measurements may cause difficulty in interpreting studies in which subjects experience large changes in weight, such as after bariatric surgery or in patients with eating disorders. Decreases of 10% in DXA BMD have been reported in patients undergoing bariatric surgery (3–5). Even moderate weight loss through dieting can lead to significant changes in BMD (6–9). There may be physiologic reasons for true bone loss to accompany weight loss, including decreased mechanical loading of the skeleton and a changing hormonal milieu. Because alterations in body composition appear to have complex effects on densitometry readings, however, it is also possible that artifacts of DXA may distort the observed changes in BMD. Studies using phantom-array simulations of bone density and varying tissue thickness, have predicted that alterations in body composition could lead to errors in BMD measurements as large as 20% (10).
Quantitative computed tomography (QCT) is another method of measuring bone mineral density. QCT measures true volumetric bone density, irrespective of bone size. In contrast, DXA makes measurements in 2 dimensions (“areal” bone density) and requires that assumptions be made about bone size and soft tissue composition around the bone. Moreover, with multi-slice helical acquisition, a newer CT technique for measuring BMD, the precision of QCT measurements is similar to that of DXA (11,12). Importantly, QCT can accurately distinguish soft tissue compartments while calculating bone density, and software corrections for beam-hardening theoretically minimize the impact of changes in body composition outside of the bone.
We sought to determine the effect of fat layering on BMD measurements by DXA in spine phantoms and in healthy volunteers and to explore whether similar changes occur when BMD is measured using quantitative computed tomography (QCT).
Materials and Methods
Anthropomorphic phantom
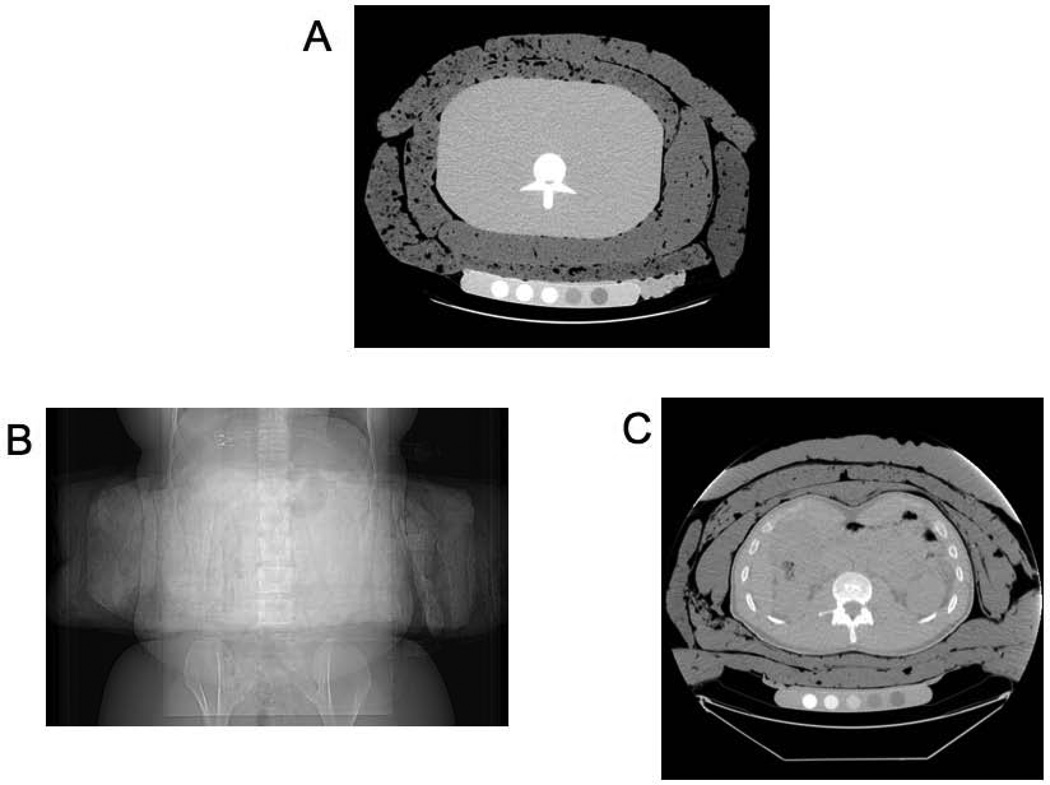
The European Spine (ES) Phantom (QRM, Mohrendorf, Germany) is an anthropomorphic phantom with three calcium hydroxyapatite vertebral inserts corresponding to densities of 50 mg/cm3 (osteoporotic), 100 mg/cm3 (osteopenic), and 200 mg/cm3 (normal) embedded into tissue-equivalent plastic. Fat layering was performed by filling rectangular plastic bags with semisolid hydrogenated vegetable oil until the mean thickness was approximately 1”, and then placing bags circumferentially around the ES phantom (Figure 1a). Each fat layering level contained 3 kg of homogenous fat material. We scanned the ES phantom by DXA and QCT at baseline and after surrounding with successive layers of these fat bags (up to 4 layers or 12 kg of the fat material). All scans with the phantom were repeated 3 times at each fat layering level.
Figure 1. Fat layering of spine phantom and adult volunteers.
(a) Cross-sectional image of European Spine Phantom with 6 kg of fat layering. Posterior-anterior (b) and cross-sectional (c) images of an adult volunteer with 7.5 kg of fat layering.
Adult volunteers
Thirteen healthy adult subjects were recruited for this study. All subjects were free from known metabolic or bone disease, and had BMIs <30 kg/m2. All subjects underwent DXA and QCT scans of the lumbar spine and proximal femur before and after 7.5 kg of fat bags were placed circumferentially around the site of interest (Figure 1b, c). For lumbar spine scans, the inferior margin of the fat layering bags was positioned 1 inch above the iliac crest. For femoral scans, the superior margin of the fat layering bags was positioned 1 inch below the iliac crest. The study was approved by the Institutional Review Board of Massachusetts General Hospital and all subjects provided written informed consent.
DXA
DXA scans were performed on a Hologic Discovery A densitometer (Hologic Inc., Waltham, MA). Enhanced mode was used during scan acquisition, which involves slower acquisition of images to improve image quality. Least significant change (LSC) was calculated as follows: LSC = 1.96 √2 CV, where CV represents the coefficient of variation. Based on prior measurements, our short-term precision CV for in vivo measurements is 1.6% for the posterior-anterior lumbar spine, 2.4% for the femoral neck, and 1.1% for the total hip (13), corresponding to an LSC of 4.4%, 6.7%, and 3.0% respectively. Coverage of the area of interest by fat bags was confirmed during post-processing analysis. Analysis of lumbar spine scans in human subjects was restricted to L2-L4 due to inconsistent coverage of L1 by the fat bags.
QCT
Trabecular BMD of the lumbar spine was measured by QCT (General Electric LightSpeed Pro scanner, General Electric Healthcare, Waukesha, WI). Scans were performed with helical acquisition at settings of 120 kV and 100 mA, with 2.5 mm slice thickness through L1 and L2 vertebrae. 3D reconstructive analysis was performed using QCT PRO software version 4.2 (Mindways Software, Inc., Austin, TX). Coverage of the area of interest by fat bags was confirmed by anterior-posterior and lateral scout scans. The density of trabecular bone was determined by means of comparison with an internal aqueous K2HPO4 standard, and the values obtained for the vertebrae were then averaged. The precision for this technique is 1.5% (11,12,14), corresponding to an LSC of 4.2%.
Statistical analysis
For analyses involving the anthropomorphic phantom, linear regression was used to compare change in BMD after sequential fat layering at each of the three vertebral density levels. For the human studies, pair-wise t-tests were used to evaluate differences in BMD before and after fat layering. Correlation coefficients were calculated for BMD, and Bland-Altman plots were used to compare measurement errors before and after fat layering. The effect of fat layering on the precision of DXA and QCT was determined by calculating the root mean square coefficient of variation (RMS CV%) of the change in BMD between the baseline and simulated fat scans (15). All statistical analyses were performed using SAS 9.2 (SAS software Inc., Cary, NC). Data are reported as mean ± standard deviation (SD).
Results
Phantom studies
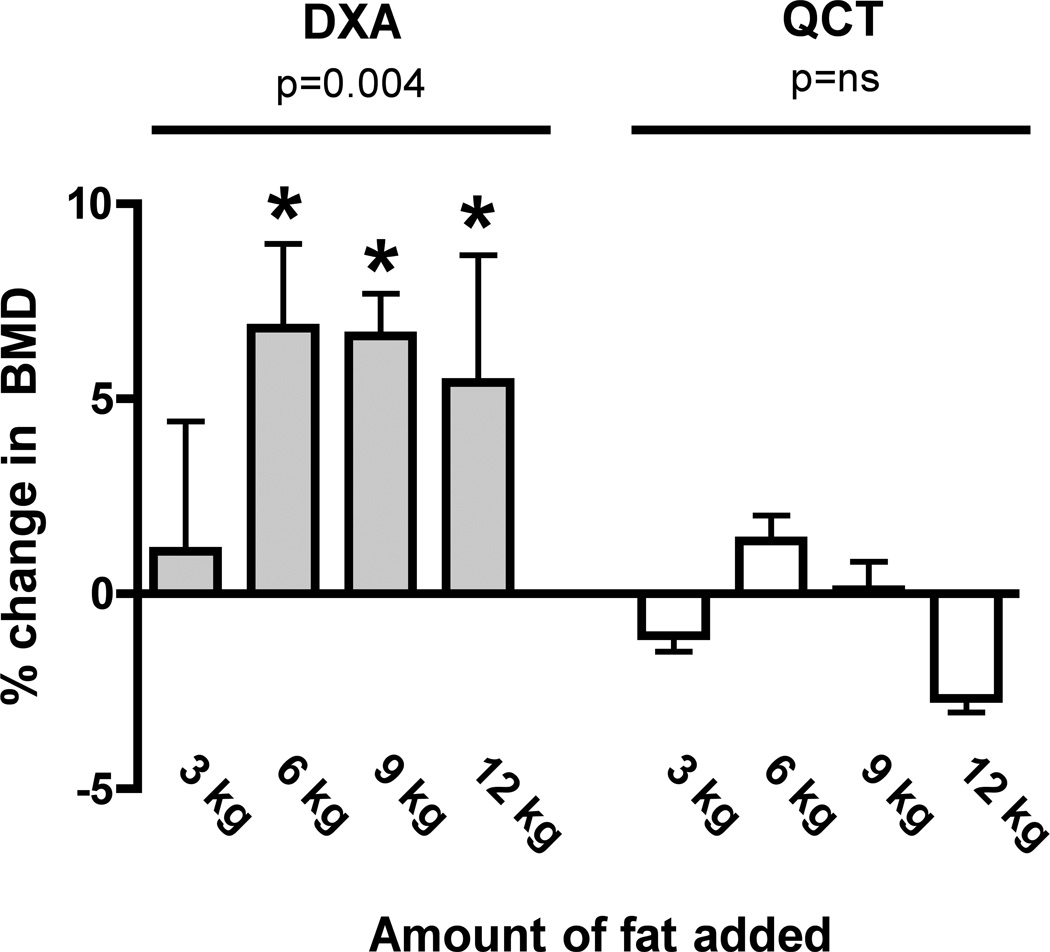
DXA BMD increased linearly with successive fat layers for phantom vertebral densities in the normal (r2 0.64, p<0.01) and osteopenic range (r2 0.55, p<0.01), but at the osteoporotic level there were erratic and bidirectional changes in BMD that did not fit a linear trend. Fat layering also increased the variability of BMD values obtained by QCT but the changes were smaller than with phantom measurements by DXA before and after fat layering. No linear trend was observed at any of the phantom vertebral densities with QCT. Figure 2 shows BMD after fat layering for both DXA and QCT at the normal bone density level, which most closely approximates the BMD range of the volunteers in our human study. Fat layering increased DXA BMD values of the spine phantom at levels of 6 kg and above, and the changes exceeded the estimated LSC for DXA. Fat layering exerted smaller effects on QCT measurements with no consistent trends, and mean differences were less than the estimated LSC.
Figure 2. Percent Change in Bone Mineral Density of the European Spine Phantom (EUS) by DXA and QCT After Fat Layering.
Fat layering in amounts of 6 kg and above increased the measured DXA BMD values of the EUS (left panel, grey bars). Fat layering had no significant effect on BMD values of the EUS measured by QCT (right panel, white bars). Data shown are for the 200 mg/cm3 insert. * Indicates that percent change from baseline BMD exceeds LSC.
Human studies
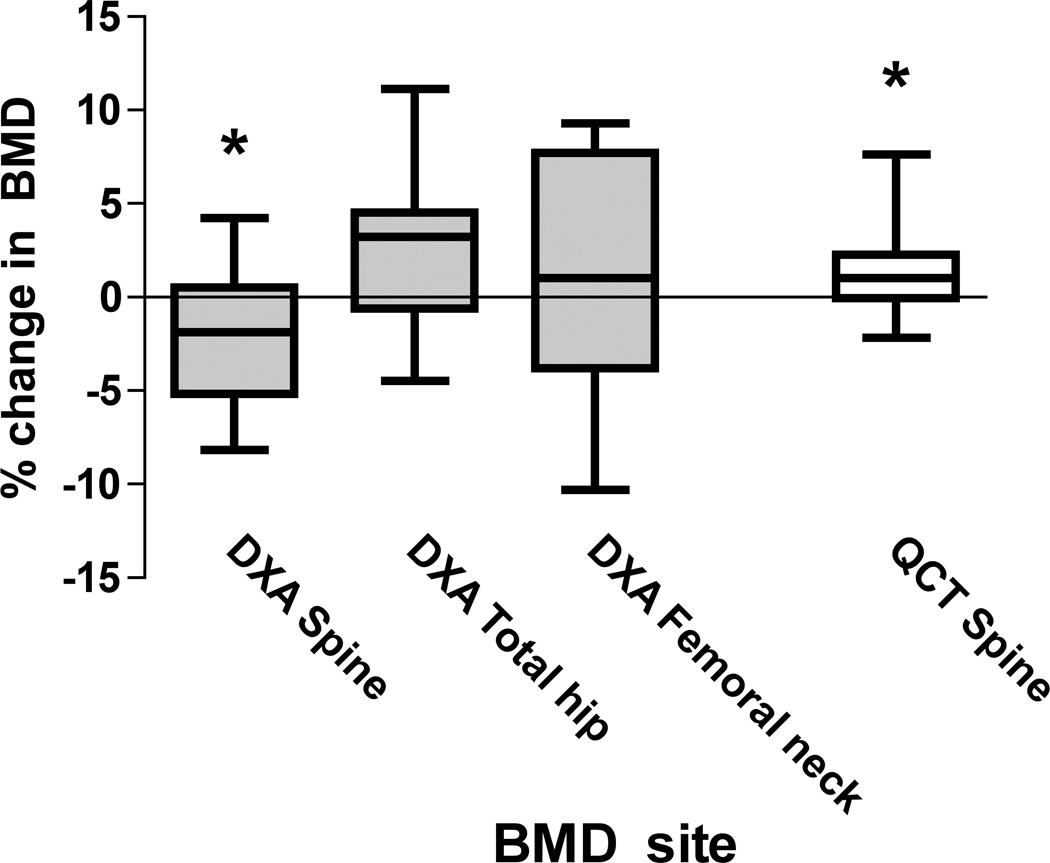
The clinical characteristics of the adult volunteers are shown in Table 1. The mean ± SD BMI was 23 ± 2 kg/m2 and all subjects had bone densities in the normal range. Results for DXA and QCT BMD measurements after fat layering are shown in Figure 3. Mean lumbar spine DXA BMD declined by −2.2 ± 3.7% (p=0.05, paired t-test) after fat layering. The correlation (r2) between DXA spine measurements made with and without fat layering was 0.87. Bland-Altman plots revealed a 95% CI of error after fat layering to be −9.8 to +5.3% (Figure 4). There were no statistically significant differences between mean DXA total hip and femoral neck measurements with and without fat layering. However, the correlation between hip measurements made with and without fat layering was somewhat weaker than for the lumbar spine (r2 = 0.75 for femoral neck DXA; r2 = 0.80 for total hip DXA) and the range of error was greater (95% CI of error: −10.9 to +13.3% for DXA femoral neck; −6.9 to +11.8% for DXA total hip). The precision error (RMS CV%) of the change in DXA BMD between baseline and simulated fat scans was 3.1% for the posterior-anterior spine, 4.3% for the femoral neck, and 3.7% for the total hip. There were no consistent effects of fat layering on DXA-measured bone area or bone mineral content (data not shown).
TABLE 1.
Descriptive characteristics of adult volunteers.
| Study subjects (N) | 13 |
| Men (%) | 53.8% |
| Age (years) | 27.6 ± 8.9 |
| Height (cm) | 173.7 ± 8.7 |
| Weight (kg) | 71.3 ± 11.0 |
| BMI | 23.4 ± 2.1 |
| Lumbar spine BMD (g/cm2) | 1.115 ± 0.112 |
| Total hip BMD (g/cm2) | 1.088 ± 0.091 |
| Femoral neck BMD (g/cm2) | 0.988 ± 0.090 |
| Trabecular spine BMD (mg/cm3) | 181 ± 23 |
Figure 3. Percent Change in Bone Mineral Density of Human Volunteers by DXA and QCT After Fat Layering.
Fat layering decreased DXA measurements of lumbar spine BMD and increased QCT measurements of trabecular BMD. Variability of bone density change in both DXA and QCT measurements was high, but the error was more pronounced for DXA measurements, especially at the total hip and femoral neck. * Indicates p<0.05 for percent change from baseline.
Figure 4. Correlation Graphs and Bland-Altman Plots After Fat Layering in Human Volunteers.
The correlation after fat layering for DXA measurements of the lumbar spine, femoral neck, and total hip was weaker than for QCT measurement of the lumbar spine. In addition, the 95% confidence intervals were wider for all DXA measurements than for QCT measurements.
Fat layering increased QCT measurements of trabecular BMD of the lumbar spine by 1.5 ± 2.5% (p = 0.05). The correlation between QCT measurements of spine BMD before and after fat layering was strong (r2 = 0.97) and the range of error (95% CI −3.4 to +6.4%) was smaller than for DXA spine. The precision error (RMS CV%) of the change in QCT BMD between baseline and simulated fat scans was 2.0% at the trabecular spine.
Discussion
In this study, we simulated the effects of increasing body fat on DXA and QCT measurements by scanning a spine phantom model and normal-weight adult volunteers with and without layers of fat. Adding fat layers around a spine phantom linearly increased the measured values for DXA spine BMD, but only minimally altered the measured values for trabecular spine QCT BMD. In human volunteers, the addition of fat layers reduced mean DXA spine BMD, did not alter mean DXA hip BMD, and increased mean QCT spine BMD. Fat layering also caused a wide range of error for both DXA and QCT measurements, but that effect was greater for DXA measurements. While the addition of fat layering caused minor deterioration of QCT precision as compared to previous reports (11,12,14), DXA precision worsened by 1–2 fold as compared to historical controls (13).
The tendency for DXA to be prone to an obesity artifact is likely related to assumptions that are made regarding fat distribution in overlying soft tissue. DXA is subject to the “two-component” limitation (10). The three components of bone mineral, fat, and lean soft tissue have different attenuation coefficients but DXA employs two photon energies and can only resolve two components at a time. Therefore, DXA makes assumptions about fat:lean tissue ratios during the calculation of bone density. Non-uniformities in this ratio can occur at extremes of weight and alterations in this ratio can occur with weight loss. Our fat simulations, which mimic such alterations and non-uniformities, demonstrate that DXA artifacts can have significant consequences in humans, and are not reliably quantified by simulations in phantoms.
Our data also demonstrate that QCT BMD is less affected than DXA BMD by simulated increases in body fat. Measurements of BMD using QCT can distinguish compartments of fat, lean, and bone mass accurately with 3D acquisition and analysis. Furthermore, only the trabecular component within the central portion of the vertebral bodies is traditionally analyzed when QCT is used to assess BMD of the lumbar spine, and the edges of this region are defined by an operator rather than by a computer algorithm. Lastly, QCT scanners make corrections for beam hardening artifact resulting from surrounding soft tissue, and further corrections are routinely applied in the analysis stage (Mindways Software, Inc., Austin, TX). In our studies, these theoretical advantages of QCT bone imaging technique translated into better reproducibility after fat layering than with DXA measurements.
Although the European Spine phantom is a well-validated tool for quality control and standardization of spinal bone measurements (16), our fat layering experiments with this spine phantom did not approximate in vivo results for DXA or QCT spine measurements. Our healthy volunteers had bone densities in the normal range, and thus are most comparable to the 200 mg/cc vertebral insert of the spine phantom. Nevertheless, while DXA BMD after layering was overestimated with the spine phantom, we observed a systematic underestimation of DXA lumbar spine BMD in our volunteers. Changes in QCT spine BMD were also somewhat discordant between the phantom and in vivo conditions. The reasons for this discrepancy are unclear. The phantom is embedded in a homogeneous material of tissue equivalent plastic, and therefore may not approximate the true complexity of heterogeneous fat:lean tissue composition that occurs in the human body. It is also possible that anatomic alterations occur in the positioning of human volunteers after fat layering (e.g. introducing lumbar lordosis), and that are not present with phantom simulations. But any alterations would be minor at best and would be unlikely to produce the large discrepancies that we observed after fat layering. Future studies exploring effects of fat simulation on DXA measurements should rely on in vivo measurements rather than phantom measurements.
Our results are similar to previous studies that have investigated the effects of fat simulation on DXA bone density measurements. Accuracy errors in the range of 5–10% due to fat in homogeneity have been reported in cadaveric studies using DXA (17–19). Other studies investigating the effects of fat layering on human volunteers have found changes in BMC and BMD across all scanner models, types, and years (20–25), but the direction of the artifact appears to depend on scan manufacturer and model. Similarly, retraction of an overlying fat panniculus significantly altered femoral BMD measurements in over half of obese patients (26). To our knowledge, however, this is the first study to compare the effects of fat layering on measurements of BMD using both DXA and QCT.
These results have important implications for the interpretation of bone density results in obese patients and in situations where changes in body composition are expected to occur. In particular, caution must be used when interpreting DXA measurements of BMD in patients before and after weight loss or weight gain. Reproducibility declined overall, and in some individuals, BMD changed by more than 10% after fat layering, even when mean errors in the entire group were either slight or not detected. This finding suggests that, on an individual basis, it may be difficult to interpret changes in DXA BMD after large changes in body composition, as the results may be under- or over-estimated. However, analysis of mean change in DXA BMD may still be reliable at a group level, even though greater imprecision suggests it will be more difficult to demonstrate that the observed charges are statistically significant. Future research examining the impact of weight loss might consider imaging other skeletal sites that are less affected by weight loss (e.g. peripheral sites) or utilizing imaging modalities that are less affected by changing body composition, such as QCT.
There are several important limitations to this study. The number of human volunteers that we studied was small. It is possible that inclusion of more volunteers would have allowed detection of significant changes in BMD at the femur, although a rather large number of subjects would have been needed to be evaluated to overcome the poor reproducibility in BMD that was induced by fat layering at the femoral sites. To limit radiation exposure in these healthy volunteers, we did not scan the proximal femur by QCT. Our QCT measurements at the lumbar spine suggest that lumbar spine QCT is a more stable imaging modality than DXA during weight change, but we cannot generalize these results to femoral sites. We studied changes with large amounts of fat material, and therefore our results are more applicable to populations in which large changes in body composition are expected to occur. Understanding the effects of large changes in body fat is important, however, given the high prevalence of obesity (1) and the rapidly increasing number of people who are undergoing bariatric surgery (27). Of note, other studies have reported similar effects on DXA with addition of just 1–2 kg of fat (22,25). Additionally, our study only assessed the impact of adding uniform layers of fat on BMD measurements. Because changes in weight are accompanied by changes in both fat and lean mass, artifactual alterations in BMD values could also be related to changes in lean mass. Weight change in humans may also be accompanied by non-homogenous or non-uniform changes in tissue composition that are not reflected in this study. Lastly, our study was not designed to assess changes in marrow fat, which could alter both DXA BMD and QCT BMD in the setting of weight change. Magnetic resonance imaging (MRI) of the bone marrow could establish whether major weight change alters fat:cell ratios in bone marrow in future studies.
In summary, our results suggest that increasing the thickness of overlying fat induces inaccuracies and measurement errors when BMD is assessed using DXA. Although overlying fat also affects QCT BMD measurements, the error is smaller and more uniform than with DXA BMD. These findings suggest that caution must be used when interpreting DXA BMD results of clinical studies in which weight and body composition are changing.
Acknowledgements
We would like to acknowledge Robbin Cleary, of the MGH Bone Density Center, for her assistance with this study. We also thank the study volunteers for their participation. Authors’ roles: Study design: EWY and JSF. Study conduct: EWY and BJT. Data collection: EWY. Data analysis: EWY and JKB. Data interpretation: EWY, BJT, JKB, and JSF. Drafting manuscript: EWY and JSF. Revising manuscript content: BJT and JKB. Approving final version of manuscript: EWY, BJT, JKB, and JSF. EWY takes responsibility for the integrity of the data analysis.
Footnotes
Author disclosures: JKB developed QCT PRO software and has a proprietary interest in Mindways Software. EWY, BJT, and JSF have no conflicts of interest to disclose.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Nelson L, Gulenchyn KY, Atthey M, Webber CE. Is a fixed value for the least significant change appropriate? J Clin Densitom. 2010;13(1):18–23. doi: 10.1016/j.jocd.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–3740. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89(3):1061–1065. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 5.von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metab Clin Exp. 2004;53(7):918–921. doi: 10.1016/j.metabol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Van Loan MD, Johnson HL, Barbieri TF. Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr. 1998;67(4):734–738. doi: 10.1093/ajcn/67.4.734. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley J, Stefanick ML, Fink HA, Lewis CE, Orwoll E Group OFiMSR. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90(4):1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 8.Tothill P. Dual-energy x-ray absorptiometry measurements of total-body bone mineral during weight change. J Clin Densitom. 2005;8(1):31–38. doi: 10.1385/jcd:8:1:031. [DOI] [PubMed] [Google Scholar]
- 9.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Int Med. 2006;166(22):2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 10.Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41(1):138–154. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Bligh M, Bidaut L, White RA, Murphy WA, Stevens DM, Cody DD. Helical multidetector row quantitative computed tomography (QCT) precision. Acad Radiol. 2009;16(2):150–159. doi: 10.1016/j.acra.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23(1):130–137. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein J. Towards a Physiologic Definition of Male Hypogonadism: How Much Testosterone Does a Man Really Need? ENDO Meeting. 2009 2009 Abstract #750132. [Google Scholar]
- 14.Engelke K, Mastmeyer A, Bousson V, Fuerst T, Laredo JD, Kalender WA. Reanalysis precision of 3D quantitative computed tomography (QCT) of the spine. Bone. 2009;44(4):566–572. doi: 10.1016/j.bone.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 16.Kalender WA, Felsenberg D, Genant HK, Fischer M, Dequeker J, Reeve J. The European Spine Phantom--a tool for standardization and quality control in spinal bone mineral measurements by DXA and QCT. Eur J Radiol. 1995;20(2):83–92. doi: 10.1016/0720-048x(95)00631-y. [DOI] [PubMed] [Google Scholar]
- 17.Svendsen O, Hassager C, Skodt V, Christiansen C. Impact of soft tissue on in vivo accuracy of bone. J Bone Miner Res. 1995;10(6):868–873. doi: 10.1002/jbmr.5650100607. [DOI] [PubMed] [Google Scholar]
- 18.Sabin MA, Blake GM, MacLaughlin-Black SM, Fogelman I. The accuracy of volumetric bone density measurements in dual x-ray absorptiometry. Calcif Tissue Int. 1995;56(3):210–214. doi: 10.1007/BF00298612. [DOI] [PubMed] [Google Scholar]
- 19.Ho CP, Kim RW, Schaffler MB, Sartoris DJ. Accuracy of dual-energy radiographic absorptiometry of the lumbar spine: cadaver study. Radiology. 1990;176(1):171–173. doi: 10.1148/radiology.176.1.2353087. [DOI] [PubMed] [Google Scholar]
- 20.Svendsen OL, Haarbo J, Hassager C, Christiansen C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am J Clin Nutr. 1993;57(5):605–608. doi: 10.1093/ajcn/57.5.605. [DOI] [PubMed] [Google Scholar]
- 21.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol. 1998;84(1):372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]
- 22.Snead DB, Birge SJ, Kohrt WM. Age-related differences in body composition by hydrodensitometry and dual-energy X-ray absorptiometry. J Appl Physiol. 1993;74(2):770–775. doi: 10.1152/jappl.1993.74.2.770. [DOI] [PubMed] [Google Scholar]
- 23.Madsen OR, Jensen JE, Sørensen OH. Validation of a dual energy X-ray absorptiometer: measurement of bone mass and soft tissue composition. Eur J Appl Physiol. 1997;75(6):554–558. doi: 10.1007/s004210050204. [DOI] [PubMed] [Google Scholar]
- 24.Tothill P, Laskey MA, Orphanidou CI, van Wijk M. Anomalies in dual energy X-ray absorptiometry measurements of total-body bone mineral during weight change using Lunar, Hologic and Norland instruments. Br J Radiol. 1999;72(859):661–669. doi: 10.1259/bjr.72.859.10624323. [DOI] [PubMed] [Google Scholar]
- 25.Evans EM, Mojtahedi MC, Kessinger RB, Misic MM. Simulated change in body fatness affects Hologic QDR 4500A whole body and central DXA bone measures. J Clin Densitom. 2006;9(3):315–322. doi: 10.1016/j.jocd.2006.04.117. [DOI] [PubMed] [Google Scholar]
- 26.Binkley N, Krueger D, Vallarta-Ast N. An overlying fat panniculus affects femur bone mass measurement. J Clin Densitom. 2003;6(3):199–204. doi: 10.1385/jcd:6:3:199. [DOI] [PubMed] [Google Scholar]
- 27.Buchwald H. Consensus Conference StatementBariatric surgery for morbid obesity: Health implications for patients, health professionals, and third-party payers. Surg Obes Rel Dis. 2005;1(3):371–381. doi: 10.1016/j.soard.2005.04.002. [DOI] [PubMed] [Google Scholar]